Abstract
A clinical trial inspired and guided by optogenetics experiments in rodents reports the outcome of targeted transcranial magnetic stimulation in patients suffering from cocaine addiction.
Addiction is a poorly understood and heavily stigmatized disease. Substance abuse– and dependence-related conditions afflict 1 in 20 people worldwide, tear individual lives and families apart, and cause hundreds of billions of dollars a year in crime, loss of productivity and health care costs in the US alone1. Potential treatments are complicated by side effects and inconsistent efficacy. New ideas are needed.
Technologies in basic neuroscience research have made substantial strides in recent years, including manipulations of activity patterns targeted to defined cells and connections in the brains of behaving animals (reviewed in ref. 2). These optogenetic interventions, in gain- or loss-of-function forms, can be delivered over any acute or chronic timescale from several milliseconds to many days or more3–5 and can also operate over many spatial scales ranging from single neurons to broad regions of the adult mammalian brain2,3. The most widely used version of this approach has not been directly translated to the clinical setting at present, as it entails brain delivery of microbial opsin genes via viral vectors, along with fiber optic neural interfaces2. Optogenetics has nonetheless made its presence felt beyond basic science discovery in the framing of clinical thinking and guiding of clinical interventions that use knowledge obtained from optogenetics experiments. Illustrating this bench-to-bedside influence, the first optogenetics-guided clinical trial has recently emerged from an Italian group that used optogenetic discoveries to design and test a targeted treatment for cocaine addiction6.
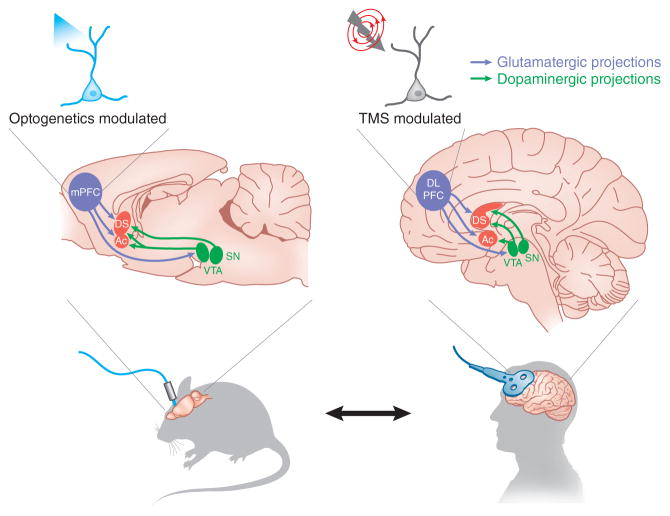
The specific clinical hypothesis of Terraneo et al.6 arose directly from an experiment by Chen et al.7 in 2013. Rats that compulsively seek cocaine have been found to have lower activity in an area of the medial prefrontal cortex known as the prelimbic cortex than non–drug-seeking rats. When Chen et al.7 optogenetically stimulated prelimbic cortex in compulsive rats, their cocaine-seeking behavior decreased (Fig. 1). Terraneo et al.6 adapted this approach to the clinical setting using a technique called transcranial magnetic stimulation (TMS). This noninvasive brain stimulation modality, approved for treatment of major depression in the US and in Europe8, employs strong, rapidly changing (millisecond timescale) magnetic fields arising from coiled wires positioned outside the head, which in turn induce electrical responses inside the brain. Depending on the design of the magnetic coils, the resulting stimulation can be targeted to a focal centimeter-scale location on or near the brain surface (Fig. 1). When applied in trains of repetitive (rTMS) pulses at around 5–10 Hz, TMS is believed to induce stable potentiation-type plasticity in the targeted circuit elements; this targeting and plasticity could have the effect of modifying activity in cells, projections or brain-wide networks, depending on the protocol.
Figure 1.
A clinical trial for patients with cocaine addiction guided by rodent optogenetics. Optogenetic stimulation of the medial prefrontal cortex (mPFC) with a channelrhodopsin (ChR) suppresses compulsive cocaine seeking in rats7. Terraneo et al.6 found that rTMS of the DLPFC appeared to prevent cocaine use relapse in cocaine-addicted patients. The mechanism of this therapeutic effect may include modulated activity in subcortical reward circuitry involving the dopaminergic midbrain (ventral tegmental area (VTA) and substantia nigra (SN)) and the striatum (dorsal striatum (DS) and nucleus accumbens (Ac)).
Although the rat brain is hundreds of times smaller than the human brain, with noteworthy differences in shape and complexity, many key brain regions appear to function similarly across species (Fig. 1). For example, the rodent prelimbic cortex has been linked to high-level cognitive control of behavior, with likely functional homologs in humans, including the dorsolateral prefrontal cortex (DLPFC). The human DLPFC, which is positioned superficially in the human brain, is accessible by TMS and can be precisely targeted across individuals using magnetic resonance imaging scans to map its location and connectivity before treatment.
Terraneo et al.6 recruited 32 cocaine-addicted individuals to participate in this open study. Half of the participants were randomly allocated to rTMS therapy (40 trains of pulses to DLPFC at 15 Hz, 13 min total) and the other half were allocated to a standard drug treatment (a cocktail of medications that have been used to treat symptoms of cocaine withdrawal). Participants in both groups were treated in an outpatient clinic daily for the first 5 d and subsequently once a week for 3 more weeks; rTMS- and control-treated individuals spent similar amounts of time with the clinical team at each visit. After the initial month of treatment, individuals were given the option to switch to the opposite treatment arm and were followed for a further 2 months.
Although the study was brief, 69% of individuals in the rTMS-treated group remained drug-free during the initial treatment phase, compared with 19% of the control group (as tracked by urine drug tests). The rTMS group also reported significantly less cocaine craving. The difference between groups emerged early during the treatment: by day 12, 50% of the control group had already relapsed, whereas not a single member of the rTMS group had relapsed. A marker of naturalistic practicality was that no rTMS patients dropped out during the first month of treatment, whereas three participants were lost from the control group. The encouraging outcomes persisted during the second stage of the study, in which ten patients in the control group chose to switch to rTMS therapy. Eight of these individuals had relapsed previously on the pharmacological therapy, but, during the subsequent rTMS treatment, only three of the ten tested positive for cocaine.
A conclusion of the study, concordant with the prior rodent experiments, was that stimulation of the DLPFC may suppress the compulsion of cocaine-addicted subjects to seek out cocaine, further supporting the hypothesis that DLPFC hypoactivity can be a pathophysiologic feature of the disorder. This result also lends support to the concept that the prefrontal cortex exerts top-down control over the brain’s reward circuitry through modulation of striatal dopamine signaling; the exact mechanism by which such an effect can occur is also the subject of investigation with optogenetics and other methods (for example, ref. 9).
The authors point out some of the key limitations of the study, including its brevity and open design. To evaluate therapeutic effects, a placebo control arm (in which patients believe they are receiving rTMS but are not) is important, although in practice this may be difficult, as patients often report mild discomfort or other sensations during genuine rTMS. In the present design, the control arm received pharmacological therapy (not given to the experimental arm), with potential side effects that could influence compliance. The authors also acknowledge a significant difference in age between the two groups, with the rTMS group being, on average, 6 years older than the controls; however, post hoc statistical analyses revealed that this difference did not account for the effect of the therapy. The authors found no difference in depression rating scales between the two groups following treatment; however, more subtle differences in specific psychiatric symptoms (such as anhedonia, motivation, or anxiety) were not described and could be important. Finally, a characteristic to note in the study was sex bias in both cohorts (only two women in the rTMS group, and zero in the control group). Although men account for the majority of drug users, cocaine use is increasing among women, with approximately one-third of cocaine users in the United States being female10. Future studies should address these demographic and design issues, as well as extend the study duration; physicians, patients and families are all too aware of the high long-term risk of relapse associated with substance abuse and dependence.
The study by Terraneo et al.6 not only stirs new hope for principled interventional treatment of addiction, but also, more fundamentally, defines a new process for neuroscience translation, as the initial published outcome of an optogenetics-guided clinical trial. The principle is generalizable; optogenetics is on the whole a basic science discovery technology, but can also guide modifications to existing therapies even beyond TMS. For example, early optogenetic studies of parkinsonism and deep brain stimulation (DBS)-like intervention in rodents highlighted the therapeutic potential of targeting white-matter projection pathways to deliver more effective neuromodulation11. Since then, a number of optogenetic studies have confirmed the potency and specificity of such projection targeting in modulating psychiatry-related phenotypes (reviewed in ref. 12), and this general principle has provided a basic foundation for clinical teams performing fiber tract mapping with diffusion tensor imaging to guide electrode placement for human DBS in Parkinson’s disease13 and depression14.
By analogy with precise genetic interventions (which even in the case of single-gene modifications can only exert their ultimate effects through cascades of perturbed downstream molecular elements), precise activity interventions in neural circuitry (whether electrical, optical, pharmacological or surgical) must also exert their behavioral effects through downstream effectors interacting across the brain9. Looking forward, clinical trials may build on basic science investigation of this natural complexity—for example, in design of optogenetically guided protocols to combine less-specific, but clinically available, neurostimulation with concomitant pharmacology to counteract inevitable off-target effects (direct non-targeted element modulation) of non-optogenetic interventions. This idea was recently demonstrated in another study of cocaine addiction15, which reported that 12-Hz optogenetic stimulation of prefrontal cortex projections to the nucleus accumbens suppressed sensitivity to a cocaine challenge in rats. Surprisingly, less-specific 12-Hz electrical DBS had no such effect unless combined with systemic administration of D1 dopamine receptor antagonists, suggesting that true off-target dopaminergic activity (that is, directly electrode-driven spurious activation of local dopaminergic projections, alongside the local desired direct target of prefrontal-to-accumbens glutamatergic projections) could be interfering with what would otherwise be a promising therapeutic strategy. Thus, two frequently used clinical treatments (DBS and pharmacotherapy) could be combined in a principled optogenetics-guided and hypothesis-driven manner for joint benefit.
A challenge for the future will be to optimize neuromodulation parameters for anatomical and temporal precision, minimally invasive access to deep brain regions, and strong safety and efficacy profiles that leverage the avalanche of insights emerging from the basic science laboratories. Meanwhile, fundamental laboratory research will continue to lay essential foundations for understanding the brain as a complex dynamical system and for probing the causal underpinnings of adaptive and maladaptive behaviors.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
Contributor Information
Emily Ferenczi, Department of Bioengineering, Stanford University, Stanford, California, USA.
Karl Deisseroth, Email: deissero@stanford.edu, Department of Bioengineering, Stanford University, Stanford, California, USA. Howard Hughes Medical Institute and Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California, USA.
References
- 1.Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States, 1992–2002. Washington, DC: Executive Office of the President; 2004. Publication No. 207303. [Google Scholar]
- 2.Deisseroth K. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goshen I, et al. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Yizhar O, et al. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt A, et al. Proc Natl Acad Sci USA. 2015 Dec 22; doi: 10.1073/pnas.1523341113. published online. [DOI] [Google Scholar]
- 6.Terraneo A, et al. Eur Neuropsychopharmacol. 2015 Nov 20; doi: 10.1016/j.euroneuro.2015.11.011. published online. [DOI] [Google Scholar]
- 7.Chen BT, et al. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 8.George MS, Taylor JJ, Short EB. Curr Opin Psychiatry. 2013;26:13–18. doi: 10.1097/YCO.0b013e32835ab46d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferenczi EA, et al. Science. 2016 Jan 1; doi: 10.1126/science.aac9698. [DOI] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. [Google Scholar]
- 11.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deisseroth K. Nature. 2014;505:309–317. doi: 10.1038/nature12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitmer D, et al. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riva-Posse P, et al. Biol Psychiatry. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creed M, Pascoli VJ, Lüscher C. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]