Abstract
We carried out a systematic review and meta-analysis to assess the efficacy and safety of propiverine for treating overactive bladder (OAB) in adult. A literature review was performed to identify all published randomized placebo-controlled trials (RCT) of propiverine for the treatment of OAB. The search included the following databases: PUBMED and EMBASE. The reference lists of retrieved studies were also investigated. A systematic review and meta-analysis were conducted. Ten publications involving nine different RCTs were used in the analysis. We found that propiverine was effective in treating OAB in our meta-analysis. The decrease in number of micturitions/24 h (P < 0.00001, the mean decrease was from 1.80 to 2.57) indicated that propiverine was more effective than the placebo. Propiverine also decrease the number of urgency, urgency incontinence, and nocturia and increase urine volume. However, the incidence of difficulty in voiding was higher with propiverine therapy compared with the placebo (P = 0.05, the mean percentage range from 0.34 to 4.93 %). The decrease of total international prostate symptom score (IPSS) (P < 0.0001, the mean decrease was from 12.5 to 16.1) indicated that propiverine add a1-adrenoceptor antagonist was more effective in decreasing the lower urinary tract symptom (LUTS). The combination therapy also decreases the voiding symptom and storage symptom scores and increases maximum flow rate. This meta-analysis shows that propiverine is a safe and effective treatment for OAB. The major adverse event associated with propiverine treatment was difficulty in voiding. Propiverine add a1-adrenoceptor antagonist was more effective in terms of decreasing difficulty in voiding.
Keywords: Propiverine, Overactive bladder, Alpha-blockers, Meta-analysis, Randomized controlled trials
Introduction
Overactive bladder (OAB) is characterized by symptoms of urgency, with or without urgency incontinence, usually with frequency and nocturia [1]. OAB symptoms are suggestive of urodynamically demonstrable detrusor overactivity [1]. A recent study showed that OAB symptoms negatively influence health-related quality of life and increase anxiety and depression [2]. Anticholinergic agents are often used to reduce detrusor overactivity and improve OAB symptoms. Propiverine is an antimuscarinic agent with a mixed mode of action in the treatment of symptoms associated with OAB. As well as blocking muscarinic receptors in the detrusor muscle, the drug also inhibits cellular calcium influx, thereby diminishing muscle spasm [3–5]. Propiverine was effective for urgency, frequency, and urgency incontinence, suggesting that it contributes to improving overall OAB symptoms, especially by improving urgency and urgency incontinence episodes; propiverine may have improved the daily living activities impaired by OAB [6]. However, the ability of propiverine to improve nocturia and the risk ofurinary retention remain controversial. Several clinical studies have demonstrated the clinical efficacy of propiverine in men with OAB. But the number of study was not enough and the main adverse was not considered.
The goal of the present study was to perform a meta-analysis to evaluate the safety and efficacy of propiverine in treating OAB, which may resolve some of the current controversies over use of the drug.
Materials and Methods
Inclusion Criteria
Randomized controlled trials (RCTs) that met the following criteria were included: (1) a study design that included treatment with propiverine; (2) the study provided accurate efficacy and safety data that could be analyzed, including the total number of subjects and the values of each index; and (3) the full text of the study could be accessed. If these inclusion criteria were not met, then the study was excluded from the analysis.
Search Strategy
MEDLINE (from 1966 to November 2014), EMBASE (from 1974 to November 2014), and the reference lists of retrieved studies were searched to identify RCTs that referred to the effects of propiverine treatment. The following search terms were used: propiverine, overactive bladder, alpha-blockers, meta-analysis, and randomized controlled trials.
Trial Selection
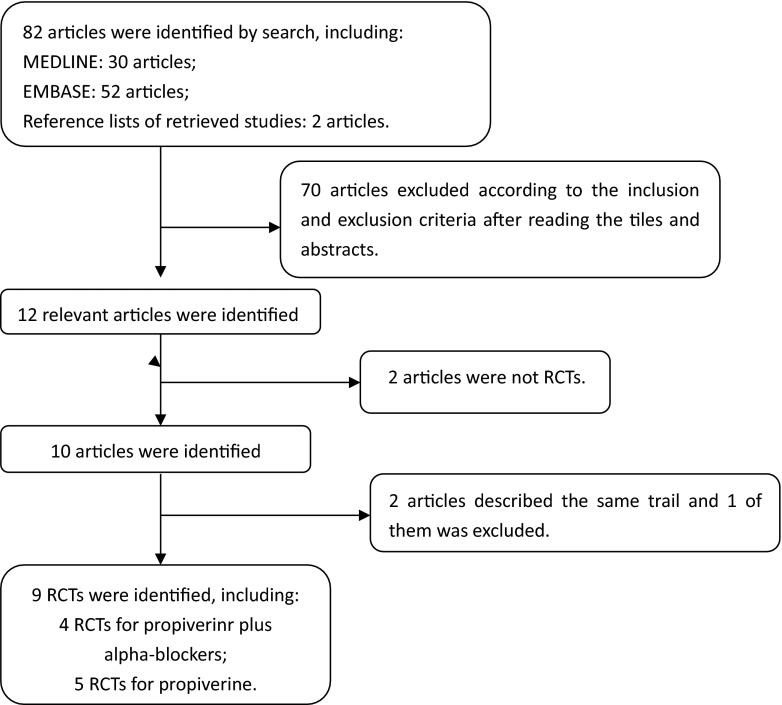
When the same study was published in various journals or in different years, the most frequently cited one was used for the meta-analysis. If the same group of researchers studied a group of subjects with multiple experiments, then each study was included. Together, we discussed each of the RCTs that were included and excluded studies that either failed to meet the inclusion criteria or could not be agreed upon by the authors. A flow diagram of the study selection process is presented in Fig. 1.
Fig. 1.
A flow diagram of the study selection process
Quality Assessment
The methodological quality of each study was assessed according to how patients were allocated to the arms of the study, the concealment of allocation procedures, blinding, and the data loss due to attrition. The studies were then classified qualitatively according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [7]. Based on the quality assessment criteria, each study was rated and assigned to one of the three following quality categories: A, if all quality criteria were adequately met, the study was deemed to have a low risk of bias; B, if one or more of the quality criteria was only partially met or was unclear, the study was deemed to have a moderate risk of bias; or C, if one or more of the criteria were not met, or not included, the study was deemed to have a high risk of bias. Sensitivity analyses were then performed on the basis of whether these quality factors were adequate, inadequate, or unclear. Differences were resolved by discussion among the authors.
Data Extraction
The following information was collected: (1) the name of the first author and the publication year; (2) the study design and sample size; (3) the therapy that the patients received; (4) the source of the patients; and (5) data including the number of micturitions/24 h, urgency episodes/24 h, urgency incontinence episodes/24 h, urine volume (ml) per micturition, nocturia episodes/24 h, total IPSS improvement, IPSS voiding improvement, IPSS storage improvement, peak urinary flow rate (Qmax), adverse events, difficulty in voiding, dry mouth, and constipation.
Statistical Analysis
The meta-analysis of comparable data was carried out using Review Manager 5.1.0. Due to the large number of plots, we combined the 6 forest plots into 1 plot using Adobe Photoshop CS (Figs. 2, 3, 4, 5, and 6).
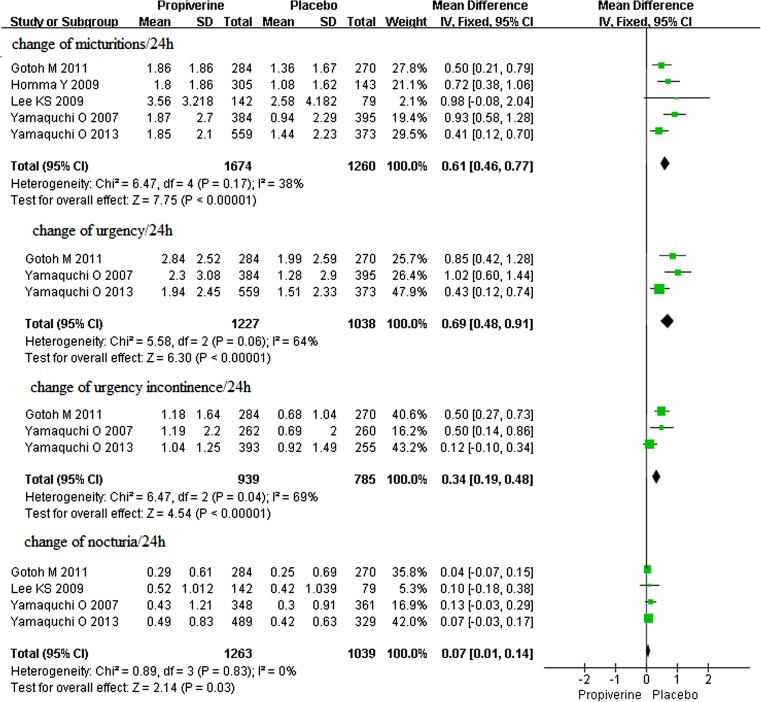
Fig. 2.
The change of the mean number of micturitions/24 h, urgency/24 h, urgency incontinence/24 h, and nocturia/24 h in propiverine versus placebo
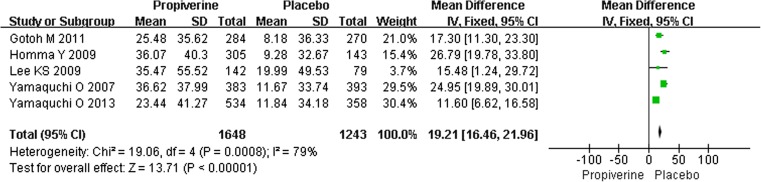
Fig. 3.
The change of the mean urine volume (ml) per micturition in propiverine versus placebo
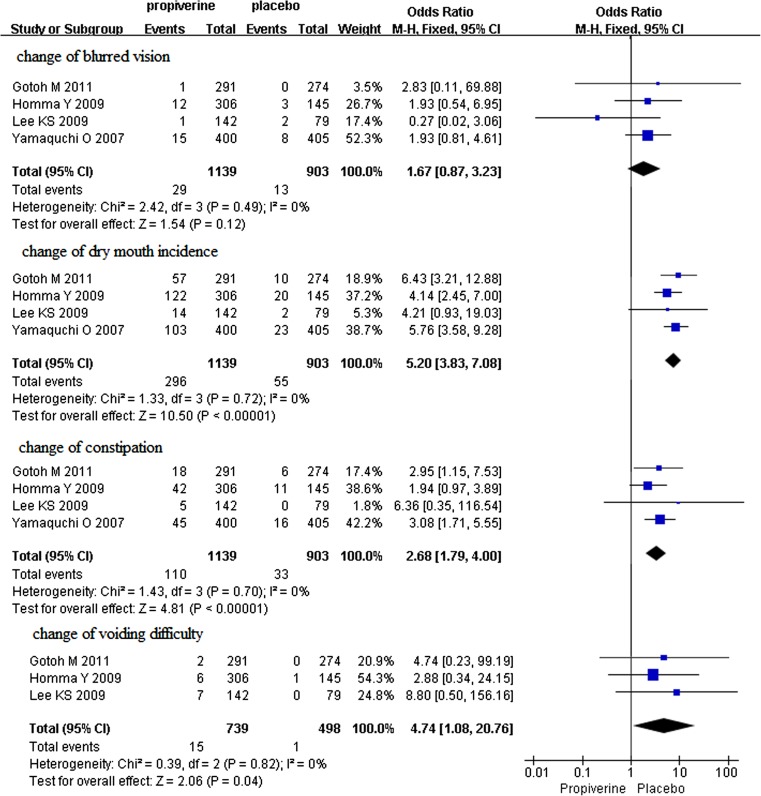
Fig. 4.
The changes of dry mouth, constipation, blurred vision, and difficulty in voiding in propiverine versus placebo
Fig. 5.
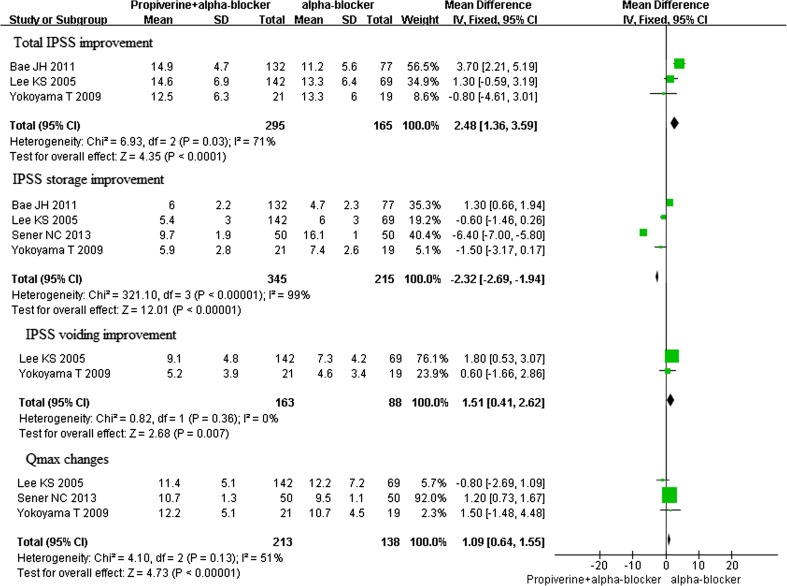
The changes of total IPSS, IPSS voiding, IPSS storage, and Qmax in propiverine + alpha-blockers versus alpha-blockers
Fig. 6.
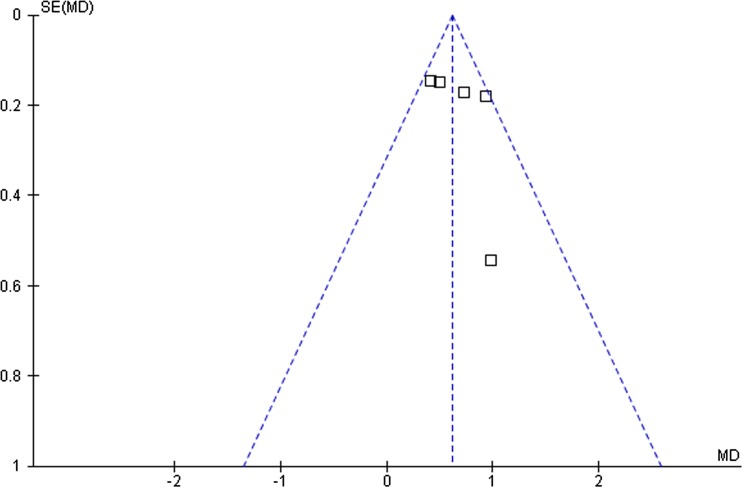
Funnel plot of the studies represented in our meta-analysis
Results
Characteristics of Individual Studies
The database search and reference lists of retrieved studies found 82 potential articles to be used in our meta-analysis. Based on the inclusion and exclusion criteria, 70 articles were excluded after simply reading the titles and abstracts of the articles and two articles were excluded because they lacked a full text. In all, 10 articles with 9 RCTs were included in the analysis, with 5 RCTs [6, 8–11] for propiverine hydrochloride for overactive bladder (Figs. 2, 3, and 4) and 4 RCTs [12–15] for propiverine hydrochloride for overactive bladder and in adult with lower urinary tract symptoms (Fig. 5). Baseline characteristics of the 9 individual studies included in our meta-analysis are listed in Table 1.
Table 1.
Study and patient characteristics
| Study | Therapy in experimental group | Therapy in control group | Country | Sample size | Duration of treatment | Inclusion population | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Gotoh M [6] | Propiverine | Placebo | Japan | 271 | 257 | 2 weeks | ≥20 years, OAB symptoms ≥12 weeks, ≥8 micturitions/24 h, and ≥1UI episodes/24 h or ≥1 urgency episodes/24 h |
| Lee KS [11] | Propiverine | Placebo | Korea | 142 | 79 | 2 weeks | ≥18 years, OAB for ≥3 months, ≥10 voids/24 h during the 3-day voiding diary period |
| Homma Y [10] | Propiverine | Placebo | Japan | 279 | 131 | 2 weeks | ≥20 years, UI (≥5 episodes/week), micturition (≥8 voids/day), and urgency (≥1 episode/day) |
| Yamaguchi O [8] | Propiverine | Placebo | Japan | 364 | 371 | 2 weeks | ≥20 years, OAB ≥6 months, ≥8 voids/24 h, ≥3 episodes of urgency, ≥3 episodes of UI for 3 days voiding diary period |
| Yamaguchi O [9] | Propiverine and placebo | Placebo | Japan | 532 | 359 | 2 weeks | ≥20 years, OAB symptoms ≥24 weeks, ≥8 micturitions daily, ≥1 urgency or UI episodes daily for 3 days voiding diary period |
| Sener NC [15] | Terazosin 2 mg/day plus propiverine HCL 15 mg/day | Terazosin 2 mg/day and placebo | Turkey | 50 | 50 | No mention | Age 50–80, OAB ≥6 months, and urodynamically proven BOO, urgency daily episode ≥1, micturition/24 h ≥8 |
| Bae JH [14] | Alfuzosin 10 mg/day, and propiverine 10 mg/day | Alfuzosin 10 mg/day | Korea | 132 | 77 | No mention | LUTS/BPH with IPSS ≥12 and IPSS storage subscore ≥4 |
| Yokoyama T [13] | Naftopidil 50 mg/day plus propiverine hydrochloride 20 mg/day | Naftopidil 50 mg/day | Japan | 21 | 19 | 4 weeks | Age ≥50 years, IPSS ≥8, ≥1 urgency episode daily, ≥8 micturitions daily, nocturia ≥1 episode/day, and PVR ≤50 ml |
| Lee KS [12] | Doxazosin 4 mg/day plus propiverine 20 mg/day | doxazosin 4 mg once daily | Korea | 142 | 69 | No mention | Age ≥40, IPSS ≥12, PSA <2.5, micturition/24 h ≥8, urgency (or UI)/24 h >3, and documented detrusor pressure >10 cm H2O |
OAB overactive bladder, UI urgency incontinence, BOO bladder outlet obstruction
Quality of Individual Studies
Among the studies included in the analysis, three described the randomization processes that they had employed. Nine studies used blinding methods, including eight double-blinded RCTs. The quality levels of the 9 identified studies are ranged from A to B (Table 2). The funnel plot provided a qualitative estimation of publication bias of the studies, and no evidence of bias was found (Fig. 6).
Table 2.
Quality assessment of individual study
| Study | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow-up | Calculation of sample size | Statistical analysis | Intention-to-treat analysis | Level of quality |
|---|---|---|---|---|---|---|---|---|
| Yamaguchi O [8] | A | A | A | 70 | Yes | The paired t test | Yes | A |
| Gotoh M [6] | A | A | A | 11 | Yes | The paired t test | Yes | A |
| Lee KS [11] | A | A | A | 7 | Yes | The paired t test | Yes | A |
| Homma Y [10] | A | A | A | 47 | Yes | The paired t test | Yes | A |
| Yamaguchi O [9] | A | A | A | 64 | Yes | The paired t test | Yes | A |
| Sener NC [15] | A | A | A | 0 | Yes | The paired t test | Yes | A |
| Bae JH [14] | A | B | B | 43 | Yes | The paired t test | Yes | B |
| Yokoyama T [13] | A | A | A | 2 | Yes | The paired t test | Yes | A |
| Lee KS 12 | A | A | A | 2 | Yes | The paired t test | Yes | A |
A all quality criteria met (adequate): low risk of bias. B one or more of the quality criteria only partly met (unclear): moderate risk of bias. C one or more criteria not met (inadequate or not used): high risk of bias
Clinical Outcome after Propiverine Hydrochloride Treatment
The Number of Micturitions per 24 h
Five studies of the effects of propiverine for overactive bladder were identified, which involved 2934 participants (1674 in the treatment group and 1260 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the five trials (P = 0.17), and thus a fixed effects model was chosen for the analysis. Based on our analysis, the decrease in the number of micturitions/24 h in the propiverine group was significantly greater than in the control group (P < 0.00001) (Fig. 2). The mean decrease in number of micturitions/24 h was from 1.80 to 2.57.
Urgency Episodes per 24 h
Three studies of the effects of propiverine hydrochloride for overactive bladder were identified, which involved 2265 participants (1227 in the treatment group and 1038 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the three trials (P = 0.06), and thus a fixed effects model was chosen for the analysis. Based on our analysis, the decreased urgency episodes per 24 h in the propiverine group was significantly greater than in the control group (P < 0.00001) (Fig. 2). The mean decrease in number of urgency episodes per 24 h was from 1.94 to 2.85.
Urgency Incontinence Episodes per 24 h
Three studies of the effects of propiverine hydrochloride for overactive bladder were identified, which involved 1724 participants (939 in the treatment group and 785 in the control group); conclusions differed across studies. Based on our analysis, the decreased urgency incontinence episodes per 24 h in the propiverine group was significantly greater than in the control group (P < 0.00001) (Fig. 2). The mean decrease in number of urgency incontinence episodes per 24 h was from 1.04 to 1.52.
Urine Volume (ml) per Micturition
Five studies of the effects of propiverine hydrochloride for overactive bladder were identified, which involved 2891 participants (1648 in the treatment group and 1243 in the control group); conclusions differed across studies. Based on our analysis, the increase of urine volume (ml) per micturition in the propiverine group was significantly greater than in the control group (P < 0.00001) (Fig. 3). The mean increase of urine volume (ml) per micturition was from 23.44 to 45.40 ml.
Nocturia Episodes per 24 h
Four studies of the effects of propiverine hydrochloride for overactive bladder were identified, which involved 2302 participants (1263 in the treatment group and 1039 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the four trials (P = 0.83), and thus a fixed effects model was chosen for the analysis. Based on our analysis, the decreased nocturia episodes per 24 h in the propiverine group was significantly greater than in the control group (P = 0.03) (Fig. 2). The mean decrease in number of nocturia episodes per 24 h was from 0.29 to 0.43.
Major Adverse Effect
Four studies of the major adverse effects of propiverine hydrochloride for overactive bladder were identified, which involved 2042 participants (1139 in the treatment group and 903 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the four trials (P = 0.72, 0.70, 0.49), and thus a fixed effects model was chosen for the analysis. Based on our analysis, there was no clinical difference in blurred vision (P = 0.12) (Fig. 4), but the increase of dry mouth and constipation in the propiverine group was significantly greater than in the control group (P < 0.00001) (Fig. 4). But their severity was mostly mild. Three studies of difficulty in voiding of propiverine for overactive bladder were identified, which involved 1237 participants (739 in the treatment group and 498 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the three trials (P = 0.82), and thus a fixed effects model was chosen for the analysis. The incidence of difficulty in voiding in the safety analysis population was 2.6 % in the propiverine group and 0.42 % in the placebo group. There were significantly higher incidence of voiding difficulty in the propiverine group than in the placebo group (P = 0.04) (Fig. 4).
Clinical Outcome with Propiverine for OAB with Lower Urinary Tract Symptoms
Four RCTs involving two multicentre trials were identified, which referred to changes in the total IPSS, maximum flow rate, storage symptom score, and voiding symptom score.
The Total IPSS
Three studies of the effects of propiverine combined alpha-blockers for the treatment of OAB associated with lower urinary tract symptoms were identified, which involved 460 participants (295 in the treatment group and 165 in the control group); conclusions differed across studies. Based on our analysis, the decreased total international prostate symptom score (IPSS) in the propiverine combined alpha-blockers group was significantly greater than in the alpha-blockers group (P < 0.0001) (Fig. 5).
The Storage Symptom Score
Four studies of the effects of propiverine combined alpha-blockers for the treatment of OAB associated with lower urinary tract symptoms were identified, which involved 560 participants (345 in the treatment group and 215 in the control group); conclusions differed across studies. Based on our analysis, the decreased storage symptom score in the propiverine combined alpha-blockers group was significantly greater than in the alpha-blockers group (P < 0.00001) (Fig. 5).
The Voiding Symptom Score
Two studies of the effects of propiverine combined alpha-blockers for the treatment of OAB associated with lower urinary tract symptoms were identified, which involved 251 participants (163 in the treatment group and 88 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the two trials (P = 0.36), and thus a fixed effects model was chosen for the analysis. Based on our analysis, the decreased voiding symptom score in the propiverine combined alpha-blockers group was significantly greater than in the alpha-blockers group (P = 0.007) (Fig. 5).
Maximum Flow Rate
Three studies of the effects of propiverine combined alpha-blockers for the treatment of OAB associated with lower urinary tract symptoms were identified, which involved 351 participants (213 in the treatment group and 138 in the control group); conclusions differed across studies. According to our analysis, no heterogeneity was found among the three trials (P = 0.13), and thus a fixed effects model was chosen for the analysis. Based on our analysis, the increased maximum flow rate in the propiverine combined alpha-blockers group was significantly greater than in the alpha-blockers group (P < 0.00001) (Fig. 5). The mean maximum flow rate changes from 10.2–10.7 ml/s to 10.7–12.25 ml/s.
Discussion
The objective of this meta-analysis was to investigate the efficacy and safety of propiverine for OAB in adult. The number of micturitions/24 h was reduced in the propiverine group compared to that in the placebo group; the difference between the groups in the change was significant, confirming the superiority of propiverine to placebo. In addition, the efficacy of propiverine for urgency, urgency incontinence, and nocturia was confirmed by comparison with placebo. And the main symptoms of the OAB are urgency, frequency, and urgency incontinence. So, our study reveals that propiverine contributes to improving OAB symptoms significantly. Furthermore, the incidence of voiding difficulty was higher with propiverine than with placebo. The main adverse effects reduced the quality of life (QOL) largely, particularly those with OAB associated with LUTS. Many studies suggested that propiverine combined a1-receptor antagonist was more effective for the treatment of both OAB and LUTS [16]. In theory, when voiding symptoms are the first priority in LUTS, an a1-receptor antagonist can be considered as a treatment of choice. When storage symptoms are the first priority in LUTS, combined therapy with an a1-receptor antagonist and an anticholinergic can be an appropriate choice. These medication therapies could theoretically improve both voiding and storage symptoms [17, 18]. In the meta-analysis, total IPSS, voiding symptom score, and storage symptom score were significantly improved in the combination group than in the a1-receptor antagonist monotherapy. Moreover, propiverine combined a1-receptor antagonist significantly increased the mean maximum flow rate. The reasons may be as follows: When combined with propiverine, alpha-blockers make up the decreased maximum flow rate of propiverine [19]. Besides, the improvements in storage symptoms after propiverine medication increase voiding volume at the time of evaluating Qmax [6]. These factors might have resulted in the marked improvements in Qmax in this study. Our results suggest it is better to use an a1-receptor antagonist first to ease the urinary symptoms and then use anticholinergics to improve the storage symptoms.
Based on our analysis, the decreased nocturia episodes per 24 h in the propiverine group was significantly greater than in the control group (P = 0.03) (Fig. 2). The mean decrease in number of nocturia episodes per 24 h was from 0.29 to 0.43. However, the ability of propiverine to decrease the number of nocturia episodes remains controversial. Some people think propiverine can decrease the number of nocturia episodes more effectively than placebo [8, 9, 11]. Others do not think that [6]. The reasons may be the following: nocturia was affected by multiple factors. The baseline number of nocturia episodes was small in this study population. About one nocturia episode is common in the elderly independent of OAB, and nocturia is affected by many factors other than OAB, such as nocturnal polyuria and sleep disorders. These may have affected the interpretation of the efficacy of propiverine for nocturia. We suggest that further high-quality prospective studies are needed to identify the efficacy of propiverine on nocturia.
This meta-analysis only includes nine studies with sample sizes that were not large. In addition, unpublished studies were not included in the analysis. These factors may have resulted in bias. Additionally, one of the RCTs did not provide maximum flow rate. According to the quality assessment scale that we developed, the quality of individual studies in the meta-analysis was variable. Quality appears to be the main reason for heterogeneity among these studies, and this heterogeneity likely arose from several factors. First, there were important differences in the adequacy of the randomization process, blinding methodology, the use of control groups, and the duration for which the drugs were used preoperatively. Second, the study outcomes may have been measured by different methods. Third, the researchers in the trials were different. Finally, potential selection biases could have influenced the homogeneity of the groups, and relatively small sample sizes limited the statistical power for identifying true associations. After the heterogeneity among individual studies is taken into account, this meta-analysis remains crucial for assessing the safety and efficiency of propiverine. More high-quality trails with larger samples are proposed to learn more about the efficacy and safety of the agent.
Conclusion
This meta-analysis shows that propiverine is a safe and effective treatment for OAB. The major adverse event associated with propiverine treatment was difficulty in voiding. Propiverine add a1-adrenoceptor antagonist was more effective in terms of decreasing difficulty in voiding. Further high-quality prospective studies are needed to confirm this observation.
Acknowledgments
Conflict of Interest Statement
The authors had no conflicts of interest to declare in relation to this article.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–96. doi: 10.1016/j.urology.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhu HL, Brain KL, Aishima M, Shibata A, Young JS, Sueishi K, et al. Actions of two main metabolites of propiverine (M-1 and M-2) on voltage-dependent L-type Ca2+ currents and Ca2+ transients in murine urinary bladder myocytes. J Pharmacol Exp Ther. 2008;324(1):118–127. doi: 10.1124/jpet.107.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, et al. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol. 2001;36:99–109. doi: 10.1016/S0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 5.Yono M, Yoshida M, Wada Y, Kikukawa H, Takahashi W, Inadome A, et al. Pharmacological effects of tolterodine on human isolated urinary bladder. Eur J Pharmacol. 1999;368:223–230. doi: 10.1016/S0014-2999(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh M, Yokoyama O, Nishizawa O. Propiverine hydrochloride in Japanese patients with overactive bladder: a randomized, double-blind, placebo-controlled trial. Int J Urol. 2011;18:365–373. doi: 10.1111/j.1442-2042.2011.02732.x. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Green S, editors (2011) Cochrane handbook for systematic reviews of interventions, v.5.1 [updated March 2011]. Cochrane Collaboration Web site. http://www.cochrane-handbook.org/
- 8.Yamaguchi O, Marui E, Kakizaki H, Itoh N, Yokota T, Okada H, et al. Japanese Solifenacin Study Group: randomized, double-blind, placebo- and propiverine-controlled trial of the once-daily antimuscarinic agent solifenacin in Japanese patients with overactive bladder. BJU Int. 2007;100(3):579–587. doi: 10.1111/j.1464-410X.2007.07031.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi O, Uchida E, Higo N, Minami H, Kobayashi S, Sato H. Efficacy and safety of once-daily oxybutynin patch versus placebo and propiverine in Japanese patients with overactive bladder: a randomized double-blind trial. Int J Urol. 2014;21(6):586–593. doi: 10.1111/iju.12372. [DOI] [PubMed] [Google Scholar]
- 10.Homma Y, Yamaguchi O. A randomized, double-blind, placebo- and propiverine-controlled trial of the novel antimuscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol. 2009;16(5):499–506. doi: 10.1111/j.1442-2042.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee KS, Lee HW, Choo MS, Paick JS, Lee JG, Seo JT, et al. Urinary urgency outcomes after propiverine treatment for an overactive bladder: the ‘Propiverine study on overactive bladder including urgency data. BJU Int. 2010;105(11):1565–1570. doi: 10.1111/j.1464-410X.2009.09050.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS, Choo MS, Kim DY, Kim JC, Kim HJ, Min KS, et al. Combination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter study. J Urol. 2005;174(4 Pt 1):1334–1338. doi: 10.1097/01.ju.0000173630.94559.fd. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama T, Uematsu K, Watanabe T, Sasaki K, Kumon H, Nagai A. Naftopidil and propiverine hydrochloride for treatment of male lower urinary tract symptoms suggestive of benign prostatic hyperplasia and concomitant overactive bladder: a prospective randomized controlled study. Scand J Urol Nephrol. 2009;43(4):307–314. doi: 10.1080/00365590902836740. [DOI] [PubMed] [Google Scholar]
- 14.Bae JH, Kim SO, Yoo ES, Moon KH, Kyung YS, Kim HJ. Efficacy and safety of low-dose propiverine in patients with lower urinary tract symptoms/benign prostatic hyperplasia with storage symptoms: a prospective, randomized, single-blinded and multicenter clinical trial. Korean J Urol. 2011;52(4):274–278. doi: 10.4111/kju.2011.52.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sener NC, Ozturk U, Goktug HN, Gucuk A, Nalbant I, Yesil S, et al. Efficacy and safety of propiverine and terazosine combination for one year in male patients with luts and detrusor overactivity. Int Braz J Urol. 2013;39(4):513–518. doi: 10.1590/S1677-5538.IBJU.2013.04.09. [DOI] [PubMed] [Google Scholar]
- 16.Madersbacher H, Mürtz G. Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic) World J Urol. 2001;19(5):324–335. doi: 10.1007/s003450100223. [DOI] [PubMed] [Google Scholar]
- 17.Engström G, Henningsohn L, Walker-Engström ML, Leppert J. Impact on quality of life of different lower urinary tract symptoms in men measured by means of the SF 36 questionnaire. Scand J Urol Nephrol. 2006;40:485–494. doi: 10.1080/00365590600830862. [DOI] [PubMed] [Google Scholar]
- 18.Sountoulides P, van Dijk MM, Wijkstra H, de la Rosette JJ, Michel MC. Role of voiding and storage symptoms for the quality of life before and after treatment in men with voiding dysfunction. World J Urol. 2010;28:3–8. doi: 10.1007/s00345-009-0480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapple CR, Carter P, Christmas TJ, Kirby RS, Bryan J, Milroy EJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol. 1994;74(1):50–56. doi: 10.1111/j.1464-410X.1994.tb16546.x. [DOI] [PubMed] [Google Scholar]