Abstract
Varicose veins have a high recurrence rate following surgery. Besides poor surgical technique, majority of these recurrences are attributable to neovascularization after both primary and repeat surgery. Authors have studied the effectiveness of a polytetrafluoroethylene (PTFE) patch interposition between the ligated vein stump and the overlying soft tissue at saphenofemoral junction in decreasing recurrence of varicose veins after initial surgery. Study was conducted on 50 patients of varicose veins with saphenofemoral junction incompetence. Patients were randomly divided into two groups, group A and group B alternately. In group A, standard surgical procedure was done followed by PTFE patch application. In group B, same surgical procedure was applied as in group A, with the exception of PTFE patch application. Patients in both groups were given similar postoperative care. A full venous duplex ultrasound assessment was performed in all the patients postoperatively. Neovascularization was observed in five patients (20 %) of group B, while it was not seen in any of the patients in group A at 1-year follow-up. This difference in neovascularization across the two groups was found to be statistically significant with a p value of 0.0251. Hence, authors concluded that patch saphenoplasty helps in reducing recurrence in varicose veins by decreasing neovascularization at saphenofemoral junction.
Keywords: Varicose veins, PTFE patch saphenoplasty, Neovascularization, Recurrent varicose veins
Introduction
Varicose vein is a common problem and affects at least 10 % of the general population [1]. Surgery has been the time-honored and gold standard treatment for varicose veins but recurrent varices after surgery (REVAS) are a common, complex, and costly problem both for the patients and the physicians who treat venous diseases. Recurrence following surgery for varicose veins remains unacceptably high with estimated rates as high as 40 % in 5 years [2]. The recurrence rate is much higher with a longer follow-up, with a study by Fischer et al. documenting a recurrence rate as high as 77 % with a 34-year follow-up [3]. It is estimated that in UK, out of the total surgeries performed for varicose veins, 20 % are for recurrent disease and technically, it is more demanding [4–6].
It has been found that besides poor surgical technique, majority of these recurrences are attributable to neovascularization, after both primary and repeat surgery [7–12]. This postoperative neovascularization at the level of the groin can readily be detected with duplex scanning [11, 13]. Thus prevention or inhibition of this angiogenesis would be a major breakthrough in reducing the rate of recurrences. Several studies have suggested that physical inhibition of angiogenesis at the ligated saphenofemoral junction (SFJ) may be effective. Introduction of a physical barrier across the site of angiogenic focus will prevent new vessels crossing back and reconnecting the venous system. Various authors have claimed the effectiveness of barrier techniques in preventing neovascularization at SFJ [5, 14–16].
Rij et al. have reported that recurrence is halved in patients in whom polytetrafluoroethylene (PTFE) patch was applied [17]. Hence, present study was planned to see whether PTFE patch is effective in decreasing neovascularization and recurrence of varicose veins after initial surgery.
Material and Methods
Study Design
The study was a prospective randomized study, single blinded at the level of ultrasonologist.
Inclusion Criteria
Patients of primary varicose veins of either sex in the age group 20–60 years with saphenofemoral incompetence with clinical severity ranging from C2-C6 (according to CEAP classification) admitted in surgical ward for surgery at Pt. B.D. Sharma PGIMS, Rohtak were included in the study.
Study
The study was conducted on 50 patients who gave informed consent to participate in the study. All patients were assessed preoperatively with a detailed history and clinical examination. Clinical severity of the disease in a given patient was assessed by standard clinical, etiologic, anatomic, and pathological (CEAP) classification.
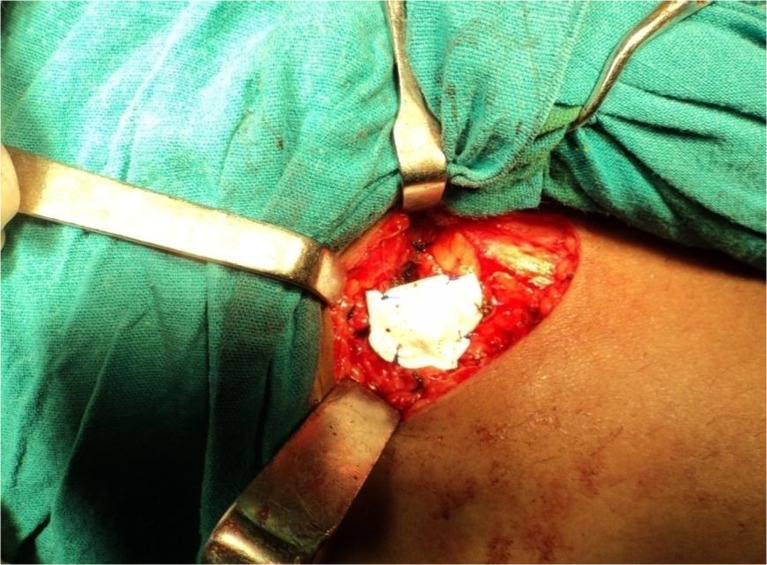
Patients were randomly divided into two groups, group A and group B. In group A, standard surgical procedure (SFJ ligation with ligation of all the tributaries with stripping of great saphenous vein (GSV) in the thigh portion) was done followed by PTFE patch application. The PTFE patch (2 × 3 cm) was secured with a 3–0 Prolene round body needle suture at each corner to adjacent tissue to lie comfortably over the stump and the adjacent common femoral vein (Fig. 1). In group B, same surgical procedure was applied as in group A, with the exception of PTFE patch application. Patients in both groups were given similar postoperative care including early mobilization and the use of elastic compression stockings 1 week after surgery.
Fig. 1.
Photograph showing PTFE patch applied over ligated SFJ Stump
The patients were assessed for evidence of neovascularization at follow-up with duplex ultrasonography.
The pattern of neovascularization at SFJ was classified as follows:
Single narrow channel (diameter ≤3 mm)
Single large channel (diameter >3 mm)
Multiple small channels
Recurrence at the SFJ was recognized on duplex ultrasonography by the reappearance of reflux for >0.5 s at the site of previous surgery and then confirmed on a subsequent reassessment. Patients were also assessed clinically for recurrence and any complication at 1-year follow-up.
Follow-up
After surgery, all patients were followed up with clinical assessment and duplex imaging, at 3, 6, and 12 months postoperatively. The results so obtained were tabulated, analyzed statistically using Student t test and Fischer’s test, and conclusions were drawn.
Observations
Two groups studied were comparable with respect to basic demographics and limb characteristics as shown in Table 1. On clinical examination, during follow-up at 1 year, dilated/visible veins were found in one patient (4 %) in group A while six patients (24 %) in group B had prominent veins. This difference in visible veins at follow-up in two groups was found to be statistically significant (p value, 0.0491). Pain in the leg persisted in five (10 %) patients (one in group A, four in group B). Edema was found in three (6 %) patients (one in group A, two in group B). Pigmentation persisted in all the patients at follow-up of 1 year irrespective of the procedure done on them. There was no statistically significant difference in symptomatic relief in two groups, with respect to pain in the leg, edema, and ulcer healing.
Table 1.
Baseline patient demographics
| Group A (patch group) | Group B (non-patch group) | ||
|---|---|---|---|
| Mean age (in years) | 31.68 | 31.44 | |
| Sex | M | 18 | 15 |
| F | 7 | 10 | |
| Side involvement | Rt. | 9 | 12 |
| Lt. | 16 | 13 | |
| Clinical severity | C2-C3 | 16 | 18 |
| C4-C6 | 9 | 7 | |
| Duration of symptoms (in years) | 0-1 | 5 | 5 |
| >1–3 | 14 | 13 | |
| >3 | 6 | 7 | |
Neovascularization
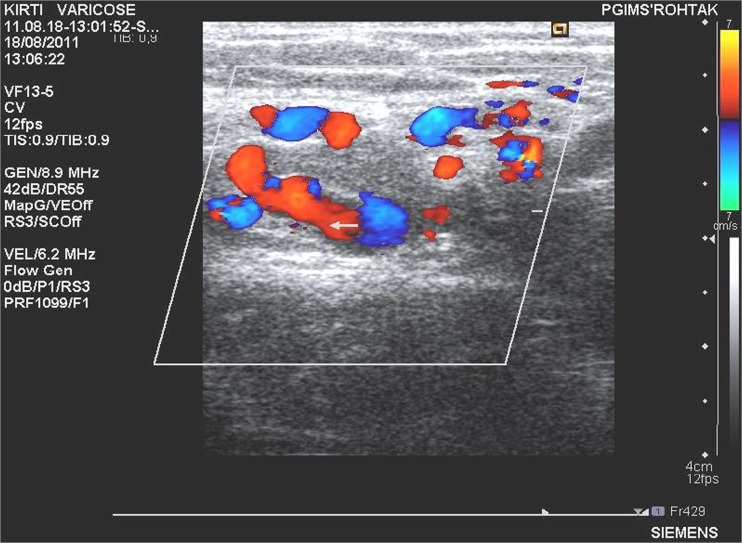
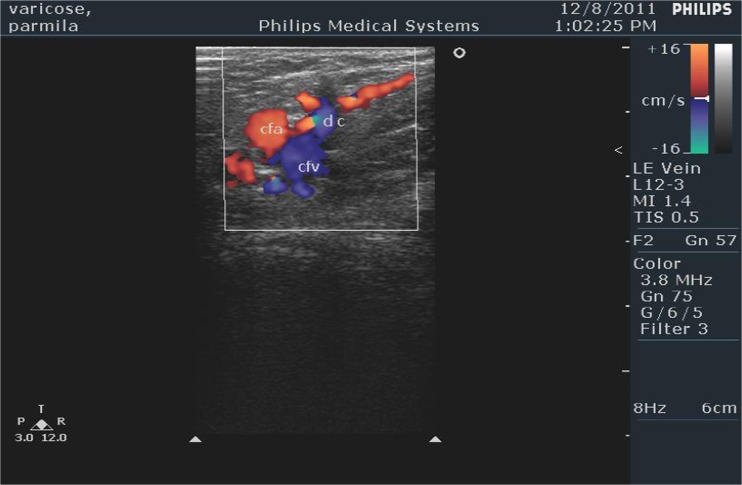
Neovascularization was not seen on ultrasonography in any of the patients in group A at 1-year follow-up. In group B, it was seen in five (20 %) patients (Table 2). This difference in neovascularization across the two groups was found to be statistically significant with a p value of 0.0251. Out of the five patients in group B, showing neovascularization at ultrasonography, majority (three out of five, i.e., 60 %) had a single large dilated channel. Rest of the patients demonstrated multiple small dilated channels (Figs. 2 and 3). Circumjunctional pattern was not seen in any of the patients in this study.
Table 2.
Pattern of neovascularization on USG examination in group B patients
| Pattern of neovascularization | No. of patients | Percentage |
|---|---|---|
| Single large dilated channel (>3 mm) | 3 | 60 |
| Multiple small dilated channels | 2 | 40 |
| Circumjunctional | 0 | 0 |
| Total | 5 | 100 |
Fig. 2.
Color Doppler showing multiple small dilated channels
Fig. 3.
Color Doppler showing single large dilated channel
Correlation of Sex with Neovascularization
In patients presenting with neovascularization, there was a female predominance. Out of 5 patients of neovascularisation, 4 were females out of 10 female patients of Group B and one was male out of 15 male patients of Group B. This difference in neovascularization among the males and females was found to be statistically significant with a relative risk (RR) of 2.769 for females [p value, 0.0400; RR, −2.769].
Correlation of Preoperative Clinical Severity of Disease with Neovascularization
In group B, two patients out of seven (28.6 %) who had disease of severe variety (C4-C6) developed neovascularization, while only 3 patients out of 18 (16.7 %) had a milder form of disease presented with neovascularization. Thus, higher percentage of patients with severe variety (C4-C6) of varicose veins presented with neovascularization as compared to patients who had milder form of disease. However, this difference was not found to be statistically significant (p value, 0.4356).
Discussion
Neovascularization at the previously ligated SFJ stump is one of the major causes of recurrence following primary varicose veins surgery. Authors have studied the effectiveness of PTFE patch saphenoplasty in reducing neovascularization.
In our study, 5 out of the 25 patients (20 %) in the group in which patch was not applied showed the presence of neovascularization on ultrasonography at 1-year follow-up while none of the 25 patients (0 %) in the PTFE patch group developed neovascularization. This difference in results was statistically significant (p value, 0.0251). Clinically prominent veins were seen in six patients in group B as compared to only one patient in group A at 1 year of follow-up. Several other researchers have shown the efficacy of barrier techniques in reducing neovascularization [14, 16, 17].
Various authors have reported the efficacy of silicone patch interposition at SFJt in reducing neovascularization, but there is high incidence of deep vein thrombosis with the use of silicone implant [14, 18].
Rij et al. reported a highly significant decrease in neovascular recurrence at the SFJ when a polytetrafluoroethylene patch (PTFE patch, 3 × 3 cm) is interposed between the ligated vein stump and the overlying soft tissue. The 3-year recurrence was halved, from 46 % to just 23 % in patients in whom PTFE patch was applied. If only the more severe limbs in each group (baseline CEAP clinical grades 4 to 6) are compared, then the recurrence after the use of the patch was reduced by >60 % [17]. Creton in his study has also obtained remarkably good results with PTFE patch saphenoplasty [16].
Several other anatomical barrier techniques are also described in the literature, like closing the cribriform fascia. After 1 year, duplex scan showed neovascularization in 15 of 223 limbs (6.7 %). The results were found to be comparable to the group of 191 limbs with silicone patch saphenoplasty and were superior to the group of 189 limbs without barrier [19].
Duplex Pattern of Neovascularization at SFJ
In the present study, out of the five patients who showed neovascularization on ultrasonography, a single dilated large channel (>3 mm) was seen in three patients (60 %) and two patients (40 %) demonstrated multiple small dilated channels. Circumjunctional pattern of neovascularization was not seen in any of the patients in this study. The study of Fischer et al., with a long follow-up of 34 years, reported single large dilated channel as the commonest pattern of neovascularization followed by multiple small dilated channels and circumjunctional connections [3]. Jones et al. reported multiple small dilated channels in the majority of patients during follow-up on ultrasonography, followed by the pattern of single large dilated channel, but single large dilated channel was the commonest pattern in patients presenting with clinically significant recurrence at SFJ [11]. Stücker et al. reported single-channel recurrence in 69 % and multichannel recurrence in 31 % of patients [20]. Rij et al. reported single large channel in 38 % of the limbs and multiple dilated channels in 62 % of the limbs [21].
Correlation of Sex of the Patient with Neovascularization
In patients presenting with neovascularization, there was a female predominance in the present study. Out of 17 female patients, 4 developed neovascularization, while only 1 male patient out of 33 male patients had evidence of neovascularization. Thus, higher percentage (23.53 %) of female patients developed neovascularization compared to their male counterparts (3.03 %). This difference in neovascularization among the sexes was found to be statistically significant with a RR of 2.769 for females (p value, 0.0400; RR, −2.769). In the literature of a seven-series compilation of patients, who presented with a reflux after a previous surgical procedure, females accounted for 505 of 722 patients, with a male to female ratio of 1:2.4 [3]. The reason for this gender bias has not been conclusively proved in the studies, possible mechanism may be some hormonal difference across the two genders.
Correlation of Preoperative Clinical Severity of Disease with Neovascularization
In the present study, a higher percentage (28.6 %) of patients with severe variety (C4-C6) of varicose veins presented with neovascularization compared to 16.7 % of patients who had milder form of disease. However, this difference was not found to be statistically significant (p value, 0.4356).
The CEAP classes 4–6 seem to be associated with higher levels of growth factors and leukokines that might facilitate the development of recurrent reflux. Pappas and colleagues found increased levels of neovascularity stimulating, transforming growth factor-β1 in punch biopsies of lower calf skin from CEAP classes 4, 5, and 6 patients, compared to skin biopsies from healthy individuals [22]. Takase and coworkers have shown that plasma from CEAP classes 4, 5, and 6 patients has markedly more granulocyte-activating activity than does plasma from CEAP classes 1 and 2 patients [23].
No complication was seen in either of the two groups in the present study. Deep vein thrombosis, wound infection, lymphocele, and lymphoedema have been reported following silicone patch application [24]. No such untoward events have been reported in studies in which PTFE patch was used instead of silicone patch [15–17].
Minimally invasive treatments including endovenous thermal ablation and ultrasound guided foam sclerotherapy are becoming increasingly popular in the treatment of varicose veins [25]. Despite apparent reduction in neovascularization and excellent occulusion rates, randomized clinical trials comparing EVLA and stripping have failed to show a significant advantage of laser at 2 years in terms of recurrence and quality of life outcomes and long-term follow-up data is awaited [26, 27]. Several factors like tactical and technical errors, disease progression, and neovascularization have been supposed to be contributing factors for recurrence [28]. Out of these factors, neovascularization can be minimized by PTFE patch saphenoplasty which is a safe procedure to prevent neovascularization in patients undergoing open surgery which is still one of the most commonly done interventions in varicose veins. The minimally invasive thermoablation procedures have been reported to reduce hospital stay and enable the patient to return to work early but are not available at all places, and cost of disposables may not be affordable for all the patients and may be associated with complications like paresthesia, failure of the procedure, and recanalization [29, 30]. Hence, if open surgery is to be done for the above reasons of cost or non-availability of minimally invasive procedures, then patch saphenoplasty may be done in open surgery to minimize the neovascularization and recurrence.
References
- 1.Burkitt DP, Liem TK, Monete GL. Venous and lymphatic diseases. In: Brunicardi FC, Anderson DK, Billair TR, Dunn DL, Hunter JG, Pollock RE, editors. Schwartz’s principles of surgery. 9. USA: McGraw-Hill; 2009. [Google Scholar]
- 2.Perrin MR, Labropoulos N, Leon LR., Jr Presentation of the patient with recurrent varices after surgery (REVAS) J Vasc Surg. 2006;43:327–334. doi: 10.1016/j.jvs.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 3.Fischer R, Chandler JG, De Maeseneer MG, Frings N, Lefebvre- Vilarbedo M, Earnshaw JJ, et al. The unresolved problem of recurrent saphenofemoral reflux. J Am Coll Surg. 2002;195:80–94. doi: 10.1016/S1072-7515(02)01188-2. [DOI] [PubMed] [Google Scholar]
- 4.Negus D. Recurrent varicose veins: a national problem. Br J Surg. 1993;80:823–824. doi: 10.1002/bjs.1800800705. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard M. A procedure for the prevention of recurrent saphenofemoral incompetence. Aust N Z J Surg. 1978;48:322–326. doi: 10.1111/j.1445-2197.1978.tb05240.x. [DOI] [PubMed] [Google Scholar]
- 6.Juhan C, Haupert S, Miltgen G, Barthelemy P, Eklof B. Recurrent varicose veins. Phlebology. 1990;5:201–211. [Google Scholar]
- 7.Glass GM. Neovascularization in recurrence of varices of the great saphenous vein in the groin. Angiology. 1988;39:577–582. doi: 10.1177/000331978803900704. [DOI] [PubMed] [Google Scholar]
- 8.Glass GM. Neovascularization in recurrence of varices of the great saphenous vein in the groin: surgical anatomy and morphology. Vasc Surg. 1989;23:435–442. doi: 10.1177/153857448902300603. [DOI] [Google Scholar]
- 9.Darke SG. Morphology of recurrent varicose veins. Eur J Vasc Surg. 1992;6:512–517. doi: 10.1016/S0950-821X(05)80626-7. [DOI] [PubMed] [Google Scholar]
- 10.Coleridge Smith PD. Recurrence at the sapheno-femoral junction [editorial] Phlebology. 1995;10:131. [Google Scholar]
- 11.Jones L, Braithwaite BD, Selwyn D, Cooke S, Earnshaw JJ. Neovascularisation is the principal cause of varicose vein recurrence: results of a randomised trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg. 1996;12:442–445. doi: 10.1016/S1078-5884(96)80011-6. [DOI] [PubMed] [Google Scholar]
- 12.Nyamekye I, Shephard NA, Davies B, Heather B, Earnshaw JJ. Clinicopathological evidence that neovascularisation is a cause of recurrent varicose veins. Eur J Vasc Endovasc Surg. 1998;15:412–415. doi: 10.1016/S1078-5884(98)80202-5. [DOI] [PubMed] [Google Scholar]
- 13.De Maeseneer MG, Tielliu IF, Van Schil PE, De Hert SG, Eyskens EJ. Clinical relevance of neovascularisation on duplex ultrasound in the long term follow up after varicose vein operation. Phlebology. 1999;14:118–122. doi: 10.1007/s005230050036. [DOI] [Google Scholar]
- 14.De Maeseneer MG, Giuliani DR, Van Schil PE, De Hert SG. Can interposition of a silicone implant after sapheno-femoral ligation prevent recurrent varicose veins? Eur J Vasc Endovasc Surg. 2002;24:445–449. doi: 10.1053/ejvs.2002.1685. [DOI] [PubMed] [Google Scholar]
- 15.Bhatti TS, Whitman B, Harradine K, Cooke SG, Heather BP, Earnshaw JJ. Causes of re-recurrence after polytetrafluoroethylene patch saphenoplasty for recurrent varicose veins. Br J Surg. 2000;87:1356–1360. doi: 10.1046/j.1365-2168.2000.01602.x. [DOI] [PubMed] [Google Scholar]
- 16.Creton D. Surgery for recurrent Sapheno-femoral incompetence using expanded polytetrafluoroethylene patch interposition in front of the femoral vein: long term outcome in 119 extremities. Phlebology. 2002;16:93–97. doi: 10.1007/s005230200011. [DOI] [Google Scholar]
- 17.van Rij AM, Jones TG, Hill BG, Amer M, Thomson IA, Pettigrew RA, et al. Mechanical inhibition of angiogenesis at the saphenofemoral junction in the surgical treatment of varicose veins: early results of a blinded randomized controlled. Trial Circ. 2008;118:66–74. doi: 10.1161/CIRCULATIONAHA.107.726869. [DOI] [PubMed] [Google Scholar]
- 18.De Maeseneer MG, Vandenbroeck CP, VanSchil PE. Silicone patch saphenoplasty to prevent repeat recurrence after surgery to treat recurrent saphenofemoral incompetence: long-term follow-up study. J Vasc Surg. 2004;40:98–105. doi: 10.1016/j.jvs.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 19.De Maeseneer MG, Philipsen TEA, Vandenbroeck CPA. Closure of the cribriform fascia: an efficient anatomical barrier against postoperative neovascularisation at the saphenofemoral junction? Prospect Study Eur J Vasc Endovasc Surg. 2007;34(3):361–366. doi: 10.1016/j.ejvs.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Stücker M, Netz K, Breuckmann F, Altmeyer P, Mumme A. Histomorphologic classification of recurrent saphenofemoral reflux. J Vasc Surg. 2004;39:816–821. doi: 10.1016/j.jvs.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 21.van Rij AM, Jones TG, Hill BG. Neovascularization and recurrent varicose veins: more histologic and ultrasound evidence. J Vasc Surg. 2004;40:296–302. doi: 10.1016/j.jvs.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Pappas PJ, You R, Rameshwar P, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor- β1 gene expression and protein production. J Vasc Surg. 1999;30:1129–1145. doi: 10.1016/S0741-5214(99)70054-6. [DOI] [PubMed] [Google Scholar]
- 23.Takase S, Schmid-SchÖnbein G, Bergan JJ. Leukocyte activationin patients with venous insufficiency. J Vasc Surg. 1999;30:148–156. doi: 10.1016/S0741-5214(99)70187-4. [DOI] [PubMed] [Google Scholar]
- 24.De Maeseneer MG, Vandenbroeck CP, Lauwers PR, Hendriks JM, De Hert SG, Van Schil PE. Early and late complications of silicone patch saphenoplasty at the saphenofemoral junction. J Vasc Surg. 2006;44:1285–1290. doi: 10.1016/j.jvs.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd AC, Gohel MS, Hamish M, Lim CS, Davies AH. Endovenous treatments for varicose veins—over taking or over rated? Phlebology. 2010;25:38–43. doi: 10.1258/phleb.2009.008091. [DOI] [PubMed] [Google Scholar]
- 26.Christenson JT, Gueddi S, Gemayel G, Bounameaux H. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow up. J Vasc Surg. 2010;52:1234–1241. doi: 10.1016/j.jvs.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079–1087. doi: 10.1002/bjs.7555. [DOI] [PubMed] [Google Scholar]
- 28.Brake M, Lim CS, Shepherd AC, Shalhoub J, Davies AH. Pathogenesis and etiology of recurrent varicose veins. J Vasc Surg. 2013;57:860–868. doi: 10.1016/j.jvs.2012.10.102. [DOI] [PubMed] [Google Scholar]
- 29.Lohr J, Kulwicki A. Radiofrequency ablation: evolution of a treatment. Semin Vasc Surg. 2010;23:90–100. doi: 10.1053/j.semvascsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Anwar MA, Lane TA, Davies AH, Franklin IJ. Complications of radiofrequency ablation of varicose veins. Phlebology. 2012;27:34–39. doi: 10.1258/phleb.2012.012S21. [DOI] [PubMed] [Google Scholar]