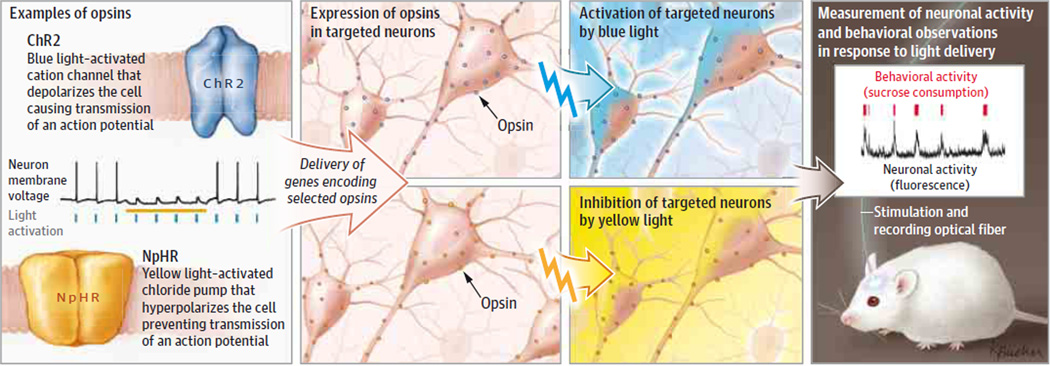
Optogenetics is a method for delivering millisecond-precision control (for activation or inhibition) to targeted cells using light within freely behaving mammals.1 The components of this method, as practiced today, involve (1) lasers and fiber optics for light delivery into the nervous system and (2) genes called microbial opsins, which encode light-activated proteins that regulate transmembrane ion conductance (Figure). Microbial opsins display diverse color sensitivity and conductance properties, with diversity generated by mutagenesis or arising naturally in the biosphere. Constituted by single genes, these microbial opsins can be targeted using a toolbox of genetic techniques, thereby specifying light-induced current flow in cells defined by function (eg, with excitatory, inhibitory, or dopaminergic properties) and anatomy (eg, with cell bodies in one location and projections going to another specific location across the brain). Light guidance with versatile fiber optics or spatial light modulators allows further resolution. Rapid advances in materials and electrical engineering have enabled opsin activation in neural tissue wirelessly using tiny LEDs.
Figure. Steps in Optogenetic Research.
Diverse opsin genes from microbes are engineered in the laboratory to allow light-activated regulation of ion flow and biochemical pathways. Targeted opsins allow cell-specific control with light with precision not achievable by electrodes. Mice can be implanted with fiberoptics that can both deliver optogenetic control and collect fluorescent readout of neural activity with fiber photometry. At left, black trace shows neuronal membrane voltage; blue ticks show pulses of light driving ChR2 to elicit action potentials (spikes in the membrane voltage); and yellow bar shows steady light providing inhibition of the neuron through NpHR. At right, red ticks indicate rewarding sucrose solution ingestion; black trace shows corresponding elevations in deep brain dopamine neuron activity.2
This control (playing in) technology has been complemented by recent advances in observing (reading out) neural circuit activity and connectivity, providing key complementary data streams. Preclinical and ex vivo methods in this regard1 have made substantial advances. For instance, it is now possible to observe detailed neural wiring diagrams across an entire intact rodent brain or large blocks of human brain tissue without slicing or disassembly (eg, using the chemical engineering method called CLARITY1), and to observe the real-time activity of specified neurons and projections within the nervous system of behaving animals (using fluorescent reporters of neural activity in combination with advanced optics).
In the clinical setting, tools for observing brain activity and connectivity are still largely limited to operating noninvasively, such as electroencephalography, contrast functional magnetic resonance imaging (MRI) dependent on blood oxygen level, and diffusional MRI for inferring the path of axonal tracts. The spatiotemporal constraints of these observational methods have placed limits on the depth of insight obtainable into circuit-level mechanisms underlying behavior; nevertheless, structural and functional neuroimaging studies have identified brainwide, circuit-level abnormalities across every psychiatric diagnosis. These abnormalities are not in single regions (each associated with an illness), but rather in sets of interconnected and distributed neural circuits, providing a clinical perspective complementary to optogenetic preclinical studies that capture clinically relevant mechanisms.
These technological advances are supported by recent discoveries focused on etiology of disease. Combinations of genes have been identified that together contribute to the occurrence of psychiatric disorders. Importantly, the circuit-level perspective now provides a source of unifying hypotheses that may help explain how discrete psychiatric symptoms can arise from a diverse array of multigene effects. For example, studies of schizophrenia and autism genetics3 have consistently pointed to genes involved in regulating the balance of excitability in the brain, concordant with optogenetic findings that excitability changes targeted to specific cell or projection types can specifically modulate social behavior in mice. Likewise, preclinical optogenetic experiments have revealed specific cells and pathways that can modulate anxiety, depression, and eating disorder–related behaviors, along with many others of great importance to psychiatry.1
How can such insights be translated to the clinical setting? This is occurring in several ways. First, circuit-level understanding of psychiatric symptoms is allowing generation of more sophisticated pathophysiological hypotheses, which is important for replacing the current symptom and subjective report-based psychiatric nosology with one based on more sophisticated understanding of etiology. Second, by combining careful patient interviews, imaging assays, and personalized genomics, diagnoses of mental illness are well poised to change substantially in a manner that could improve both prevention and treatment. Third, direct knowledge of cells and projections causally involved in psychiatric symptoms is facilitating identification of clinically relevant circuit biomarkers, which could revolutionize not only diagnosis but also prediction of treatment outcomes.
Fourth, this rapid identification of novel circuit targets for medications or brain stimulation treatments4,5 may lead to interventions that more specifically ameliorate causal circuit pathophysiology. Even without direct translation of research tools to patients, human brain stimulation can leverage circuit-level insights (such as those arising from optogenetics).6,7 For example, transcranial magnetic stimulation (TMS), with which centimeter-scale brain regions can be stimulated noninvasively, can be integrated with functional MRI and electroencephalography, creating an opportunity in which activity in targeted brain regions can be monitored and modulated.5 Specific neural circuits, such as those more active at rest or during attention-demanding tasks, have been localized and then selectively stimulated or inhibited in humans.4 Transient modulation of these circuits has provided information about causal circuit regulatory mechanisms in humans, as well as elucidated how long-lasting plasticity in these circuits could form the basis of therapeutic applications of TMS.
Treatments (either using or informed by neurostimulation techniques) based on these methods can only be expected to increase. Despite US Food and Drug Administration clearance for TMS treatment in patients with depression, its mechanistic underpinning has been poorly understood and hence is likely far from optimal (in fact, relatively very few locations in the human brain have yet been explored with neurostimulation). For deep structures that TMS cannot currently reach, additional noninvasive tools (eg, focused ultrasound) will be required. Knowledge of neural plasticity mechanisms8 (including long-term potentiation, long-term depression, spike timing-dependent plasticity, and biochemical pathways supporting each of these in turn) can be used to elicit lasting changes in causally important circuit components that had been identified with optogenetics and other modern neuroscience tools, such as diffusion MRI, CLARITY, and computational modeling of network dynamics. Even behavioral manipulations, such as psychotherapy or computerized cognitive training methods, can be combined with noninvasive neurostimulation through common grounding in circuit-level mechanisms. For example, neurostimulation methods aimed at enhancing emotion regulation and fear extinction can be combined with psychotherapies targeting traumatic memories in patients with posttraumatic stress disorder.
Optogenetics and other recent methodological advances in basic research have leveraged the deep knowledge imparted by neuroscience research, which provides critical guidance on anatomy, definition of cell types, and neurophysiology. Neural circuit dynamic properties are now revealing unifying principles that can causally determine the expression and modulation of complex behavioral phenotypes relevant to psychiatry. Over the next decade, guided by deliberate alignment of circuit-based methods from basic and clinical neuroscience, advances in genetics, brain imaging, and neurostimulation will continue to translate these insights into improved understanding of pathophysiology and development of novel and more effective treatments.
Acknowledgments
Drs Deisseroth and Malenka are consultants and shareholders of Circuit Therapeutics Inc, a start-up company building platforms for drug discovery and peripheral nervous system intervention with optogenetics.
Footnotes
Conflict of Interest Disclosures: No other disclosures were reported.
Additional Information: Dr Deisseroth is the recipient of the 2015 Albany Medical Center Prize in Medicine and Biomedical Research. Optogenetic tools and methods are freely available on request at http://www.optogenetics.org.
Contributor Information
Karl Deisseroth, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California; Department of Bioengineering, Stanford University, Stanford, California; and Howard Hughes Medical Institute, Stanford University, Stanford, California.
Amit Etkin, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California.
Robert C. Malenka, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California.
REFERENCES
- 1.Deisseroth K. Circuit dynamics of adaptive and maladaptive behaviour. Nature. 2014;505(7483):309–317. doi: 10.1038/nature12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunaydin LA, Grosenick L, Finkelstein JC, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iossifov I, O’Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen AC, Oathes DJ, Chang C, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen BT, Yau HJ, Hatch C, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 7.Creed M, Pascoli VJ, Lüscher C. Addiction therapy: refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347(6222):659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- 8.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]