Abstract
Vulva cancer is the fourth leading gynaecological malignancy, accounting for approximately 4 % of all gynaecological cancers. Surgery represents the treatment of choice, and cases of advanced or recurrent vulvar cancers are to date a major challenge to multidisciplinary teams. Abdominoperineal excision (APE) in combination with vulvectomy and inguinal lymphadenectomy is the only curative treatment option. Patients’ files of all women with squamous cell carcinoma of the vulva who underwent abdominoperineal resection were retrospectively reviewed with special regards to technical challenges the general surgeon will face. Seven women were enrolled in this retrospective study with a median age of 71 years (range 56–79 years). In six patients, the pelvic floor after abdominoperineal excision could be closed by direct suture of the levator muscles. One woman underwent abdominoperineal resection with closure of the defect using a vertical rectus abdominis myocutaneous (VRAM) flap. All women underwent radical vulvectomy, in five patients in combination with bilateral inguinal lymph node dissection. Operation time was 377 min (range 130–505 min). The median overall survival after surgery was 27 months (range 4–84 months), with a calculated 5-year survival rate of 42 %. Women with negative lymph nodes revealed a longer survival time after surgery compared to women with lymph node metastases (15.5 vs. 72 months; p = 0.09). Abdominoperineal excisions represent a powerful tool in the multidisciplinary treatment regimen of advanced or recurrent vulvar cancer. Reconstruction of the pelvic floor usually does not require myocutaneous flaps, even when facing large tumours. Despite high complication rates, radical surgery was a feasible treatment with long-term survival potential without mortality.
Keywords: Vulvar cancer, Abdominoperineal excision, Salvage surgery, Exenteration, Gynaecological cancer
Introduction
Vulvar cancer is a rare disease and affects 1.2 per 100,000 women annually, accounting for 4 % of all gynaecological cancers. In 75 %, the age at diagnosis is above 60 years and older, with a peak incidence of 20 per 100,000 women aged 70 and older [1, 2].
Surgery is the cornerstone for correct staging and treatment of vulvar cancer. Numerous studies have been published to address the issue of surgery in localized vulvar cancer and surgery for vulvar cancer developed from “en bloc” techniques with radical vulvectomy plus bilateral inguinal lymphadenectomy as described by Taussig to a triple incision technique with wide local vulvar excision plus bilateral groin surgery using three separate incisions, reducing overall morbidity while providing negative resection margins. Furthermore, sentinel node dissections has evolved to be the standard treatment for patients with clinically negative lymph nodes [3, 4].
However, 25 % of all patients either present with advanced or inoperable disease. Taken together, the 5-year survival rate for stage III disease is 43 %, for patients suffering from stage IV squamous cell carcinoma of the vulva 13 %, respectively [5].
Despite the extensive data published addressing surgery in locally confined vulvar cancer, only few publications provide insight into extensive surgical procedures for advanced or recurrent disease regarding outcome and complications, often in a subset of different cancer entities (cervical, vulvar, vaginal cancer). Parks and colleagues reported a series of 46 patients undergoing pelvic exenteration for a variety of gynaecological cancers (including 3 vulvar cancers), revealing a substantial rate of major complications in 15 patients and minor complications in 21 patients. Negative resection margins and the absence of lymph node metastases emerged as a prognostic factor in a multivariate analysis [6].
A recent meta-analysis by Ang and colleagues of the Cochrane Gynecological Cancer Group revealed no evidence that would support decision-making about extensive surgery in cervical, endometrial, vaginal or vulvar malignancies due to the heterogeneity of the published literature [7].
Abdominoperineal excisions (APE), with or without the use of myocutaneous flaps, are still an accepted salvage procedure, preventing the affected women from developing mutilating ulcerations evoked by a progressive vulvar cancer. The role of extralevator abdominoperineal excisions (ELAPE) has not been addressed in the context of vulvar cancer.
Therefore, we analysed the role of abdominoperineal resections in the management of advanced and recurrent vulvar cancer with special regards to complications and technical challenges the general surgeon of the multidisciplinary team will face during such procedures.
Methods
Patients’ files of all women with squamous cell carcinoma of the vulva who underwent abdominoperineal excision performed by a general surgeon of the Department of General and Visceral Surgery of the University Hospital Frankfurt between January 2002 and December 2013 were retrospectively reviewed after approval of the study by the Internal Review Board (Protocol No. 315/14).
All women with a histologically proven squamous cell carcinoma of the vulva underwent APE, accompanied by resection of the respective vulva and bilateral inguinal lymphadenectomy. The defect of the pelvic floor after APE was closed either by direct suture or vertical rectus abdominis myocutaneous (VRAM) flap. The procedures have been performed by a multidisciplinary team consisting of abdominal surgeons and gynaecologists. All cases had been discussed by a multidisciplinary tumour board prior to surgery.
Demographic and clinical data, specifically length of hospital stay, number of complications and revision procedures, operation time, time to recurrence and overall survival were collected. Postoperative complications have been classified using the Dindo–Clavien classification [8].
The Mann–Whitney test, Fisher’s exact test, t test and Spearman correlation were used to evaluate the relationships between clinical features. Survival curves and rates were calculated using the Kaplan–Meier method. A p value <0.05 was considered statistically significant. Statistics were calculated using MedCalc® version 13.2.2. (MedCalc Software, Belgium).
Results
Patients’ Characteristics
Seven women were enrolled in this retrospective study. The median age at operation was 71 years (range 56–79 years), and the median body mass index (BMI) was 25 (range 24–33). All patients had a locally advanced, sphincter infiltrating and histologically proven squamous cell carcinoma of the vulva. Four women had a history of a secondary malignancy (two breast cancers, one lung cancer). Three women had a history of diabetes, but none of the patients suffered from HIV. All women underwent local excision or biopsy prior to surgery. Two women presented with recurrent vulvar cancer. One woman received preoperative chemoradiation therapy with mitomycin C/5-FU in combination with 50.4 Gy radiation with an additional boost of 7.2 Gy.
Surgical Aspects
In six patients, the pelvic floor after abdominoperineal resection could be closed by direct suture of the levator muscles. One woman underwent abdominoperineal resection with closure of the defect using a vertical rectus abdominis myocutaneous (VRAM) flap. All women underwent radical vulvectomy, in five patients in combination with bilateral inguinal lymph node dissection. Operation time was 377 min (range 130–505 min), and none of the women required postoperative surveillance on an intensive care unit.
In five patients, histology revealed a R0 resection with margins >8 mm, whereas two patients had positive resection margins on the vulvar resection site. Of these two patients, one underwent immediate re-resection during the same hospital stay. The median tumour size was 7.5 cm (range 2.4 cm −8.5 cm) and did not correlate with the necessity to employ a flap for reconstruction. Three out of five women that underwent inguinal lymphadenectomy were positive for bilateral inguinal lymph node metastases (Table 1).
Table 1.
Patients’ characteristics
| Patient | Age (years) | Tumour size (cm) | LN metastases (R:L) | Operation time (min) | Technique | Outcome |
|---|---|---|---|---|---|---|
| 1 | 60 | 8.5 | – | 130 | APE | DURC |
| 2 | 70 | 7.5 | – | 377 | APE | DURC |
| 3 | 71 | 2.4 | 2:1 | 354 | APE | DOD |
| 4 | 79 | 8.0 | 0:0 | 370 | APE | NED |
| 5 | 76 | 5.0 | 1:1 | 465 | APE | DOD |
| 6 | 74 | 8.5 | 1:1 | 499 | APE | DOD |
| 7 | 56 | 2.8 | 0:0 | 505 | APE + VRAM | NED |
LN lymph node, R:L right:left, APE abdominoperineal excision, VRAM vertical rectus abdominis myocutaneous flap, DURC dead of unrelated cause, DOD dead of disease, NED no evidence of disease
Outcome and Complications
The median hospital stay was 46 days (range 29–77), with a median preoperative stay of 8 days (range 5–16 days) and a median postoperative stay of 36 days (range 22–44 days). All women developed complications in the postoperative course. Four women suffered from surgical site infections in the groin (Dindo–Clavien: I–II), three women had a breakdown of their perineal wound leading to reoperations (Dindo–Clavien: IIIa and IIIb) and one women developed a perineal hernia as well as a parastomal hernia and underwent laparoscopic hernia repair 5 years after the APE. None of the patients developed life-threatening complications in the course of the hospital stay (Dindo–Clavien: ≥IV).
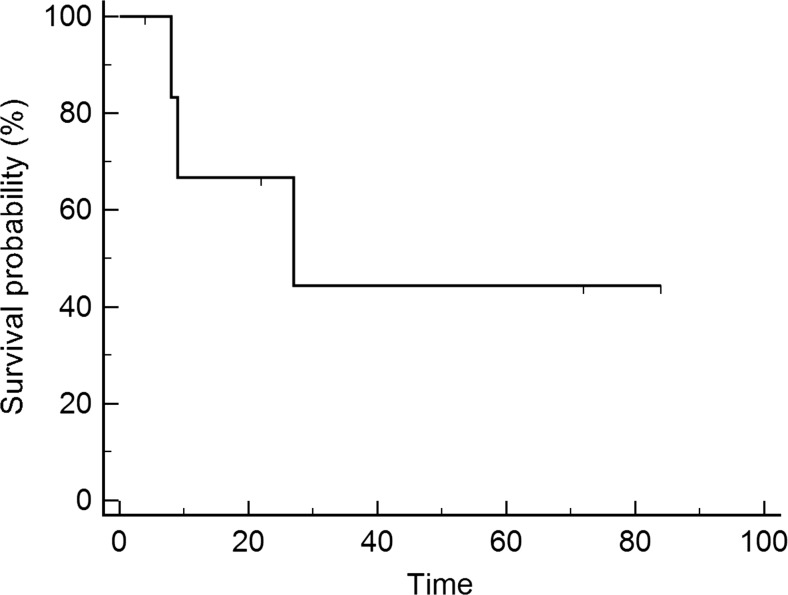
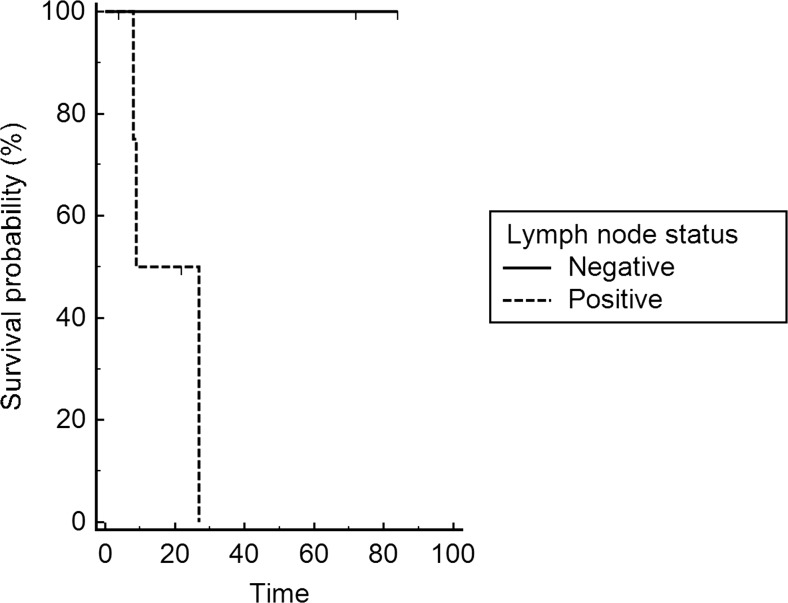
In total, 2 women underwent up to 5 revision procedures due to wound complications in either the groin, the vulvar resection site or the perineal wound. After discharge from the hospital, 3 of the 7 (43 %) patients developed recurrences, whereas 4 (57 %) revealed no evidence of disease. Three woman died of the disease, and 2 women died of different causes. The median overall survival after surgery was 27 months (range: 4–84 months) (Fig. 1), with a calculated 5-year survival rate of 42 %. Women with negative lymph nodes revealed a longer survival time after surgery compared to women with lymph node metastases (18 vs. 72 months; p = 0.09; Fig. 2).
Fig. 1.
Overall survival after surgery
Fig. 2.
Overall survival after surgery for lymph node metastasis (negative, n = 2 vs. positive, n = 5)
Discussion
In this series, we demonstrate the safety of abdominoperineal resections with or without reconstruction using a flap specifically in advanced vulvar cancer. Although every woman in our study group developed complications in the course of the hospital stay, local tumour control could be achieved with R0 resections in 71 % of all patients. Furthermore, radical surgery provided long-term survival in two women. Despite a high perioperative morbidity and complication rate, none of the women developed life-threatening complications.
As surgery represents the foundation of vulvar cancer treatment, several topics need to be addressed. The extent of R0 resection margins is still under debate, and some authors even consider local recurrences as second primary tumours. Several authors favour a minimum margin of 8 mm, revealing no local recurrences beyond 8 mm, whereas 22.5–50 % of women developed local recurrences if the margin was <8 mm [9]. In contrast, Woelber and colleagues published a study on 102 patients showing no impact of margin distance on the progression-free survival [10]. In the seven patients presented here, only one woman revealed resection margins <8 mm but exhibited bilateral inguinal lymph node metastases, whereas the other two women with lymph node metastases had >8 mm margins and subsequently died due to progressive disease. Therefore, the outcome of these three women with lymph node metastases was determined by their lymph node status rather than by the resection margins.
Lymph node metastases of vulvar cancer are associated with a poor prognosis. As presented here, all patients with lymph node metastases died and had a significantly shorter survival compared to women without lymph node metastases. Two large studies revealed that sentinel node procedures can be performed in women presenting with a unifocal disease, a tumour size below 4 cm and clinically negative lymph nodes [11, 12]. All cases but one in this study had tumour sizes exceeding 5 cm, and five women had clinically enlarged lymph nodes, with two women revealing no metastases in the final histology report. Therefore, five patients underwent bilateral inguinal lymphadenectomy, which by itself is a procedure with complication rates up to 70 %, leading to the aforementioned wound complications resulting in exceptionally long hospital stays. In malignant melanoma, lymphatic fistula rate and wound breakdown can occur in up to 65 % of all patients that underwent radical unilateral inguinal lymphadenectomy due to lymph node metastases [13].
Abdominoperineal excision is the standard procedure for sphincter-infiltrating rectal cancers and is still commonly used for rectal cancers of the lower third. With the increasing use of anterior resections performing intersphincteric stapled anastomosis, the amount of APE decreased over the last decades. Furthermore, extralevator abdominoperineal excisions (ELAPE) emerged to reduce the risk of local recurrences via providing a cylindrical specimen in order to avoid the typical waist of APE specimen. The pelvic floor in this procedure is then mostly closed using a gluteal myocutaneous flap [14, 15].
Considering the median tumour size of 7.5 cm, it is noteworthy that only one woman required a VRAM flap for reconstruction of the pelvic floor in our study. In the remaining six patients, it was possible to perform a standard APE with closure of the pelvic floor by suturing the levator muscles. The positive resection margins in two women were located on the vulvar site, which could not have been avoided with an ELAPE. From a technical point of view, vulvar cancers mainly develop laterally of the midline, leaving at least one levator muscle unaffected which can then be used for the reconstruction of the pelvic floor. Park and colleagues employed myocutaneous flaps in pelvic floor reconstruction on 23 % of their 46 patients undergoing pelvic exenteration for a variety of gynaecological cancers (four cases of vulvar cancer) [6]. Similar findings were presented by Benn and coworkers, analysing 54 patients undergoing pelvic exenteration for different types of gynaecological malignancies, including nine cases of vulvar cancers. Interestingly, three patients underwent total pelvic exenteration due to vulvar cancer [16].
The major disadvantage of all aforementioned studies regarding technical details is the mixture of different types of cancers, requiring different surgical procedures without presenting data separating each tumour type, its adequate surgical procedure, reconstruction techniques and outcome.
Radical surgery for advanced gynaecological and especially vulvar cancer is associated with a high complication rate. In this study, all patients developed wound complications either at the perineal wound, the groin or at both sites. The combination of three procedures, which by themselves harbour high complication rates, i.e. radical vulvectomy, inguinal lymphadenectomy and APE, explains the complication rates and the resulting long hospital stays. Jäger and colleagues reported similar complication rates with every patient revealing at least one complication and 43 % undergoing reoperation in their study on 28 women presenting with a variety of gynaecological cancers [17]. In their study on 107 women with different gynaecological cancers, Baiocchi reported a rate of 53 % early and 44 % late complications. Benn and colleagues reported early complication in 50 % and late complication in 61 % of all cases [18]. Again, all aforementioned studies did not distinguish between cancer type and procedure performed regarding the complication rate. Despite the elevated complication rates reported in several studies, the mortality of these procedures is mostly below 3 % and increases in cases with reconstruction of the urinary system [6, 16–18].
One woman in our study developed a perineal as well as a parastomal hernia and underwent repair 5 years after the initial APE. A recently published meta-analysis of the current literature addressed this issue, concluding that the use of prophylactic prosthetic mesh at the time of primary stoma formation reduces the incidence of parastomal hernias [19]. Therefore, we recommend the use of a prophylactic mesh in women undergoing APE for vulvar cancer.
In the data presented here, the overall survival was 22 month, with a calculated 5-year survival of 28 %. Lymph node metastasis emerged as a prognostic factor, lowering the median survival time in patients with lymph node metastasis to 15.5 months. This outcome is comparable to a study by Miller and colleagues, who reported a 5-year survival rate of 38 % in their analysis of 21 cases over a period of 33 years [20]. Similar findings were published by Blecharz and coworkers, revealing a 5-year overall survival rate of 30.5 % in patients over 70 years of age [21]. The impact of lymph node metastasis on the overall survival as presented by Sznurkowski et al. was comparable to our series, with 50 % of all patients dying due to the disease within the first 20 months [22]. In the studies mentioned before, 5-year survival rate after pelvic exenteration was 54, ∼30 and 27,4 %, respectively [6, 16–18]. As these studies analysed the outcome of different types of gynaecological malignancies; thus, the comparability with our study is limited.
Conclusions
In this present series, abdominoperineal resections in the treatment regimen of advanced and recurrent vulvar cancer were a feasible surgical option with long-term survival potential without mortality, despite the fact that these procedures are associated with high complication rates. In patients with lymph node metastasis, extended surgery provides local tumour control, often preventing mutilating cancer growth.
Technically, reconstruction of the pelvic floor does usually not require myocutaneous flaps, even when facing large tumours. Furthermore, prophylactic intraperitoneal mesh placement should be performed to prevent parastomal hernias.
Nevertheless, this study is clearly limited due to the small number of patients analysed, and the statistical analysis subsequently lacks power. Randomized studies or at least merged data from several centres are needed to overcome this problem in order to provide robust data on which the surgeon facing these challenging tumour situations can rely on.
Acknowledgments
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–302. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Sturgeon SR, Brinton LA, Devesa SS, Kurman RJ. In situ and invasive vulvar cancer incidence trends (1973 to 1987) Am J Obstet Gynecol. 1992;166:1482–1485. doi: 10.1016/0002-9378(92)91623-I. [DOI] [PubMed] [Google Scholar]
- 3.Taussig FJ. Cancer of the vulva: an analysis of 155 cases. Am J Obstet Gynecol. 1940;40:764–779. doi: 10.1016/S0002-9378(15)30802-4. [DOI] [Google Scholar]
- 4.Baiocchia G, Rocha RM. Vulvar cancer surgery. Curr Opin Obstet Gynecol. 2014;26:9–17. doi: 10.1097/GCO.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 5.Raspagliesi F, Zanaboni F, Martinelli F, Scasso S, Laufer J, Ditto A. Role of paclitaxel and cisplatin as the neoadjuvant treatment for locally advanced squamous cell carcinoma of the vulva. J Gynecol Oncol. 2014;25(1):22–29. doi: 10.3802/jgo.2014.25.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JY, Choi HJ, Jeong SY, Chung J, Park JK, Park SY. The role of pelvic exenteration and reconstruction for treatment of advanced or recurrent gynecologic malignancies: analysis of risk factors predicting recurrence and survival. J Surg Oncol. 2007;96(7):560–568. doi: 10.1002/jso.20847. [DOI] [PubMed] [Google Scholar]
- 7.Ang C, Bryant A, Barton DP, Pomel C, Naik R. Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev. 2014;2 doi: 10.1002/14651858.CD010449.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 9.De Hullu JA, Hollema H, Lolkema S, et al. Vulvar carcinoma. The price of less radical surgery. Cancer. 2002;95:2331–2338. doi: 10.1002/cncr.10969. [DOI] [PubMed] [Google Scholar]
- 10.Woelber L, Choschzick M, Eulenburg C, Hager M, Jaenicke F, Gieseking F, et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann Surg Oncol. 2011;18(13):3811–3818. doi: 10.1245/s10434-011-1778-0. [DOI] [PubMed] [Google Scholar]
- 11.Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26(6):884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 12.Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol. 2012;30(31):3786–3791. doi: 10.1200/JCO.2011.41.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habbe N, Reinisch A, Bechstein WO, Hannes S. Lymph fistulas after inguinal lymph node dissection—assessment of risk factors and future treatment strategies. Surg Sci. 2014;5:193–199. doi: 10.4236/ss.2014.54033. [DOI] [Google Scholar]
- 14.Prytz M, Angenete E, Ekelund J, Haglind E. Extralevator abdominoperineal excision (ELAPE) for rectal cancer–short-term results from the Swedish Colorectal Cancer Registry. Selective use of ELAPE warranted. Int J Color Dis. 2014;29(8):981–987. doi: 10.1007/s00384-014-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer G, Anderin C, Martling A, Holm T. Local control and survival after extralevator abdominoperineal excision for locally advanced or low rectal cancer. Color Dis. 2014;16(7):527–532. doi: 10.1111/codi.12610. [DOI] [PubMed] [Google Scholar]
- 16.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011;122(1):14–18. doi: 10.1016/j.ygyno.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jäger L, Nilsson PJ, Rådestad AF. Pelvic exenteration for recurrent gynecologic malignancy: a study of 28 consecutive patients at a single institution. Int J Gynecol Cancer. 2013;23(4):755–762. doi: 10.1097/IGC.0b013e318287a874. [DOI] [PubMed] [Google Scholar]
- 18.Baiocchi G, Guimaraes GC, Rosa Oliveira RA, Kumagai LY, Faloppa CC, Aguiar S, et al. Prognostic factors in pelvic exenteration for gynecological malignancies. Eur J Surg Oncol. 2012;38(10):948–954. doi: 10.1016/j.ejso.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Shabbir J, Chaudhary BN, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Color Dis. 2012;14(8):931–936. doi: 10.1111/j.1463-1318.2011.02835.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller B, Morris M, Levenback C, Burke TW, Gershenson DM. Pelvic exenteration for primary and recurrent vulvar cancer. Gynecol Oncol. 1995;58(2):202–205. doi: 10.1006/gyno.1995.1211. [DOI] [PubMed] [Google Scholar]
- 21.Blecharz P, Karolewski K, Bieda T, Klimek M, Pudelek J, Kojs E, et al. Prognostic factors in patients with carcinoma of the vulva—our own experience and literature review. Eur J Gynaecol Oncol. 2008;29(3):260–263. [PubMed] [Google Scholar]
- 22.Sznurkowski JJ, Milczek T, Emerich J. Prognostic factors and a value of 2009 FIGO staging system in vulvar cancer. Arch Gynecol Obstet. 2013;287(6):1211–1218. doi: 10.1007/s00404-012-2683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]