Abstract
Laparoscopic distal pancreatectomy (LDP) has gained large popularity in recent years, although the choice of whether to preserve the spleen has remained inconsistent. The aim of our study was to report our experiences with LDP and to provide evidence for the safety of the operative technique and an evaluation index of splenic function. We retrospectively evaluated all LDPs performed at our institution between March 2008 and February 2012. Cases were divided into a laparoscopic spleen-preserving distal pancreatectomy (LSPDP) group (n = 14) and an LDP with splenectomy (LDPS) group (n = 19). Parametric and nonparametric statistical analyses were used to compare perioperative and oncologic outcomes. Demographic characteristics, operating time, length of stay, estimated blood loss, transfusion requirement, pathologic diagnosis, and complication rate were similar between groups. Patients who underwent LDPS tended to have larger masses and lower pancreatic fistula rates, but these differences were not significant. White blood cell (WBC) counts were significantly higher in the LDPS group than in the LSPDP group on postoperative days 1 and 7. To avoid splenectomy-associated complications, preservation of the spleen and especially the splenic vessels are preferred. This procedure can be performed safely and feasibly. Lower postoperative WBC counts may imply better splenic function.
Keywords: Laparoscopic distal pancreatectomy, Spleen preserving, Pancreatectomy, Minimally invasive surgery
Introduction
Laparoscopic distal pancreatectomy (LDP) is gradually becoming the gold standard treatment for benign and malignant neoplasms in the body and tail of the pancreas [1, 2]. Traditionally, splenectomy was performed in association with LDP because the spleen lies adjacent to the tail of pancreas and shares the same blood supply. Since the discovery of the important immunologic functions of the spleen and the risks for overwhelming post-splenectomy infection (OPSI), hypercoagulability, and hematological malignancies after splenectomy [3], splenic preservation has increasingly been advocated.
Historically, splenic preservation has been controversial. Spleen-preserving techniques have been associated with outcomes equivalent to those of splenectomy [4–6] in open distal pancreatectomy. Furthermore, splenectomy may lead to immediate postoperative complications, such as OPSI, subphrenic abscess formation, and hypercoagulability [7]. However, criticisms of splenic preservation include increased operating risk and time, and postoperative complications [8, 9]. Thus, the superiority of a spleen-preserving or splenectomy approach remains a matter of debate. In this retrospective case–control study, splenic function, and perioperative and oncologic outcomes were compared between patients managed by spleen-preserving [laparoscopic spleen-preserving distal pancreatectomy (LSPDP)] and splenectomy [LDP with splenectomy (LDPS)] techniques. The aims of the study were to report our experience with LDP, to identify any significant difference in function or outcomes between treatment groups, and to provide evidence for the safety of the operative technique and an evaluation index of splenic function.
Materials and Methods
Patients and Definitions
We retrospectively evaluated all LDPs (n = 34) performed at our institution from the time that we began to perform this operation from March 2008 to February 2012.
One patient who underwent LDP to treat an invasive malignant tumor and required en bloc resection of other intra-abdominal organs was excluded from the study. Another patient who underwent LDP with cholecystostomy was not excluded. Thus, our cohort comprised 33 patients who underwent LDP with or without splenectomy (LSPDP, n = 14; LDPS, n = 19). Three patients who underwent LDPS had malignant masses classified as tumor, nodes, and metastases (TNM) stage Ia (n = 1) or IIb (n = 2) [10, 11].
The following data were recorded for each patient: demographic information (age, sex, and body mass index), operative technique, operating time, conversion to open operation, estimated blood loss (EBL), mass size, postoperative length of stay (LoS), pathologic diagnosis, white blood cell (WBC) count and neutrophil percentage (NE%) on postoperative days 1 and 7, complications, and follow-up findings.
The calculation of mass size (height × length × width) was based on pathologic or radiologic measurements, depending on the properties of the neoplasm. The operating time was measured from the injection of the first trocar to final skin suturing. The presence and grade of postoperative pancreatic fistula (PF) were classified according to the definitions of the International Study Group on Pancreatic Fistula [12].
Operative Techniques
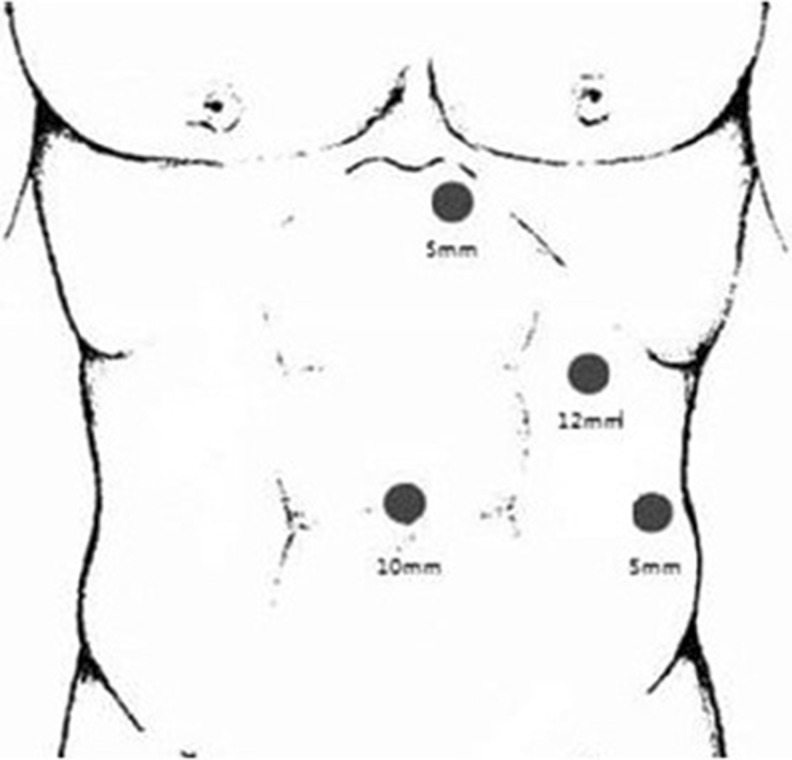
Two teams of surgeons performed operations in both groups, and the choice of operative approach was based on the surgeons’ and patients’ preferences. Each patient was placed in a supine position on an operating table that allowed his/her position to be changed easily. Two surgeons, one of whom operated the laparoscope, and a nurse stood to the right of the patient, and the first assistant stood on the left side. After pneumoperitoneum was established in the umbilicus (10 mm), a 30° angled laparoscope (HD ENDOEYE, 10 mm; Olympus Corporation, Tokyo, Japan) was inserted. We then explored the abdominal cavity for undetected pathologies and determined the position of the lower extremity of the spleen, and the other three trocars were inserted as shown in Fig. 1.
Fig. 1.
Port positions
Laparoscopic Distal Pancreatectomy with Splenectomy
The entry to the lesser sac was gained by dividing the greater omentum and gastrocolic, and gastrosplenic ligaments with Ligasure™ Vessel Sealing System (Valleylab, Boulder, CO, USA) or staples. The body and tail of the pancreas were explored to determine the properties, mobility, and invasiveness of the neoplasm. Laparoscopic ultrasound was used to delineate the neoplasm and identify its relationship to the splenic vessels and surrounding organs. The posterior tissue was divided along the lower border of the pancreas. The site of pancreatic transection was determined by the location and size of the tumor; we generally chose a site 2 cm from the neoplasm. The EndoGIA system (Echelon 60; Ethicon EndoSurgery, Cincinnati, OH, USA) containing appropriate staples was inserted through the 12-mm trocar to transect the pancreas. The tail of the pancreas was lifted, and the splenorenal ligaments were divided. By retracting the pancreas inferiorly, the superior pole of the spleen and the retrogastric vessels were divided. If the pancreas was difficult to lift, we inserted a #8 catheter through its posterior portion, as shown in Fig. 2. The resected pancreas and spleen specimen were placed into a drainage bag or a 3-L bag prepared in our laboratory. The 12-mm port was extended to enable extraction of the specimen without rupture. The procedure was completed by placing a closed drain in the left subphrenic cavity.
Fig. 2.
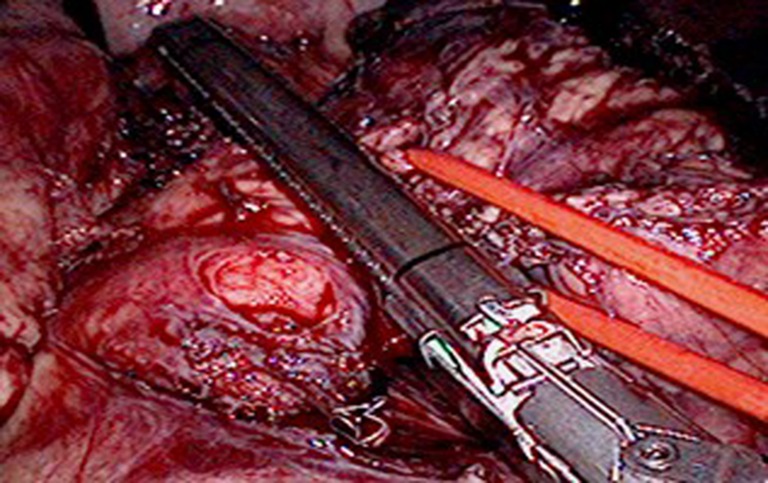
Division of the body of the pancreas using an EndoGIA system (Echelon 60; Ethicon EndoSurgery, Cincinnati, OH, USA)
Laparoscopic Spleen-Preserving Distal Pancreatectomy
The lesser sac was entered via the gastrocolic ligament, and the greater curvature was mobilized carefully to ensure that the gastric vessels remained intact. The body and tail of the pancreas were explored and mobilized, and the pancreas was transected using the EndoGIA system. The splenic vessels were divided at the hilum. The pancreas and proximal splenic vessels were then divided and secured with staples, LigaSure, or clips if necessary. The other procedures were the same as those described above for LDPS.
Statistical Analysis
Statistical analyses were performed using SPSS software (ver. 16.0; SPSS Inc., Chicago, IL, USA). Characteristics and outcomes were compared between the two patient groups. Continuous and normal variables were expressed as means and compared using t-tests. Continuous and non-normal variables were expressed as medians and compared using the Mann–Whitney U-test. Categorical variables were expressed as proportions and compared using Fisher’s exact test. Differences were considered statistically significant when P < 0.05.
Results
A total of 33 cases (14 LSPDPs, 19 LDPSs) were analyzed. Patient characteristics and diagnoses are presented in Table 1. Demographic characteristics were similar between the two groups. The indications for surgery differed; no malignant tumors were treated using LSPDP, whereas three (9 %) patients in the LDPS group had malignant tumors (one TNM stage Ia and two TNM stage IIb). The excised masses tended to be larger in the LDPS group than in the LSPDP group (38.5 vs. 18.8 cm3), but this difference was not significant.
Table 1.
Demographic characteristics of patients undergoing laparoscopic spleen-preserving distal pancreatectomy (LSPDP) and laparoscopic distal pancreatectomy with splenectomy (LDPS)
| Variable | LSPDP (n = 14) | LDPS (n = 19) | P |
|---|---|---|---|
| Age(years) | 39.1 ± 18.5 | 43.2 ± 13.6 | 0.47 |
| Sex, n(%) | 0.39 | ||
| Male | 4(29) | 3(16) | |
| Female | 10(71) | 16(84) | |
| Body mass index | 22.4 ± 4.1 | 23.5 ± 4.1 | 0.42 |
| Mass size(cm3) | 18.8 ± 27.5 | 35.5 ± 48 | 0.25 |
| Diagnosis | |||
| Serous cystadenoma | 2 | 1 | |
| Neuroendocrine tumor | 3 | 2 | |
| Cyst | 2 | 1 | |
| Solid pseudopapillary tumor | 3 | 5 | |
| Pseudocyst | 1 | 1 | |
| Mucinous tumors | 1 | 6 | |
| Malignant mass | 0 | 3 | |
| Accessory spleen | 2 | 0 | |
Table 2 presents intraoperative and postoperative data. Intraoperatively, the LSPDP and LDPS groups showed no difference in EBL, operating time, or transfusion requirement. Two LDPSs (6.1 %) and no LSPDP were converted to open procedures, but this difference was not significant. One conversion was due to uncontrollable bleeding, and the other was combined with multiple organ resection.
Table 2.
Outcomes of patients undergoing laparoscopic spleen-preserving distal pancreatectomy (LSPDP) or laparoscopic distal pancreatectomy with splenectomy (LDPS)
| Variable | LSPDP (n = 14) | LDPS (n = 19) | P |
|---|---|---|---|
| Operating time (min) | 137(70–160) | 149(85–250) | 0.4 |
| Estimated blood loss (ml) | 179(50–700) | 187(50–700) | 0.9 |
| Length of stay (d) | 11(5–28) | 10(7–14) | 0.5 |
| Received blood transfusion (n,%) | 2(14.3) | 3(15.8) | 1.0 |
| Conversion to open (n, %) | 0 | 2(10.5) | 0.49 |
| Overall complications (n, %) | 5 (35.7) | 6 (31.6) | 1.0 |
| Pancreatic fistula (n, %) | 4 (28.5) | 3 (15.9) | 0.42 |
| Splenic infarction (n, %) | 1 (7.1) | 0 | 0.42 |
| WBC count at PoD 1 | 11.7 ± 4.5 | 17 ± 5.3 | 0.005 |
| NE% at PoD 1 | 0.82 ± 0.11 | 0.87 ± 0.06 | 0.1 |
| WBC count at PoD 7 | 8.5 ± 4.4 | 13.5 ± 5.5 | 0.008 |
| NE% at PoD 7 | 0.68 ± 0.1 | 0.73 ± 0.08 | 0.1 |
| Follow-up (months) | 16.7(3–30) | 12.7(6–29) | 0.4 |
WBC white blood cell, PoD postoperative day, NE% neutrophil percentage
Overall, 11 (33 %) patients had 15 complications, with no significant difference in complication rate between groups. Seven (21 %) patients had pancreatic leaks; the rate of this complication was higher in the LSPDP group (28.6 %; n = 4) than in the LDPS group (15.8 %; n = 3), but this difference was not significant (P = 0.42). The leaks were managed nonoperatively with persistent drainage for grade B leaks or percutaneous drainage for grade C leaks. Four patients had serosal effusions, but only one patient required treatment with percutaneous drainage. One patient in each group suffered infectious complications. Another patient had multiple premature ventricular contractions that were not related to the operation. Patients in the LDPS group had significantly higher median WBC counts on postoperative days1 and 7(P = 0.005 and P = 0.008, respectively), but NE% was similar.
All but one patient (32/33, 97 %) attended a follow-up appointment within 3 months of surgery. No patient complained of persistent left upper quadrant pain. One patient (7.1 %) in the LSPDP group had a splenic infarction. No patient who underwent LDPS has developed OPSI to date. The three patients with malignant masses who underwent LDPS remain alive at 7, 11, and 27 months, respectively, after the operations.
Discussion
Doubts have been raised about the safety of splenic preservation. In this study, we demonstrated that LSPDP is a feasible and comparably safe, efficient approach. LSPDP was associated with an operating time, EBL, LoS, and transfusion requirement similar to those of LDPS. Both open and LSPDP have been reported to be more technically challenging than procedures with concomitant splenectomy [13]. However, Rodriguez et al. [14] reported that SPDP was associated with a shorter hospital stay and operating time, less blood loss, and fewer complications. Kristin et al. [15] confirmed these findings for laparoscopic operations. In contrast, our series showed similarities in these parameters between operative techniques. Although some may argue that our LSPDP cases were technically easier due to smaller mass size, this trend was not statistically significant. All operations were performed by two teams, and the operators’ learning curves and skills may have affected our results. With the development of the technique, our surgeons may have been able to operate more quickly and safely.
Splenic preservation can be accomplished with the preservation of splenic vessels or by the Warshaw technique, which preserves the short gastric vessel arcade while sacrificing both splenic vessels. Although many groups have advocated this method because of the shorter operating time and less blood loss, we do not routinely use the Warshaw technique due to the risk of splenic complications, such as splenic infarction. Symptomatic splenic infarction has been reported to occur in 12–20 % of cases [16, 17]. In the present series, we performed the Warshaw technique in only two patients, one of whom developed splenic infarction.
In our series, surgical intervention for malignant lesions was performed in only three cases, all of which included splenectomies for fear of oncologic outcomes. Further research is needed to determine the adequacy of LDP as an oncologic procedure in the resection of malignant lesions.
The overall complications and PF rates were similar in both of our study groups. Goh et al. [18] demonstrated that splenic preservation may reduce the rate of clinically significant PFs. Although this finding was not confirmed in our series, we proved that LSPDP did not increase the incidence of this complication. Damage to the pancreas stump may be more likely to occur during splenic preservation, and the accumulation of microdamage may result in a PF. This topic requires further investigation.
Infectious complications were rare and did not differ between groups, but a significant difference in splenic function was observed. Tsiouris et al. [19] used WBC and platelet counts as surrogates for measures of immunological competence and demonstrated better preservation of splenic function after LSPDP. We did not use platelet counts as markers because they may be high in patients who have undergone splenectomy, as platelets are mainly destroyed in the spleen. Instead, we used neutrophil percentages, but these did not differ between groups. The differences in WBC counts between groups imply that splenic function is preserved in LSPDP patients, but this effect requires further study and long-term outcome data.
In conclusion, our data suggest that LSPDP is a safe procedure with an operating time, EBL, and complication rate similar to those of LDPS. Postoperative WBC counts suggest that patients undergoing LSPDP may benefit from some preservation of splenic function. Spleen preservation should thus be attempted during LDP.
Acknowledgments
Ethics Committee Letter
All participants provided informed consent, and the study was approved by the Institutional Review Board of Jilin University. This study was conducted according to the principles and guidelines expressed in the declaration of Helsinki.
References
- 1.Martin FM, Rossi RL, Munson JL, ReMine SG, Braasch JW. Management of pancreatic fistulas. Arch Surg. 1989;124:571–573. doi: 10.1001/archsurg.1989.01410050061012. [DOI] [PubMed] [Google Scholar]
- 2.Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc. 2012;264:904–913. doi: 10.1007/s00464-011-2016-3. [DOI] [PubMed] [Google Scholar]
- 3.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;75:577–583. doi: 10.1002/1097-0142(19950115)75:2<577::AID-CNCR2820750222>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi K, Noshiro H, Yokohata K, Nakano K, Watanabe M, Ohtani K, Chijiiwa K, Tanaka M. Is there any benefit of preservation of the spleen in distal pancreatectomy? Int Surg. 2001;86:162–168. [PubMed] [Google Scholar]
- 5.Lee SY, Goh BK, Tan YM, Chung YF, Cheow PC, Chow PK, Wong WK, Ooi LL. Spleen-preserving distal pancreatectomy. Singapore Med J. 2008;49:883–885. [PubMed] [Google Scholar]
- 6.Aldridge MC, Williamson RC. Distal pancreatectomy with and without splenectomy. Br J Surg. 1991;78:976–979. doi: 10.1002/bjs.1800780827. [DOI] [PubMed] [Google Scholar]
- 7.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;5:577–573. doi: 10.1002/1097-0142(19950115)75:2<577::AID-CNCR2820750222>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Mo M, Mauro Z, Franco M, Lorenzo C, Enzo Z, Giorgio R, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery. 1995;117:26–31. doi: 10.1016/S0039-6060(05)80225-9. [DOI] [PubMed] [Google Scholar]
- 9.Benoist S, Dugué L, Sauvanet A, Valverde A, Mauvais F, Paye F, et al. Is there a role of preservation of the spleen in distal pancreatectomy? Jam College Surg. 1999;188:255–260. doi: 10.1016/S1072-7515(98)00299-3. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind C. TNM classification of malignant tumours. 6. Baltimore: Wiley-Liss; 2002. [Google Scholar]
- 11.[No authors listed] (2002)Exocrine pancreas. In: Freene FL, Page DL, Felming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual, 6th ed. New York: Springer-Verlag 157–64
- 12.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition Postoperative pancreatic fistula: an international study group (ISGPF) Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Benoist S, Dugué L, Sauvanet A, Valverde A, Mauvais F, Paye F, Farges O, Belghiti J. Is there a role of preservation of the spleen in distal pancreatectomy? Jam College Surg. 1999;188:255–260. doi: 10.1016/S1072-7515(98)00299-3. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez JR, Madanat MG, Healy BC, Thayer SP, Warshaw AL, Fernández-del Castillo C. Distal pancreatectomy with splenic preservation revisited. Surgery. 2007;141:619–625. doi: 10.1016/j.surg.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekeel KL, Moss AA, Reddy KS, Mulligan DC, Harold KL. Laparoscopic distal pancreatectomy: does splenic preservation affect outcomes? Surg Laparosc Endosc Percutan Tech. 2011;215:362–365. doi: 10.1097/SLE.0b013e31822e0ea8. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Cruz L, Martinez I, Gilabert R, Cesar-Borges G, Astudillo E, Navarro S. Laparoscopic distal pancreatectomy combined with preservation of the spleen for cystic neoplasms of the pancreas. J Gastrointest Surg. 2004;8:493–501. doi: 10.1016/j.gassur.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 18.Goh BK, Tan YM, Chung YF, Cheow PC, Ong HS, Chan WH, Chow PK, Soo KC, Wong WK, Ooi LL. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143:956–965. doi: 10.1001/archsurg.143.10.956. [DOI] [PubMed] [Google Scholar]
- 19.Tsiouris A, Cogan CM, Velanovich V. Distal pancreatectomy with or without splenectomy: comparison of postoperative outcomes and surrogates of splenic function. HPB. 2011;1310:738–744. doi: 10.1111/j.1477-2574.2011.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]