Abstract
Chronic wounds are a common problem faced by health care professionals, both in the community and in the hospital setting. The aim of this study was to evaluate the use of honey and phenytoin with respect to the process of wound healing, eradication of infection, pain relief and hospital stay. The study included 150 patients, 3 groups of 50 each (group A, honey dressing; group B, phenytoin dressing; group C, saline dressing). The appearance of granulation tissue was faster with significant wound area reduction after 3 weeks in groups A and B compared to group C. Eradication of infection was evident earlier in the honey- and phenytoin-treated groups along with significant pain relief as compared to that of group C. The outcomes of the use of honey and phenytoin as wound dressings are beneficial and comparable. Honey provides quicker pain relief and removes malodour more effectively.
Keywords: Chronic wound, Honey, Phenytoin, Infection, Pain relief
Introduction
Wound healing is the process of restoration of the physical integrity of internal and external body structures and involves complex interactions between the cells and several other factors [1]. Chronic wounds are those that have not proceeded through orderly and timely reparation to produce anatomic and functional integrity after 3 months [2]. Sharma and John opined that “chronicity may be considered when there is no complete healing after 6 weeks or if there is poor response to a treatment change” [3]. Chronic wounds are a common problem faced by health care professionals, both in the community and in the hospital setting. The quest for better wound-healing agents is perhaps one of the oldest challenges for medical practice.
High osmolarity has been considered a valuable tool in the treatment of infections because it prevents the growth of bacteria and encourages healing [4]. This can be achieved by topical use of honey which contains up to 40 % fructose, 30 % glucose, 5 % sucrose and 20 % water [5].
Kimball in 1939 first observed that gingival hyperplasia occurred in some patients treated with phenytoin, an antiepileptic [6]. The apparent stimulatory effect of phenytoin on connective tissue has suggested an exciting possibility for its use in wound healing [7]. This study is undertaken to compare the wound-healing properties of honey and phenytoin in chronic wounds.
Aims and Objectives
The aim of this study was to evaluate the use of honey and phenytoin with respect to the process of wound healing, eradication of infection, pain relief, and hospital stay.
Materials and Methods
This was a prospective comparative study over a period of 36 months done at a tertiary care centre. The study included 150 patients (group A, 50 patients treated with honey; group B, 50 patients treated with phenytoin; group C, 50 patients treated with saline dressings). The study was approved by the institute review board, and written and informed consent of all the patients was obtained before their enrollment into the study. The cases included in the study were patients in the age group of 20 to 80 years with wound infections, trophic ulcers, diabetic ulcers, venous ulcers and pressure sores. All the cases were examined and investigated, wounds were debrided and the ulcer dimensions as well as surface area were assessed using a transparent sheet of graph paper before the dressings were applied. The cases were randomised into three groups using sealed envelopes. The wounds were comparable in all respects.
Group A patients were treated with commercially available sterilised honey. It was made fluid by stirring/light warming. It was spread on a dressing pad, and even coverage of the wound surface with honey was ensured. For moderately to heavily exuding wounds, a secondary dressing was applied to absorb the seepage of diluted honey from the primary dressing.
Group B patients were treated with phenytoin dressings. Phenytoin for wound healing is based on a rational scientific theory and within the guidelines set forth by the Food and Drugs Administration. Phenytoin powder was directly applied to wounds in a thin, uniform layer and then covered with gauze. The dosage of phenytoin was calculated as per the surface area of the wound: 0 to 5 cm2—100 mg, 5.1 to 9 cm2—150 mg, 9 to 15 cm2—200 mg and >15 cm2—300 mg. Group C patients were treated with normal saline dressings. All dressings were done once daily. Antibiotics administered: inj. Taxim 1 g bd and inj. Flagyl 100 cc I/V 8 hrly for initial 3 days followed by oral route administration for 4 days.
Evaluation was done by assessing the time required for wound healing (appearance of granulation tissue, comparison of initial and final wound areas, percentage reduction of the wound after 3 weeks of treatment), eradication of infection (a negative culture report) (swabs from wounds of all three groups before start of treatment and then on days 3, 6 and 9), pain relief (as per pain scores using the visual analogue scale on days 5, 10, 15 and 20) and the hospital stay. The results were analysed using chi-square test.
Results
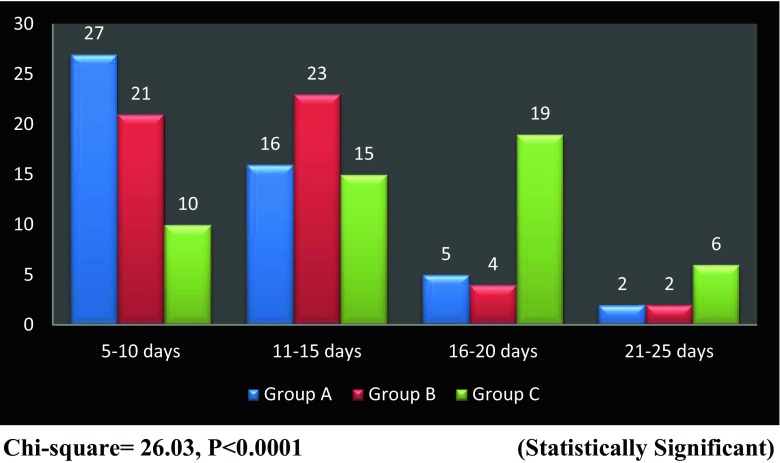
Maximum patients (52 %) were in the age group of 41 to 60 years. Sixty-four percent were males and 36 % were females. The different cases included in the study were wound infections (32 cases), trophic ulcers (33 cases), diabetic foot ulcers (51 cases), venous ulcers (22 cases) and pressure sores (12 cases). The appearance of granulation tissue was faster in groups A and B compared to group C (Fig. 1). The wounds in the study and control groups were comparable in all respects. There was significant reduction in the wound area at the end of 3 weeks of treatment in the honey- and phenytoin-treated groups compared to the saline-treated group. The difference in wound area reduction between the honey and phenytoin groups was not statistically significant (Table 1). The percentage reduction of wound achieved at the end of 3 weeks was 20.66 % for the honey-treated group, 15.80 % for the phenytoin-treated group and 8.07 % for the saline-treated group (Fig. 2). Methicillin-resistant Staphylococcus aureus (MRSA) was the most common organism isolated in the study (16 %) along with other organisms like Pseudomonas and Klebsiella. Eradication of infection was evident earlier in the honey-treated group (mean 8.4 days ± 1.71) and phenytoin-treated group (mean 9.28 days ± 2.03) as compared to the group treated with saline dressing (mean 14.94 days ± 2.56). The results were statistically significant (group A vs group C: P < 0.0001 and group B vs group C: P < 0.0001). There was no change in the colonising organism during the study period. Ninety percent of the wounds were free from infection with the topical treatment regime. Pain relief was significant in patients treated with honey and phenytoin as compared to saline dressings. However, honey provided maximum analgesia (Table 2). Only three patients complained about a transient stinging sensation after honey was applied. None of the patients in groups A and B described dressing removal as painful, no matter how high the perceived baseline pain level. The hospital stay was less in patients in the honey group as compared to those treated with phenytoin and normal saline (Table 3). At the end of the study period, complete healing of wounds was observed in 10 % of patients in groups A and B. Four patients were taken up for skin grafting.
Fig. 1.
Graph showing time of appearance of granulation tissue in the three groups
Table 1.
Comparison of wound area reduction
| Group | Initial wound area (mm2) | Final wound area (mm2) | Wound area reduction (mm2) | P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Group A | 663.4 | 294.1 | 526.4 | 286.3 | 137.03 | 56.71 | P < 0.0001 |
| Group B | 658.9 | 214.4 | 554.8 | 179.2 | 104.04 | 69.76 | |
| Group C | 582 | 221.2 | 534.1 | 215 | 47.96 | 19.01 | |
P < 0.0001 (statistically significant)
Fig. 2.
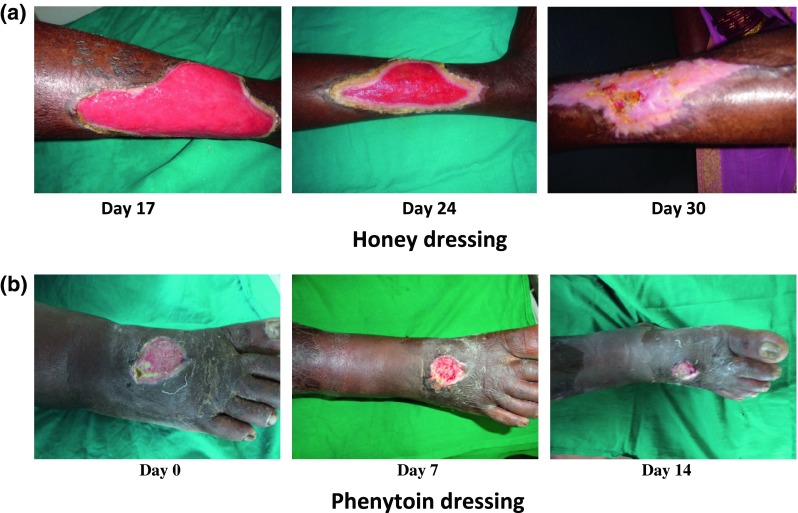
Wound healing with honey and phenytoin dressings. a Honey dressing. b Phenytoin dressing
Table 2.
Comparison of pain score at the 5th, 10th, 15th and 20th days
| Parameter | Number of patients | Pain score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5th day | 10th day | 15th day | 20th day | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Group A | 50 | 5.10 | 0.79 | 3.06 | 0.91 | 2.06 | 0.65 | 1.20 | 0.45 |
| Group B | 50 | 5.54 | 0.68 | 3.76 | 0.77 | 2.68 | 0.65 | 1.90 | 0.51 |
| Group C | 50 | 6.72 | 0.64 | 5.02 | 0.77 | 4.32 | 0.79 | 3.14 | 0.76 |
| F value | 70.65 | 73.30 | 138.09 | 140.42 | |||||
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| A vs B | <0.01 | <0.0001 | <0.0001 | <0.0001 | |||||
| A vs C | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| B vs C | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
Table 3.
Hospital stay
| Range | Hospital stay (days) | F value | P value | ||
|---|---|---|---|---|---|
| Mean | SD | ||||
| Group A | 18–41 days | 24.62 | 5.20 | 134.29 | <0.0001 |
| Group B | 21–49 days | 29.6 | 5.64 | ||
| Group C | 39–74 days | 47.58 | 10.19 | ||
Group A vs group B: P < 0.005, group A vs group C: P < 0.0001, group B vs group C: P < 0.0001 (statistically significant)
Discussion
Pure honey is a semi-translucent concentrated mixture of glucose, fructose, proteins, fatty acids, minerals, vitamins and water produced by worker bees from the nectar of flowers. The Egyptians, Chinese, Greeks, and Romans also used honey in combination with herbs to treat wounds. Aristotle (350 BC) wrote of honey being a salve for wounds, and Discorides wrote of honey being “good for all rotten and hollow ulcers”. Honey was displaced from common use in the medical profession when antibiotics came into use in the 1940s [7]. The antibacterial property of honey was first recognised in 1892 by Van Ketel [8]. Honey contains an enzyme that produces hydrogen peroxide when diluted. This agent was referred to as “inhibine” prior to its identification as hydrogen peroxide [9]. The harmful effects of hydrogen peroxide are further reduced because honey sequesters and inactivates the free iron which catalyses the formation of oxygen free radicals produced by hydrogen peroxide and its antioxidant components help to mop up oxygen free radicals [10]. The osmosis facilitated by the high sugar concentration draws exudate and lymph from the wound bed and the tissues, maintaining a moist wound environment. The enzyme glucose oxidase converts glucose into gluconolactone and results in the production of gluconic acid and hydrogen peroxide. The slightly acidic pH of honey assists in epithelialisation. The acidic environment increases the rate of wound granulation, as more oxygen is released from the haemoglobin in the wound bed [11]. Glucose and fructose provide nutrients for wound healing. Honey feeds the wound not only with a cellular source of energy but also with minerals, amino acids and vitamins. The moist environment helps in the contractile activity of myofibroblasts, pulling the edges of the wound together [12]. The anti-inflammatory property of honey inhibits the formation of serous exudate, which has a negative effect on the bacterial growth, as the exudate is a rich medium for bacterial colonisation [13]. Honey has a de-odourising action on wounds. The glucose present in honey is readily used as an energy source within the wound, which results in lactic acid production, as opposed to malodourous compounds that arise during the metabolism of amino acids [14]. The anti-inflammatory activity of honey provides a soothing effect on the wound. The painfulness of wounds results from factors released in the inflammatory response sensitising the nerve endings. The release of these factors is decreased if inflammation is suppressed [7]. The risk of hyperglycaemia occurring in diabetic patients, even with the treatment of large wound surfaces, appears purely hypothetical. The glucose is partially absorbed in the wound, but an increase of the blood glucose has not been observed [15].
Phenytoin stimulates the proliferation of fibroblasts, decreases the collagenase activity, enhances granulation tissue formation, exhibits antibacterial activity and assists neovascularisation [16, 17]. It is the duty and responsibility of both the wound care specialists and the dispensing pharmacist to make certain that any off-label use of a drug is supported by credible, evidence-based medicine. In a 1984 paper by an investigator in the FDA office of Orphan Products Development, wound healing was listed as an important application for which phenytoin appears to be potentially useful. The general guidelines for the off-label use of pharmaceuticals are that “it is entirely proper if the proposed use is based on rational scientific theory, expert medical opinion, or controlled clinical studies”. Hence, the topical use of phenytoin for wound healing is within the guidelines set forth by the FDA [16]. It is not known if phenytoin has intrinsic antibacterial activity, or whether the effect of phenytoin on the bacterial load of wounds is medicated indirectly by effects on inflammatory cells and neovascularisation [18]. The local pain relief can be explained by its membrane-stabilising action and the reduced inflammatory response [19]. Facilitation of nerve regeneration has also been reported with phenytoin [18].
The principles of local management of chronic wounds include effective debridement, stimulation of the intrinsic process of wound healing and wound support with the use of appropriate dressing techniques till the wound bed is ready for final closure.
This study evaluates the use of honey and phenytoin in chronic wounds. The results clearly show the beneficial properties of both, with honey exhibiting a more positive response compared to phenytoin.
In a meta-analysis of five observational and ten randomised controlled trials using honey, complete healing was reported with 2–9 weeks’ time and indicated that 99 % patients showed improvement in terms of wound healing [20]. In a study by Vijaya Kumari and Nishteswar, pain relief was observed after the 15th day in 80 % of cases and healthy granulation developed after the 7th day in 50 % of patients [21]. These are comparable to the results of our study. Udwadia reported that ghee and honey dressings markedly reduced the foul odour and discharge from infected wounds, significantly improving the quality of life [22]. Efem reports that microbiological examination of swabs from the wounds showed that 51 out of 59 wounds with bacterial contamination became sterile within 1 week [12]. In our study, wounds treated with honey were free from infection on the 8th day. The transient stinging sensation experienced by three patients after honey application does not seem to be associated with damage to the wound. There is evidence that honey stimulates the nociceptors. In some patients, nerve endings are sensitised and thus more responsive to honey’s acidity and/or its organic chemicals [23]. No changes in blood sugar levels of diabetic patients treated with honey were seen in our study. Rhodes et al. have shown significant reduction in healing time for pressure ulcers with phenytoin as opposed to hydrocolloid [19]. Anstead et al. mentioned that phenytoin promotes the healing of massive necrotising soft tissue wounds that are unresponsive to conventional treatment. Fresh granulation was apparent within 2 days of application of topical phenytoin in their study [16]. In our study, the phenytoin group showed evidence of appearance of granulation tissue within 5 to 10 days in 21 patients. Bansal and Mukul compared topical phenytoin with sodium chloride (0.9 %) dressing in the treatment of leprosy trophic ulcers. In the phenytoin group, granulation tissue was well established, and wound discharge was eliminated within 1 week [24]. Pendse et al. achieved negative cultures in 50 % of phenytoin-treated wounds compared to 17 % of controls by day 7 [25]. No side effects of phenytoin were observed in our study.
Proper protocols for wound assessment, identification of infection and increasing range of antimicrobial dressings can bring about a revolutionary change in wound care. This study has evaluated the use of honey and phenytoin which have the potential to cure the commonest surgical ailment—the infected wound. This will offer the clinicians the opportunity to achieve faster clinical outcomes.
Conclusions
Therapies for wound healing address a huge unmet need. Chronic wounds are a cause of mental distress to the patient and cause a financial burden to the patient and health services. The outcomes of the use of honey and phenytoin as wound dressings are beneficial and comparable. Honey provides quicker pain relief and removes the malodour more effectively. These medications are cheap and easy to use, providing valuable alternatives for the costly wound dressings especially in an era of antibiotic resistance. It will be welcomed by the patient who increasingly seeks a “natural” approach to wound care.
Acknowledgments
Conflict of Interest
Nil
References
- 1.Hasamnis AA, Mohanty BK, Muralikrishna, Patil S. Evaluation of wound healing effect of topical phenytoin on excisional wound in albino rats. J Young Pharm. 2010;2:59–62. doi: 10.4103/0975-1483.62215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werdin F, Tenenhaus M, Rennekampff HO. Chronic wound care. Lancet. 2008;372:1860–1862. doi: 10.1016/S0140-6736(08)61793-6. [DOI] [PubMed] [Google Scholar]
- 3.Sharma RK, John JR. Role of stem cells in the management of chronic wounds. Indian J Plast Surg. 2012;45:237–243. doi: 10.4103/0970-0358.101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer HG, Barnett S, Irving S, Middleton KR, Seal DV. A controlled model of moist wound healing; comparison between semipermeable film, antiseptics and sugar paste. J Exp Pathol. 1990;71:155–170. [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence JC. Honey and wound bacteria: editorial. J Wound Care. 1999;8(4):155. doi: 10.12968/jowc.1999.8.4.26172. [DOI] [PubMed] [Google Scholar]
- 6.Kimball OP, Horan TN. The use of Dilantin in the treatment of epilepsy. Ann Intern Med. 1939;13:787–793. doi: 10.7326/0003-4819-13-5-787. [DOI] [Google Scholar]
- 7.Molan PC. Using honey in wound care. Int J Clin Aromather. 2006;3(2):21–24. [Google Scholar]
- 8.Dustmann JH. Antibacterial effect of honey. Apicata. 1979;14(1):7–11. [Google Scholar]
- 9.White JW, Subers MH, Schepartz AI. The identification of inhibine, the antibacterial factor in honey as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta. 1963;73:53–70. doi: 10.1016/0006-3002(63)90359-7. [DOI] [PubMed] [Google Scholar]
- 10.Frankel S, Robinson GE, Berenbaum MR. Antioxidant capacity and correlated characteristics of 14 uniforal honeys. J Apic Res. 1998;37(1):27–31. [Google Scholar]
- 11.Kaufman T, Eichenlaub EH, Angel MF, Levin M, Futrell JW. Topical acidification promotes healing of experimental deep partial thickness skin burns: a randomised double-blind preliminary study. Burns Incl Therm Inj. 1985;12(2):84–90. doi: 10.1016/0305-4179(85)90032-4. [DOI] [PubMed] [Google Scholar]
- 12.Efem SE. Clinical observations on the wound healing properties of honey. Br J Surg. 1988;75(7):679–681. doi: 10.1002/bjs.1800750718. [DOI] [PubMed] [Google Scholar]
- 13.Molan PC. Re‐introducing honey in the management of wounds and ulcers—theory and practice. Ostomy Wound Manage. 2002;48:28–40. [PubMed] [Google Scholar]
- 14.Haynes JS, Callaghan R. Properties of honey: its mode of action and clinical outcomes. Wounds UK. 2011;7(1):50–57. [Google Scholar]
- 15.Visavadia BG, Honetsett J, Danford MH. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2008;46(1):55–56. doi: 10.1016/j.bjoms.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Anstead GM, Hart LM, Sunahara JF, Liter MF. Phenytoin in wound healing. Ann Pharmacother. 1996;30:768–775. doi: 10.1177/106002809603000712. [DOI] [PubMed] [Google Scholar]
- 17.Tauro LF, Shetty P, D’souza NT, Mohammed S, Sucharita S. A comparative study of efficacy of topical phenytoin vs conventional wound care in diabetic ulcers. Int J Mol Med Sci. 2013;3(8):65–71. [Google Scholar]
- 18.Modaghegh S, Salchian B, Tavassoli M, Djamshidi A, Rezai AS. Use of phenytoin in healing of war and non-war wounds. A pilot study of 25 cases. Int J Dermatol. 1989;28:347–350. doi: 10.1111/j.1365-4362.1989.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes RS, Heyneman CA, Culberston VL, Wilson SF, Phatak HM. Topical phenytoin treatment of stage II decubitus ulcers in the elderly. Ann Pharmacother. 2001;35(6):675–681. doi: 10.1345/aph.10267. [DOI] [PubMed] [Google Scholar]
- 20.Medhi B, Puri A, Upadhyay S, Kaman L. Topical application of honey in the treatment of wound healing: a meta-analysis. J K Sci. 2008;10(4):166–169. [Google Scholar]
- 21.Vijaya Kumari K, Nishteswar K. Wound healing activity of honey: a pilot study. AYU. 2012;33(3):374–377. doi: 10.4103/0974-8520.108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udwadia TE. Ghee and honey dressing for infected wounds. Indian J Surg. 2011;73(4):278–283. doi: 10.1007/s12262-011-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004;13(9):353–356. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- 24.Bansal NK, Mukul Comparison of typical phenytoin with normal saline in the treatment of chronic trophic ulcers in leprosy. Int J Dermatol. 1993;32:210–213. doi: 10.1111/j.1365-4362.1993.tb02798.x. [DOI] [PubMed] [Google Scholar]
- 25.Pendse AK, Sharma A, Sodani A, Hada S. Topical phenytoin in wound healing. Int J Dermatol. 1993;32:214–217. doi: 10.1111/j.1365-4362.1993.tb02799.x. [DOI] [PubMed] [Google Scholar]