Abstract
Percutaneous endoscopic gastrostomy (PEG) is an important technique for the provision of nutrition. The present study presents data from our analysis of the PEG procedure. Patients administered with PEG at the endoscopy unit of the 19 Mayıs University General Surgery Department between 2007 and 2013 were analyzed retrospectively, and technical problems, indications, and complications related to the PEG procedure in 221 patients were evaluated. Of the patients, 60 % were male and the median age was 61 years (18–92 years). The most frequent indication was admittance to the intensive care unit, accounting for 46 % of the total, followed by neurological disease, with 41 %. The success rate of the procedure was 98 %, and the overall rate of complications was 22 %. No mortalities were reported as resulting from the procedure. The most common complication was the development of granulomas around the tube (8 %). PEG is a safe method of long-term feeding but is associated with a high rate of morbidity that can be treated easily using conservative treatment methods.
Keywords: Gastrostomy tube, Percutaneous, Indication, Complication, Management
Introduction
Percutaneous endoscopic gastrostomy (PEG) is a suitable feeding procedure for patients who are unable to be fed orally. In patients who have lost their deglutition reflex, PEG provides continuous nutrition via a tube inserted into the stomach. The procedure was first described by Gauder et al. in 1980, and since then, it has seen to have increasing use as a less invasive procedure than surgical gastrostomy [1, 2].
As the knowledge of hospital malnutrition has increased, in addition to chronic nutritional disorders, enteral feeding practices in trauma and intensive care patients has also gained importance [3]. Feeding by way of a nasogastric tube is easy and cheap; however, there are many clinical studies citing PEG as a more comfortable technique that is associated with fewer complications than the nasogastric catheter [4–6]. Accordingly, PEG is recommended in patients who require long-term feeding by tube [7].
Methods
Study Design
For the present study, patients who were administered with PEG at the endoscopy unit of 19 Mayıs University Medical Faculty General Surgery Department were analyzed retrospectively. The patients’ age, gender, ailment, tube application procedure, PEG tube fixing distance, morbidity, and mortality were extracted from the hospital automation system and recorded.
A total of nine patients who had undergone a planned PEG application, but who could not tolerate the procedure and in whom the stomach could not be accessed, were excluded from the study.
Patients
Two hundred twenty-one patients who were administered with PEG between 2007 and 2013 were included in the study.
Procedure
The patients were given no food for 8 h prior to the procedure. Treatments for additional diseases were maintained. The use of low-molecular-weight (LMWH) heparin, clopidogrel, or aspirin was not considered a contraindication. In patients using warfarin, the procedure was carried out after international normalized ratio (INR) values were under control (<2.5). Previous abdominal surgeries (including gastric surgery) were not considered a contraindication.
The tubes were inserted at the bedside for patients who had been hospitalized in the intensive care unit and for the rest of the patients in the endoscopy unit. The length of the tube to the skin entry point was recorded. Prophylactic antibiotic treatments were not administered.
The patients’ pulse rate, blood pressure, and oxygen saturation were monitored during the procedure. The procedure was carried in all patients out using the “pull” method. The tubes used were 16–20 F standard silicon PEG kits. An oral local anesthesia (10 % lidocaine spray) in addition to sedoanalgesia with midazolam (Dormicum, Roche, Basel, Switzerland) and propofol (Diprivan; AstraZeneca, Hamburg, Germany) was administered to conscious patients and those responding to pain stimuli. In patients for whom sedoanalgesia was insufficient, the PEG procedure was carried out under general anesthesia. A local anesthesia was given before the skin incision. The location of the tube insertion point was determined using a diaphanoscope and by finger indentation of the abdominal wall.
The tubes of the patients administered with PEG were in free drainage for 24 h, and gastric decompression was applied. Feeding was initiated at the end of this period.
Results
Among the 221 patients who had undergone PEG procedures at the endoscopy unit of the 19 Mayıs University General Surgery Department between 2007 and 2013, 132 were male (60 %) and 89 were female (40 %). The median age was 61 years (18–92 years).
The PEG procedure was successful in 217 out of 221 patients (98.2 %). In four patients, diaphanoscopy and palpation did not reveal a safe zone between the abdominal wall and the stomach, and so, surgical gastrostomy was applied to these patients. In one of the patients, who was found to have a small stomach, the gastrostomy tube was inserted from the right under the arcus costa, while another patient experienced adhesions as a result of an earlier gastric surgery. In another two patients, a laparotomy did not reveal any problems related to the position of the stomach.
The most frequent indication was hospitalization in the intensive care unit, with a rate of 46 %, followed by neurologic diseases, with 41 %. The PEG indications are presented in Table 1.
Table 1.
PEG indications
| Intensive care patients after trauma or surgery n = 101 (46 %) |
Neurologic diseases n = 89 (41 %) |
Malignant diseases n = 15 (7 %) |
Others n = 12 (6 %) |
| Trauma, 43 (20 %) | CVD, 48 (22 %) | HNC 9 (4 %) | |
| SDH, SAH 31 (14 %) |
Alzheimer 26 (12 %) |
Other 6 (2 %) |
|
| Aspiration pneumonia, COPD 27 (12 %) |
Parkinson 12 (6 %) ALS 3 (1 %) |
CVD cerebrovascular disease, HNC head and neck cancer, SDH subdural hematoma, SAH subarachnoid hemorrhage, COPD chronic obstructive pulmonary disease, ALS amyotrophic lateral sclerosis
The procedure in unconscious, immobile, and intubated patients was carried out in supine position and in reverse Trendelenburg position when possible. The insertion of the endoscope into the esophagus was partially more difficult in such patients. In patients with a nasogastric tube, the tube was removed during the removal of the guide wire via an endoscope. Inserting the endoscope into the esophagus was found to be easier and faster in patients with nasogastric catheters.
The duration of the procedure was between 2.5 and 16 min, with the majority of the time spent pushing the endoscope along the esophagus. Cervical collars and chin fixators complicated the procedure in some patients.
In four cases, the patients were unable to open their mouths wide enough to fit the mouthpiece due to neurological problems, and so, the PEG was inserted under general anesthesia (1.8 %).
The median distance of the outer bumper of the PEG tube to the skin was 2.6 cm (1.5–6 cm).
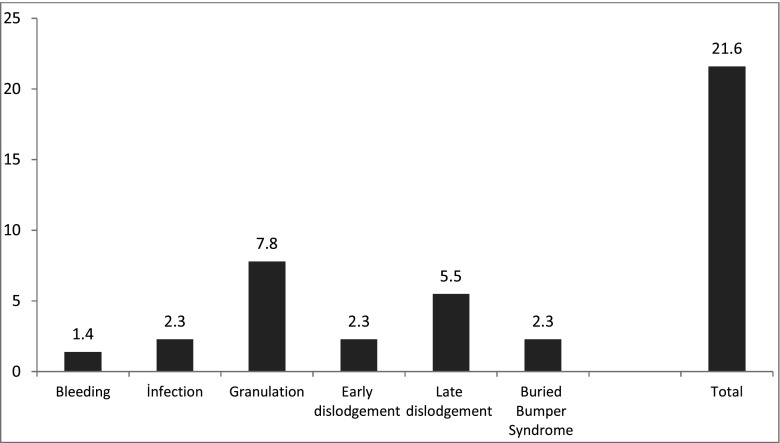
Within the first 7 days after the procedure, the tubes of five patients had become accidentally dislodged due to traction (2.3 %); two patients had pulled and dislodged their own tubes, 8 and 11 h after the procedure. In the second of these patients, the gastrostomy tube was immediately advanced through its tract, and abdominal ultrasonography was used to confirm the tube in the stomach. The patient was treated with antibiotics and kept under observation to monitor any abdominal findings. The other patient was examined and fitted surgically with a gastrostomy tube. The tubes of three additional patients became dislodged due to traction, respectively, 2, 3, and 6 days after the procedure. The gastrostomy tubes were successfully replaced in these patients by pushing them through their tracts. The tube position and the intra-abdominal region were checked with abdominal US. The patients whose tubes were dislodged within the first 7 days experienced no further problems.
The tubes of 12 patients (5.5 %) were dislodged after the initial week, and after which, surgical gastrostomy tubes were inserted into these patients by pushing them through the tube tract.
In a total of three (1.4 %) patients who developed bleeding, two suffered gastric bleeding and one experienced bleeding at the skin level. Skin compression was increased transiently in these patients. The bleeding stopped within 24 h in the first patient but lasted for 48 h in another. None of the patients required a blood transfusion.
An infection developed at the PEG site in five patients (2.3 %). Of these, one patient has drainage through a skin incision at the edge of the tube, while the other patients recovered after wound care and antibiotic treatments (Fig. 1).
Fig. 1.
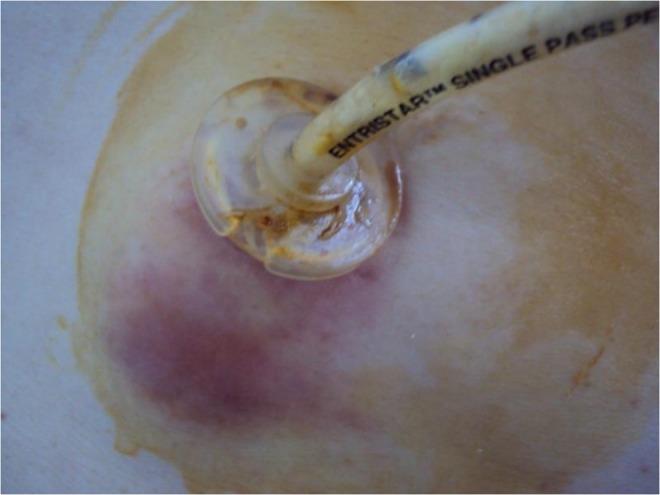
PEG site infection
Granulation developed at the PEG site in 17 patients (7.8 %), who experienced pain and bleeding in the form of leakage. These patients were treated with chemical cauterization using silver nitrate.
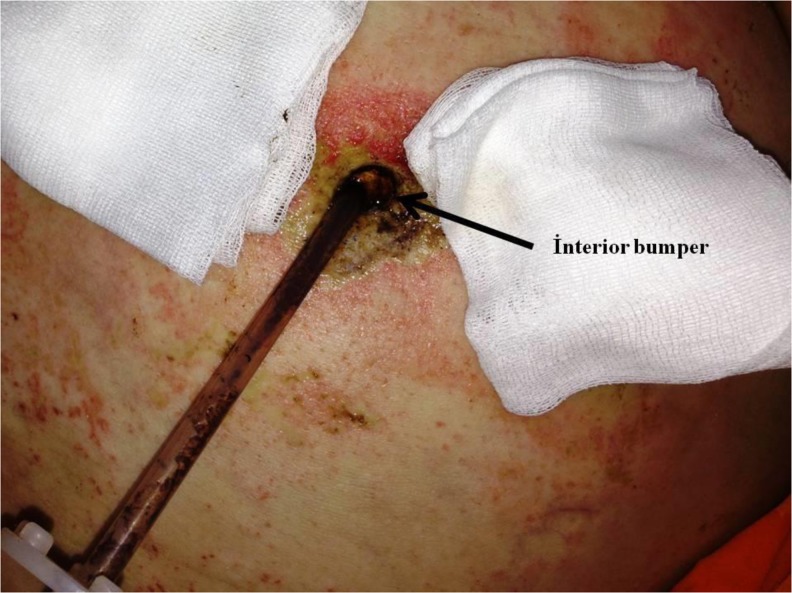
Buried bumper syndrome developed in five patients (2.3 %), who were admitted with tube obstructions and discharges from the tube site (Fig. 2). Examinations revealed that the tube was close to the skin surface for less than 1 cm. In a total of five patients, three have tubes that were at a subcutaneous level and were removed by traction. The remaining two patients were diagnosed by endoscopy. The PEG tubes were disconnected at the skin level, pushed into the stomach, gripped using a snare, and removed by endoscopy. The tube site was drained, and a new PEG tube was inserted into these patients from another location.
Fig. 2.
Buried bumper syndrome
Among the eight patients that had undergone previous gastrointestinal surgeries, a PEG tube was inserted into seven (87.5 %). A surgical gastrostomy was applied to one patient for whom the PEG had been unsuccessful.
The PEG complications are presented in Fig. 3. Among the patients reporting complications associated with PEG, only one had undergone surgical treatment due to early-phase tube removal (0.5 %). The other patients were treated successfully using conservative methods. There were no reported mortalities related to the PEG procedure.
Fig. 3.
PEG complications
Since our endoscopy unit provides the third line of care, the majority of the patients administered with PEG had posttreatment follow-ups at the health-care centers in their own regions. As a result, the long-term medical records of the patients after tube insertion could not be accessed.
Discussion
With the advent of a better understanding of the clinical importance of nutrition, gastrostomy practices have become a major component of treatments. Since enteral feeding offers significant advantages over parenteral feeding, it is more often recommended in patients with functional gastrointestinal systems. Short-term feeding can be provided by way of a nasoenteral tube in patients with insufficient oral uptake [8]. Although PEG is a more invasive procedure, it is said to offer more advantages over nasogastric feeding [4–8]. In clinical practice, PEG is recommended for long-term feeding. ESPEN (The European Society for Clinical Nutrition and Metabolism) guidelines recommend PEG for patients who require feeding for more than 2 to 3 weeks [7], whereas various other studies advise the use of PEG for feedings that last longer than 4 weeks [8–10].
While PEG is common in patients with neurologic diseases, its use in intensive care patients after trauma or surgery has increased since feeding has been considered a part of treatment [3, 10, 11]. In patients with predicted long-term treatment needs, the PEG can be applied shortly after the initiation of nasoenteral feeding. The nutritional strategies followed by different clinics can lead to differences in the frequency of PEG application. The frequency of PEG applications among the patients who require intensive care support after trauma or surgery is also higher in the present study.
The use of prophylactic antibiotics in the PEG procedure is controversial. Although most studies report that the use of antibiotics may reduce peristomal infections [12–14], there are also studies claiming the opposite [15–17]. It has been reported that the use of antibiotics may increase bacterial resistance [18], although the ESPEN guidelines indicate that their use is not mandatory [7]. In the present study, no antibiotic prophylaxis was used, and the rate of peristomal infections observed in the present study was 2.3 %, lower than the rates (5–36 %) reported in earlier literature [19–21]. Risk factors include cancer, cirrhosis, radiation exposure, diabetes, the size of the tube, and experience [17–22].
Different durations have been recommended regarding the initiation of feeding after the procedure. In the present study, feeding was initiated 24 h after the procedure, and during this period, the PEG tube was left to allow drainage. Feeding was not initiated earlier so as to allow the epithelialization of defects in the skin, muscles, and peritoneum, in accordance with the principles of drainage. We ensured that the gas insufflation and the gastric fluid in the stomach were decompressed through drainage during the procedure, and in this way, we also monitored bleeding complications. We believe that the late initiation of feeding may have led to the low rate of peristomal infections experienced. Current literature implies that the early initiation of the feeding does not necessarily increase the rate of complications when compared to a late initiation, and furthermore, nutritional guidelines do not include a recommendation on this subject [23, 24].
Complications resulting from PEG can be encountered both during and after the procedure, and different rates of complication have been reported in the literature. Fröchlich et al. reported the rates of PEG-associated complications, morbidity, and mortality as 4.9–50, 3–12, and 0.5–1.2 %, respectively [25]. In the present study, no mortality associated with the procedure was encountered, and the most frequent complication, although not commonly listed among complications related to PEG, was granulation at the edge of ostomy. Exophytic granulation tissue may develop around the stoma in cases of long-term tube use as a result of leakage from the edge of the tube or insufficient care [21]. Leakage and bleeding may be seen at the edge of the tube, where maceration may develop when sufficient care is not provided. Regularly changing the PEG tube is not recommended [7]. The PEG tube was changed only in the event of malfunction in the present study, and the high rate of granulation that was observed can be associated mainly with long-term tube use. The granulating tissue can be treated with surgical or chemical cauterization and wound care, and all patients reported here were successfully treated using silver nitrate sticks.
Buried bumper syndrome relates to the dislocation of the PEG tube from the stomach mucosa towards the skin. The rate of buried bumper syndrome reported in the literature was between 0.3 and 4 %, concurring with the findings of the present study of 2.3 %. The most common cause of buried bumper syndrome included over-traction, hard interior bumper, malnutrition, and weak wound healing [21]. Tube obstruction may develop as a result of leakage from the edge of the tube as well as wound infection, and the diagnosis can be generally confirmed from the tube distance and the status of the ostomy, although radiological and endoscopic assessments may also be required in some cases. Out of five patients in the present study, the diagnosis was confirmed by endoscopy in two and by examination in three. Differences between the distances measured during the tube insertion and during the follow-up, as recorded in the patient records, may be an indicator of buried bumper syndrome. No radiological assessment was required in any of the cases presented here.
Although reported complications associated with bleeding are frequent, the rate of such complications was relatively low in the present study. While the ESPEN guidelines recommend maintaining an INR of below 1.5 in order to reduce bleeding complications [7], in a study of 1041 patients, Richter-Schrag et al. reported that the use of anticoagulants and increased INR values do not increase the rate of bleeding complications [22]. In the present study, the patients were provided with continuous anticoagulant treatment prior to the procedure but kept their INR level below 2.5.
The accidental dislodgement complication rate observed in the present study was higher than that reported in the previous literature (0–5.3 %) [26]. The recovery of a gastrocutaneous fistula takes generally 1 or 2 weeks, and dislodgements before this period may result in a high mortality risk. Rosenberger et al. reported a dislodgement rate of 4.1 % within 7 days and 12.8 % within the lifespan and a mortality rate of 7.8 % [26]. In the present study, the rates of early and late dislodgement were 2.3 and 5.5 %, respectively. Furthermore, four of the five patients (80 %) that experienced dislodgement within the 7 days were those that had been admitted to the intensive care unit after trauma or surgery. These patients were unconscious and suffered involuntary movements, but of these, only one required emergency surgical treatment after pulling the tube. In the remaining four patients, the gastrostomy tube was successfully inserted through the skin defect. No mortalities occurred in any of the patients as a result of the accidental dislodging of the tube. We believe that immediate intervention in the case of tube dislodgements had a role in this finding.
Previous abdominal surgeries are not considered to be contraindications to PEG insertions [7–27]. Of the cases reported here, eight (3.7 %) had undergone previous gastrointestinal surgery, and the procedure was unsuccessful in one of them (12.5 %). The rate of failure after previous gastrointestinal surgery was reported to be between 2.7 and 12 % in the literature [27, 28]. We believe that the rate of failure observed in the present study was high since the number of patients was limited.
Conclusions
Our experience with the application of PEG reveals that the rate of morbidity is remarkable; however, morbidities such as tube dislodgement within the first week of application of PEG can mostly be treated using conservative methods. As such, PEG can be considered a safe method of long-term feeding.
Acknowledgments
Conflict of Interest
None
Source of Funding
None
References
- 1.Gauderer MW. Percutaneous endoscopic gastrostomy-20 years later: a historical perspective. J Pediatr Surg. 2001;36:217–219. doi: 10.1053/jpsu.2001.20058. [DOI] [PubMed] [Google Scholar]
- 2.Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27:9–11. doi: 10.1016/S0016-5107(81)73133-X. [DOI] [PubMed] [Google Scholar]
- 3.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945–948. doi: 10.1136/bmj.308.6934.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–1790. doi: 10.1002/1097-0142(20010501)91:9<1785::AID-CNCR1197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Saha S, Bose A. Percutaneous endoscopic gastrostomy (PEG)—an useful ‘surgical’ measure. Indian J Otolaryngol Head Neck Surg. 2006;58(3):235–238. doi: 10.1007/BF03050827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norton B, Homer-Ward M, Donnelly MT, Long RG, Holmes GKT. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13–16. doi: 10.1136/bmj.312.7022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, Rollins H, Singer P, Skelly RH. ESPEN guidelines on artificial enteral nutrition-percutaneous endoscopic gastrostomy (PEG) Clin Nutr. 2005;24(5):848–861. doi: 10.1016/j.clnu.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recommendations. J Parenter Enteral Nutr. 2009;33:122–167. doi: 10.1177/0148607108330314. [DOI] [PubMed] [Google Scholar]
- 9.Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JL, Martin ND. Complications related to percutaneous endoscopic gastrostomy tubes. A comprehensive clinical review. J Gastrointest Liver Dis. 2007;16:407–418. [PubMed] [Google Scholar]
- 10.Nicholson FB, Korman MG, Richardson MA. Percutaneous endoscopic gastrostomy: a review of indications, complications and outcome. J Gastroenterol Hepatol. 2000;15(1):21–25. doi: 10.1046/j.1440-1746.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes CA, Lustosa SA, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2012;3:CD008096. doi: 10.1002/14651858.CD008096.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Sharma VK, Howden CW. Meta-analysis of randomised, controlled trials of antibiotic prophylaxis before percutaneous endoscopic gastrostomy. Am J Gastroenterol. 2000;95:3133–136. doi: 10.1111/j.1572-0241.2000.03283.x. [DOI] [PubMed] [Google Scholar]
- 13.Akkersdijk WL, van Bergeijk JD, van Egmond T, et al. Percutaneous endoscopic gastrostomy (PEG): comparison of push and pull methods and evaluation of antibiotic prophylaxis. Endoscopy. 1995;27:313–316. doi: 10.1055/s-2007-1005699. [DOI] [PubMed] [Google Scholar]
- 14.Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647–656. doi: 10.1111/j.1365-2036.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 15.Jonas SK, Neimark S, Panwalker AP. Effect of antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1985;80:438–441. [PubMed] [Google Scholar]
- 16.Sturgis TM, Yancy W, Cole JC, Proctor DD, Minhas BS, Marcuard SP. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1996;91:2301–2304. [PubMed] [Google Scholar]
- 17.Zopf Y, Konturek P, Nuernberger A, et al. Local infection after placement of percutaneous endoscopic gastrostomy tubes: a prospective study evaluating risk factors. Can J Gastroenterol. 2008;22:987–991. doi: 10.1155/2008/530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins G, Hull M. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy insertion in patients with non-malignant disease. Aliment Pharmacol Ther. 2006;23:1276–1277. doi: 10.1111/j.1365-2036.2006.02821.x. [DOI] [PubMed] [Google Scholar]
- 19.Faias S, Cravoi M, Claro I, et al. High rate of percutaneous endoscopic gastrostomy site infections due to oropharyngeal colonization. Dig Dis Sci. 2006;51:2384–2388. doi: 10.1007/s10620-006-9216-z. [DOI] [PubMed] [Google Scholar]
- 20.Gossner L, Keymling J, Hahn EG, Ell C. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG). A prospective randomized clinical trial. Endoscopy. 1999;31:119–124. doi: 10.1055/s-1999-13658. [DOI] [PubMed] [Google Scholar]
- 21.McClave SA, Chang WK. Complications of enteral access. Gastrointest Endosc. 2003;58:739–751. doi: 10.1016/S0016-5107(03)02147-3. [DOI] [PubMed] [Google Scholar]
- 22.Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25(4):201–206. doi: 10.1155/2011/609601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali T, Le V, Sharma T, Vega KJ, Srinivasan N, Tierney WM, Rizvi S. Post-PEG feeding time: a web based national survey amongst gastroenterologists. Dig Liver Dis. 2011;43(10):768–771. doi: 10.1016/j.dld.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Corkins MR, Fitzgerald JF, Gupta SK. Feeding after percutaneous endoscopic gastrostomy in children: early feeding trial. J Pediatr Gastroenterol Nutr. 2010;50:625–627. doi: 10.1097/MPG.0b013e3181bab33d. [DOI] [PubMed] [Google Scholar]
- 25.Fröhlich T, Richter M, Carbon R, Barth B, Köhler H. Review article: percutaneous endoscopic gastrostomy in infants and children. Aliment Pharmacol Ther. 2010;31:788–801. doi: 10.1111/j.1365-2036.2010.04246.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307–3311. doi: 10.1007/s00464-011-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eleftheriadis E, Kotzampassi K. Percutaneous endoscopic gastrostomy after abdominal surgery. Surg Endosc. 2001;15(2):213–216. doi: 10.1007/s004640000250. [DOI] [PubMed] [Google Scholar]
- 28.Foutch PG, Talbert GA, Waring JP, Sanowski RA. Percutaneous endoscopic gastrostomy in patients with prior abdominal surgery: virtues of the safe tract. Am J Gastroenterol. 1988;83(2):147–150. [PubMed] [Google Scholar]