Abstract
Poor consistency of the ice thickness from one area of a cryo-electron microscope (cryo-EM) specimen grid to another, from one grid to the next, and from one type of specimen to another, motivates a reconsideration of how to best prepare suitably thin specimens. Here we first review the three related topics of wetting, thinning, and stability against dewetting of aqueous films spread over a hydrophilic substrate. We then suggest that the importance of there being a surfactant monolayer at the air-water interface of thin, cryo-EM specimens has been largely underappreciated. In fact, a surfactant layer (of uncontrolled composition and surface pressure) can hardly be avoided during standard cryo-EM specimen preparation. We thus suggest that better control over the composition and properties of the surfactant layer may result in more reliable production of cryo-EM specimens with the desired thickness.
Introduction
Ever since the initial publication of a method to prepare macromolecular particles embedded in vitreous ice (1, 2), thin samples have continued to be made by blotting excess liquid from grids with filter paper. The use of computerized control of key parameters (such as the ambient temperature and humidity, the blotting pressure and duration, and the interval between blotting and final vitrification) has improved the sophistication and the reproducibility with which cryo-specimens can be prepared (for a review from 2010, see Dobro et al. (3)). Even so, the results still remain less consistent from trial to trial and over the entire area of a grid than one might have thought. In particular, preparation of specimens at a desired thickness of ∼30–50 nm remains quite unreliable.
Achieving specimen thicknesses well below ∼100 nm becomes increasingly important as the resolution of cryo-electron microscope (cryo-EM) images is increased (4, 5). This is because a single image may contain particles located at different focal heights if the vitrified ice is significantly thicker than the particle size. Merging such data leads to an unwanted envelope function (6) that is equivalent to the one produced by varying the focus of the objective lens by the same amount.
Even if all particles are tethered at a common Z-height, as is possible when using a continuous support film, an ice-film thickness significantly greater than the size of the particle will necessarily cause an increase in the fraction of electrons that are scattered inelastically. This is undesirable because it leads to a corresponding loss of useful signal in the image.
It thus is timely to ask whether contemporary practices of cryo-EM specimen preparation make optimal use of what is known about the subjects of substrate wetting, film thinning, and the stability of thin aqueous films. Quantitative understanding of these topics, briefly reviewed here, can be traced mainly to 19th century physics, while practical developments remain an active area of research in the fields of foams, colloids, emulsions, microfluidics, and nanoscience. There is therefore reason to hope that improvements can be made in cryo-EM specimen preparation by evaluating both the physical principles and the technical opportunities associated with making thin, aqueous films.
Throughout this review we consider mainly the case that the support film on the EM grid is continuous and hydrophilic. This is expected to provide a good model for describing what happens when cryo-EM samples are prepared on continuous, glow-discharge-treated carbon film. There will be additional effects, of course, when holey carbon grids are used (i.e., without a continuous carbon film). Even in this case, however, we believe the basic principles covered in this review will still apply to the formation and the stability of thin aqueous films.
In the section titled The Initial Formation of Thin Aqueous Films on EM Grids, we first review familiar background about the wetting of substrates (surfaces) under equilibrium conditions. We also address the question of the maximum amount of liquid that can be removed when a grid is drained (wicked) from an edge, and why it is necessary to press the filter paper directly on the face of the grid if there is to be any chance of producing a suitably thin sample.
In the section titled Uniformly Thin Films of Pure Water Are Inherently Unstable, we explain the fact that uniformly thin films of pure water, in the shape of a flat pancake for example, are unstable with respect to dewetting the substrate. Dewetting results in the formation of puddles, whose thickness is determined by possibly very small, but still finite contact angles, and by the diameter and volume of the puddle.
In the section titled A Surfactant Monolayer Suppresses the Thickness Fluctuations That are Required to Nucleate Dewetting, we then go on to review the fact that a surfactant monolayer can act to suppress the thickness fluctuations that nucleate dewetting within the interior rather than at the edge of a uniformly thin pancake shape. When such internal nucleation events are suppressed, a uniformly thin aqueous film may become metastable, as are soap bubbles. This means, in the context of making cryo-EM specimens, that the presence of a surfactant monolayer at the air-water interface can be a good thing.
We further review, in the section titled Surfactant Monolayers Also Affect the Disjoining Pressure, Which Determines the Thickness of Metastable, Aqueous Films, the fact that additional interactions emerge between apposed interfaces as the separation between them becomes very small. The thickness of a thin liquid film, when stabilized by a surfactant monolayer, for example, is ultimately determined by the range and magnitude of these interfacial interactions. Thus, by controlling the chemical makeup of this surfactant monolayer, it may be possible to engineer a local minimum in the total energy of the system at a thickness that is optimal for a particular cryo-EM specimen.
Finally, we conclude by reviewing, in the section titled Possible Use of Surfactant Monolayers as Electron-transparent Coverslip Structures When Preparing Cryo-EM Specimens, some of the ways in which a surfactant monolayer of defined composition might be intentionally applied to the air-water interface during the preparation of cryo-EM grids. We include a description of preliminary work in which we observed that one such method, delivery of a phospholipid dissolved in chloroform, is accompanied by thinning of an aqueous puddle even before there is direct, liquid-liquid contact. This example of the Marangoni effect, well known in other applications such as the drying of silicon wafers, has the potential to serve as an alternative to blotting with filter paper, as a way to produce thin aqueous films.
The initial formation of thin aqueous films on EM grids
A small amount of water that is placed on an extended, clean surface, such as freshly cleaved mica, normally forms a puddle whose contact angle, θ, with the substrate satisfies the expression attributed to (7)
| (1) |
where γSG, γSL, and γLG are the surface tensions for the solid-gas, solid-liquid, and liquid-gas interfaces, respectively. The surface tension of water at the water-air interface is ∼70 mN/m (or 70 erg/cm2). The other two values of surface tension are necessarily dependent on the nature of the solid substrate. For the purposes of this Review, there is no need to distinguish between the contact angles when the interface is either advancing or receding, as long as it is recognized that in neither case is it likely that the angle will be zero.
When the thickness of a liquid puddle is small enough, the effect that gravity has on its shape can be ignored. In this case, the equilibrium shape is that of the topmost portion (the cap) of a sphere (see Fig. 1 for an illustration). The length scale below which the influence of gravity is negligible is known as the capillary length, λC, and it is given by
| (2) |
where ρ is the density of water, and g is the gravitational acceleration. For pure water, the capillary length is ∼2 mm at 1 g. As a practical illustration, little, if any, change in the shape of a 3-μL drop can be noticed when a 3-mm grid is held in either a horizontal or vertical orientation.
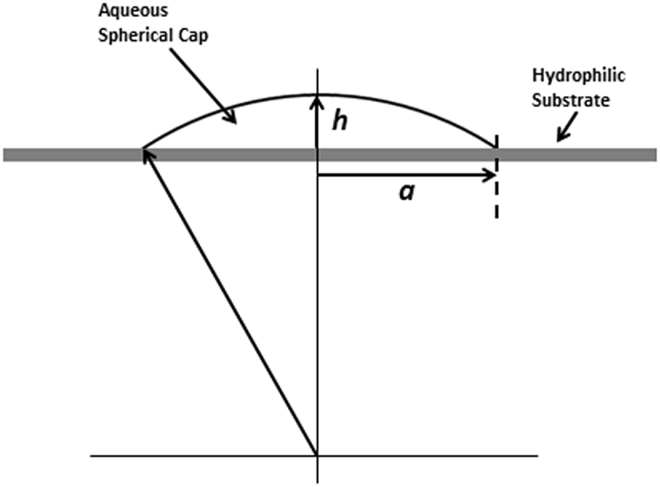
Figure 1.
Spherical cap on a hydrophilic surface. As is illustrated by the side view that is shown in this cartoon, the phrase “spherical cap” refers to the topmost portion of a sphere. For completeness, the radius of the sphere, and its center, are shown below the plane of the hydrophilic substrate. In the top view (not shown), the perimeter of the cap would be a circle of radius a. The thickness of the spherical cap (at the center of the plano-convex lens), h, is given by Eq. 3.
When the available surface area is not limiting, the diameter (puddle size) of a water drop, and its thickness (at the center of the puddle), are determined by the volume of liquid placed on the hydrophilic surface. The puddle thickness, h, is of special interest to us, and it can be expressed as a function of the contact angle and the radius, a, of the puddle as
| (3) |
with the latter approximation applying when the contact angle is small.
When the available surface area is limiting, as it is when excess sample is placed on an EM grid, the initial droplet can be thicker than the value described in Eq. 3, because the water cannot spread beyond the edge of the grid. Nevertheless, the droplet will still form a spherical cap, as long as the thickness of the drop is smaller than the capillary length. As a result, when sample is first applied to a hydrophilic grid, the height of the spherical cap will generally be greater than what is specified in Eq. 3. In this case, excess liquid can be drained (wicked) away by touching the edge of the EM grid with filter paper. Draining will stop at the point when the contact angle at the rim of the spherical cap is determined by how hydrophilic the surface is, rather than by how much area is available. The volume, V, of liquid that remains after draining can be expressed in terms of 1) the radius, a, of the puddle that remains, which should be equal in size to the radius of the EM grid; and 2) the thickness, h, of the puddle
| (4) |
with the latter approximation again applying when the contact angle is small, thus allowing us to substitute the thickness parameter by the expression on the far right of Eq. 3.
As stated in Eq. 3, the thickness of the puddle (at the middle of the spherical cap) is proportional to the contact angle when the latter is small. If the grid is made hydrophilic by exposure to a glow discharge, the contact angle is, in fact, so small that it is not practical to measure it visually. The volume of water that remains after wicking can be estimated gravimetrically, however. In our hands, this volume is at least 20 nL (as measured with a microbalance). If the radius is assumed to be 1.5 mm (i.e., the size of the entire EM grid), this volume corresponds to a contact angle, as expressed in Eq. 4, of ∼0.4°. As a result, the thickness at the middle, as expressed in Eq. 3, is estimated to be ∼6 μm or more.
An important conclusion is that blotting excess liquid from the edge of a vertically held EM grid does not produce a film of aqueous sample that is thin enough to use as a cryo-EM specimen. Furthermore, blotting from the bottom of the grid cannot result in a significant gradient of thickness from the top to the bottom of the grid, as has sometimes been stated (e.g., Frederik et al. (8)).
On the other hand, areas of thin ice, usually accompanied by other areas that are too thick to be used, are routinely obtained by pressing filter paper to the face of the grid. Blotting against the face of a grid is bound to remove more water than blotting from the edge because the puddle of water is mechanically flattened as well as being wicked away simultaneously at many points. The hope is that such face-blotting initially leaves a uniformly thin aqueous film (pancake) covering at least some parts, if not all, of the grid, but it is perhaps impossible to observe, before freezing, whether that really happens. Nevertheless, supposing that a uniformly thin pancake actually is produced, whose thickness is <100 nm, the next issue to review is how stable such a liquid film is likely to be.
Uniformly thin films of pure water are inherently unstable
While it is possible to briefly spread a small volume of water into a thin pancake, e.g., by face-blotting, doing so means that the surface/volume is greater than if the same volume adopted the shape of a spherical cap. If the thickness of the flattened volume is still relatively large (>∼100 nm, as we will explain below), one can expect the spread film of water to dewet the substrate only at the outer rim of the pancake, quickly contracting the entire volume of water to a spherical cap covering a smaller area than that of the previous pancake. It is only if the same volume of water is spread into a much thinner pancake that one can expect dewetting, discussed in de Gennes et al. (9), to occur at one or more points within the film as well as at the outer edges. Note that, as a consequence of dewetting, departures in puddle shapes from the ideal spherical caps imagined above can be attributed to inhomogeneities or defects on the substrate that are able to pin the contact line (where solid, gas, and liquid meet).
It may seem surprising that van der Waals forces (characterized in the late 1800s) are responsible for the internal dewetting events that occur when a film of liquid is still much thicker than molecular dimensions. This is nevertheless possible because the total interaction energy of molecules within a thin film of liquid, due to van der Waals forces, actually scales as the square of the film thickness (see, for example, Berg (10)), even though the interaction energy between any pair of molecules scales as the sixth power of their separation. As a result, once a liquid film becomes thinner than a certain value, generally believed to be ∼100 nm (11, 12), the magnitude of the van der Waals interaction energy of molecules within the thin film, relative to that of molecules within a thick film, decreases significantly.
Local thinning of such a liquid film thus leads to an unstable, runaway situation in which molecules in an initially thinner area move to the adjacent, thicker area, where their van der Waals energy is more favorable. There is, in fact, a second term in the van der Waals energy that also scales as the square of the thickness of the liquid film, and which again serves to drive the thickness to zero. This second term is the van der Waals attraction between the semiinfinite half-spaces on either side of the liquid film (see Parsegian (13)). When, however, the material on either side of the liquid film is a gas at atmospheric pressure, as it is when making a thin specimen for cryo-EM, the low density of gas makes the scaling-constant, known as the Haymaker constant, very small compared to that of the first term discussed above.
The final result is that the thinnest areas of a liquid film act as nucleation sites for events that subsequently dewet the substrate, leaving thicker puddles (e.g., spherical caps) in equilibrium with dry areas. Restating this in simpler terms, a liquid pancake that is thinner than ∼100 nm is inherently unstable with respect to dewetting at one or more points across the face of the pancake. Once dewetting begins, the same volume of liquid will minimize its surface/volume by spontaneously coalescing into possibly quite complex patterns of wet and dry areas (14). The practical implication, for cryo-EM specimen preparation, is that some areas of the grid are bound to become air-dried—even in the hypothetical case when the ambient humidity is so high that evaporation is not a contributing factor—while at the same time adjacent areas may be much thicker than desired.
A surfactant monolayer suppresses the thickness fluctuations that are required to nucleate dewetting
It is well known that thin aqueous films are greatly stabilized against rupture or dewetting when there is a diffusible surfactant at the air-water interface. Soap bubbles provide a good example of this. The literature says relatively little, however, about how the surfactant monolayer might tend to prevent such events from getting started.
Some workers believe that Marangoni flow—the bulk transfer of a liquid due to a gradient in surface tension (described in Berg (10), for example)—opposes any fluctuations in thickness that may nucleate the dewetting process. Although the following argument may be too simplistic, the idea is that formation of a locally thinner area, i.e., occurrence of a nascent dewetting event, is accompanied by an increase in surface area, and thus a local decrease in surface coverage by the surfactant. To the extent that this is true, local thinning would also be accompanied by a local increase in surface tension. Marangoni flow would then cause mass transfer back into the thinner area.
Ignoring the question of the detailed mechanism, the suppression of thickness fluctuations (capillary waves) by a surfactant monolayer is believed to prevent nucleation of the process required for dewetting or rupture of thin, liquid layers. It is tempting to say that the mechanism by which a surfactant monolayer opposes thickness fluctuations of a thin aqueous film may be the same as that by which it calms the turbulent seas.
Surfactant monolayers also affect the disjoining pressure, which determines the thickness of metastable, aqueous films
As an aqueous film gets thinner than ∼100 nm, under the influence of van der Waals forces, previously ignored interactions come into play between the two, apposed interfaces. These interactions produce what is referred to as a disjoining pressure, defined as the gradient of the Gibbs free energy per unit area, normal to the surface. At very close distances (generally only a few nanometers), the value of the disjoining pressure starts to change rapidly as a function of the distance. Depending upon its sign, the disjoining pressure either promotes or resists dewetting or rupture of the thin film. It thus is important to know the physical origins of these interfacial interactions, and the extent to which they can be manipulated by controlling the physical and chemical properties at the two, respective interfaces.
In general, polar groups at the aqueous interfaces are responsible for a hydration (solvation) force that resists complete dehydration (15). Hydrophilic surfaces that are brought into close apposition are thus likely to maintain a gap of water between them that is at least 1 nm or possibly 2 nm thick (see Israelachvili (16)).
If the polar headgroups are charged, electrostatic forces between the interfaces may also become important. Nevertheless, the range over which electrostatic forces are exerted, under normal biochemical-buffer conditions, is limited by shielding of the surface charge by counterions. The distance over which this shielding occurs depends upon the ionic strength, of course. When the ionic strength is 10 mM or more, electrostatic repulsion between like-charged surfaces may not contribute much to maintaining a liquid gap between opposed interfaces, beyond what solvation itself would ensure.
When preparing thin specimens for cryo-EM, however, one would no doubt prefer to stabilize the water film at a thickness greater than that maintained by the combination of hydration and repulsive electrostatic forces. As of this writing, it is not clear how to do this, of course. One suggestion is to use some type of steric spacer. This steric spacer could be the specimen particles themselves, or it could be even larger spacer particles that are added to the specimen. In addition, one can consider using a surfactant that has long flexible headgroups, such as polyethylene glycol chains, as a steric spacer (an entropic spring). For more on this latter possibility, see, for example, Israelachvili (16) for a general discussion of polymer-mediated contributions to the disjoining pressure, or Kenworthy et al. (17) for a specific example.
Possible use of surfactant monolayers as electron-transparent coverslip structures when preparing cryo-EM specimens
The idea of sandwiching macromolecular specimens between a continuous carbon film, which serves as a type of electron-transparent “slide”, and either a fatty acid monolayer or a second carbon film (effectively, a “coverslip”), was explored early in the development of cryo-EM specimen preparation (18, 19, 20, 21). A retrospective (19) describes this and other early work, which first established that high-resolution information is preserved in frozen-hydrated specimens, and that adequate contrast is available in such unstained specimens. The attempt to use an electron-transparent coverslip was not pursued further when the far simpler technique of blotting excess liquid with filter paper (1, 2) proved to be all that was needed. Now, however, as macromolecular structures are being achieved at higher and higher resolution, for example by Dobro et al. (3), Bartesaghi et al. (5), Cao et al. (22), Scheres (23), Li et al. (24), and Campbell et al. (25), it again becomes a priority to determine whether further improvements can be found in the methods of preparing thin specimens.
Surfactants are likely to be present in abundance, without even trying
As was noted previously by Frederik et al. (8), it seems likely that the thin cryo-EM specimens that are routinely produced, which are 100 nm thick or less, are actually covered by a monolayer of surfactant, even though no specific effort is made to ensure that it will be there. Indeed, one must take extreme measures if one wants to remove such surfactants from a sample, as the literature of surface-science makes abundantly clear. When one thinks about the steps that must be taken to prepare a clean air-water interface on a Langmuir trough (26) (see also the description in https://en.wikipedia.org/wiki/Langmuir%E2%80%93Blodgett_trough), the standard preparation of cryo-EM specimens must be considered to be a relatively dirty process.
To amplify this point, volatile organics in the atmosphere are bound to contaminate the surface of EM grids before use, ready to spread over the air-water interface when an aqueous sample is applied. For example, Xu et al. (27) found that such contamination accumulates on the surface of freshly cleaved mica within a few hours, greatly affecting the rate of evaporation of water that condenses (due to capillary action) between an AFM tip and mica. Any piece of laboratory equipment (tweezers, protective gloves, the plastic barrier used to prevent samples from wetting the foam on the Vitrobot paddles (FEI, Hillsboro, OR) will similarly be coated with a monolayer of surfactant. Indeed, in a laboratory environment where multiple users share the same equipment, one must think about the questions of who has been here before, and what they have left behind. It has also been mentioned, but not proven experimentally, that the familiar glow-discharge process may itself generate surfactant species on the EM grid. Even macromolecules within the specimen should be regarded as likely surfactants, because proteins can easily denature and spread at an air-water interface.
One response to this dismal situation is to simply carry on doing the sample preparation as we do it now, and accept whatever results we normally get, variable as they may be from one grid to the next.
Another response might be to reconsider applying a known, engineered coverslip to the air-water interface. Because it is impractical to eliminate the native surfactant that is already there, however, perhaps the most that one might hope to accomplish is to overwhelm the unknown surfactant by applying excess engineered surfactant. The potential benefits of this approach might be, in turn, to produce thin samples with greater consistency, and possibly even to engineer the ice thickness to match the size of the particle being studied.
Surfactants can be added directly to the sample of interest
The addition of phospholipid vesicles to the sample of interest is a promising way to deliver a chemically defined “surfactant coverslip” to the air-water interface, as was suggested by Frederik et al. (8). This technique seems not to have been regarded by the community as a good thing to do, perhaps because unwanted, excess liposomes remain in the thin, aqueous film. We nevertheless believe that it may be profitable to revisit this idea. It seems likely that one could easily find an optimal concentration of liposomes that would deliver enough surfactant to completely cover the air-water interface while leaving only a minimal number of intact vesicles still suspended in the bulk of the thin, vitrified sample.
The addition of detergent to the sample might be another approach, provided that the sample is not adversely affected. Many purification protocols actually include a detergent in the buffer to prevent unwanted aggregation. The addition of detergent is also one of the standard tricks to try when a sample is found to adsorb to the air-water interface. The main shortcoming of having detergent in the sample is that the specimen may then be more sensitive to any evaporation that might occur after blotting but before freezing. In addition, some authors report that it is difficult to find areas of the grid where the ice is as thin as is desired, when there is detergent in the sample buffer.
There are many other surfactant materials that can be considered. Amphipols (28), for example, have just recently gained attention in the context of cryo-EM of membrane proteins (29). A number of relatively low-molecular weight proteins, such as cytochrome c or β-casein, are also known for their ability to stabilize bubbles.
Surfactant materials can be applied to the air-water interface in a volatile-solvent carrier
When doing experiments with a Langmuir trough, a surfactant of interest is normally dissolved in a volatile, organic solvent (e.g., chloroform), which is then delivered to the air-water interface. The organic solvent quickly evaporates, leaving a monolayer of the solute at the air-water interface. In principle, much the same technique might be used to deliver a well-defined surfactant to the surface of an EM grid, possibly doing so after first blotting off excess liquid.
In preliminary experiments described below (the section titled Thinning by the Marangoni Effect May Even Be an Alternative to Blotting with Filter Paper), we confirmed that touching a thin, aqueous puddle with phospholipid dissolved in chloroform permanently forms an apparently dry, hydrophobic patch in the center of the puddle of water. A similar, but not identical thing happens when chloroform alone is used, however. This experiment thus raises a new question. Why does the aqueous puddle become thin when touched by pure chloroform? Indeed, one can easily observe that an aqueous puddle already begins to thin as the liquid chloroform approaches the puddle, but does not yet touch it. As we explain in the section titled Thinning by the Marangoni Effect May Even Be an Alternative to Blotting with Filter Paper, it is the Marangoni effect that causes the observed thinning, and the phospholipid monolayer (if present) acts as a coverslip to stabilize the resulting, thin aqueous film.
Thinning by the Marangoni effect may even be an alternative to blotting with filter paper
As mentioned in the section titled A Surfactant Monolayer Suppresses the Thickness Fluctuations that are Required to Nucleate Dewetting, water experiences a mass transfer or bulk flow whenever there is a gradient in surface tension parallel to the surface. Although this effect may be unfamiliar in the context of making cryo-EM samples, most people have seen it in the form of the “tears of wine” effect (illustrated on the cover of Berg (10), and see also http://en.wikipedia.org/wiki/Tears_of_wine). Marangoni, after whom such mass-transfer effects are named, is acknowledged to be the first to publish experimental studies (in 1865), and Gibbs is credited with providing the theoretical explanations (in 1878).
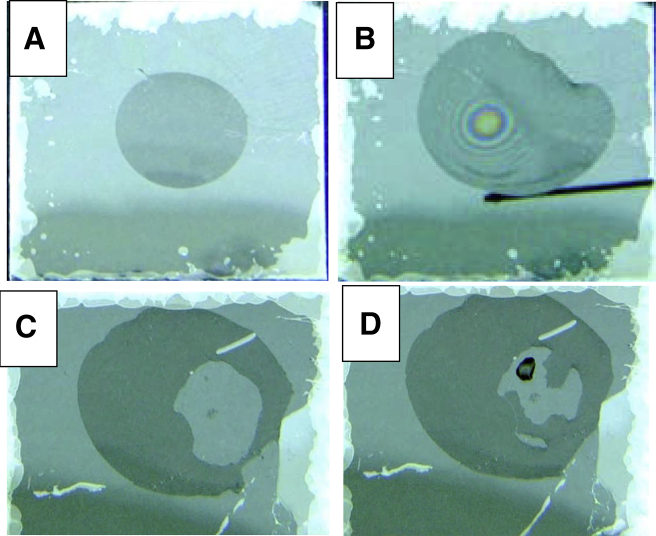
A practical demonstration of how Marangoni thinning, rather than blotting with filter paper, might be used to make thin specimens for cryo-EM is shown in Fig. 2, with a more detailed demonstration being given in Movies S1 and S2 in the Supporting Material. Fig. 2 A shows a still image of a 10 μL puddle (aqueous sample) on the surface of freshly cleaved mica, and Fig. 2 B shows that the center of the puddle can be made thinner than 100 nm by bringing a point-source of chloroform vapor close to the surface. Thinning is revealed by the interference fringes in the transitional region between the thinner middle and the thicker surrounding region.
Figure 2.
Marangoni flow on freshly cleaved mica. Individual frames extracted from real-time movies, included in the Supporting Material, in which we recorded how Marangoni flow, rather than blotting with filter paper, might be used to thin an aqueous specimen before vitrification. Frames (A) and (B) are extracted from Movie S2, while frames (C) and (D) are from Movie S1. (A) A thin puddle, ∼1 cm in diameter, forms when 10 μL of water is applied to the surface of freshly cleaved mica. Imperfections in the reflectance of light near the periphery of the mica are due to air gaps created by inadvertent cleavage of planes of the mica during cutting the one-inch square from stock. (B) Interference fringes, centered around the point of closest approach to the aqueous puddle, are formed when a syringe needle, made of Teflon (DuPont, Wilmington, DE) and loaded with chloroform, is brought close to the center of the puddle. As Movie S1 shows, this pattern of local thinning is largely reversible when the tip of the syringe is removed. On the other hand, a permanently dry area forms when liquid chloroform touches the aqueous puddle. As is shown in Movie S2, this dry area is again wetted relatively well when a new droplet of water is applied to it, but wetting is no longer as perfect as is the case for freshly cleaved mica. (C) A permanently dry area again forms when a solution of 1 mg/mL of phospholipid in chloroform touches an aqueous puddle. Note a small amount of residue is left at the point where the transferred chloroform sat as it evaporated. (D) In this case, the dry area is largely hydrophobic, as indicated by the fact that an added droplet of water does not spread. Exceptions occur when the applied droplet contacts surrounding areas of the puddle. To see this figure in color, go online.
As is the case for the tears-of-wine phenomenon, the system shown here is in a nearly steady state rather than being at equilibrium. We suggest that the mechanism involved in establishing this seeming steady state is that chloroform first adsorbs to the air-water interface at the point closest to the source of vapor, and in so doing lowers the surface tension there. The resulting gradient in surface tension drives mass transfer to the region of higher surface tension, carrying the monolayer of adsorbed chloroform along with the flow of water. Once removed some distance from the source of vapor, the chloroform again evaporates from the water surface, thus keeping the surface tension high in the region farther removed from the source of vapor.
When the puddle of water is actually touched by the chloroform solution, however, phospholipid instantly spreads over the air-water interface as a coverslip. As is shown in Fig. 2 C, in this case the puddle becomes permanently thinned over a large area surrounding the point of contact. Furthermore, much of the thinned area is now hydrophobic, as is seen by applying a new droplet of water, shown in Fig. 2 D. For the reasons discussed in the section titled Surfactant Monolayers Also Affect the Disjoining Pressure, Which Determines the Thickness of Metastable, Aqueous Films, above, we assume that a relatively thin layer of water necessarily remains between the mica and the polar headgroups of the phospholipid monolayer. This is in contrast to what happens when a puddle of water is touched by chloroform alone, in which case the dry area is still hydrophilic. Real-time videos, Movies S1 and S2 showing the two behaviors, are included in the Supporting Material.
Although we have used chloroform to illustrate the Marangoni effect in Fig. 2, we think that a different volatile surfactant would be a better choice to use for preparing cryo-EM specimens. The reason is that the solubility of chloroform in water is quite high, ∼1%. This means that dissolved chloroform may easily partition into the hydrophobic interior of the macromolecule of interest, and in so doing it may compromise the structure and biochemical function of the specimen. Finding a more favorable solvent with low solubility in water should be a productive future direction to exploit Marangoni flow for thinning cryo-EM grids.
Acknowledgments
This work was supported in part by National Institutes of Health grants No. R01-GM083039 and R01-GM065050.
Editor: Edward Egelman.
Footnotes
Two movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00766-3.
Supporting Material
The movie shows that a permanently dry patch forms when a small amount of chloroform solution, containing 1 mg/mL of phospholipid, touches a thin puddle of water on the surface of freshly cleaved mica. As is shown in this movie, the dry patch is largely hydrophobic.
The movie shows that a 10 mL volume of water spreads over an area of about 10 cm when applied to the surface of freshly cleaved mica. When a “point source” of chloroform vapor approaches but does not touch this puddle, the water thins appreciably, giving rise to interference fringes (Newton’s rings). When the point source is removed, the water returns. When chloroform liquid touches the puddle of water, however, it leaves a dry spot, which nevertheless can be wetted again quite easily.
References
- 1.Adrian M., Dubochet J., McDowall A.W. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- 2.Dubochet J., Adrian M., McDowall A.W. Cryo-electron microscopy of vitrified biological specimens. Trends Biochem. Sci. 1985;10:143–146. [Google Scholar]
- 3.Dobro M.J., Melanson L.A., McDowall A.W. Plunge freezing for electron cryomicroscopy. Methods Enzymol. 2010;481:63–82. doi: 10.1016/S0076-6879(10)81003-1. [DOI] [PubMed] [Google Scholar]
- 4.Agard D., Cheng Y., Subramaniam S. Single-particle cryo-electron microscopy (Cryo-EM): progress, challenges, and perspectives for further improvement. In: Hawkes P.W., editor. Advances in Imaging and Electron Physics. Elsevier; New York: 2014. pp. 113–137. [Google Scholar]
- 5.Bartesaghi A., Merk A., Subramaniam S. 2.2 Å resolution cryo-EM structure of β-galactosidase in complex with a cell-permeant inhibitor. Science. 2015;348:1147–1151. doi: 10.1126/science.aab1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen G.J. Alignment error envelopes for single particle analysis. J. Struct. Biol. 2001;133:143–155. doi: 10.1006/jsbi.2001.4334. [DOI] [PubMed] [Google Scholar]
- 7.Young T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805;95:65–87. [Google Scholar]
- 8.Frederik P.M., Stuart M.C.A., Busing W.M. Phospholipid, nature’s own slide and cover slip for cryo-electron microscopy. J. Microsc. 1989;153:81–92. doi: 10.1111/j.1365-2818.1989.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 9.de Gennes P.-G., Brochard-Wyart F., Quéré D. Springer; New York: 2004. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. [Google Scholar]
- 10.Berg J.C. World Scientific; Hackensack, NJ: 2010. An Introduction to Interfaces & Colloids: the Bridge to Nanoscience. [Google Scholar]
- 11.Wyart F.B., Daillant J. Drying of solids wetted by thin liquid films. Can. J. Phys. 1990;68:1084–1088. [Google Scholar]
- 12.Vrij A. Possible mechanism for spontaneous rupture of thin, free liquid films. Discuss. Faraday Soc. 1966;42:23–33. [Google Scholar]
- 13.Parsegian V.A. Cambridge University Press; New York: 2006. van der Waals Forces: a Handbook for Biologists, Chemists, Engineers, and Physicists. [Google Scholar]
- 14.Elbaum M., Lipson S.G. How does a thin wetted film dry up? Phys. Rev. Lett. 1994;72:3562–3565. doi: 10.1103/PhysRevLett.72.3562. [DOI] [PubMed] [Google Scholar]
- 15.Parsegian V.A., Zemb T. Hydration forces: observations, explanations, expectations, questions. Curr. Opin. Colloid Interface Sci. 2011;16:618–624. [Google Scholar]
- 16.Israelachvili J.N. Elsevier Science; San Diego, CA: 2011. Intermolecular and Surface Forces. [Google Scholar]
- 17.Kenworthy A.K., Hristova K., McIntosh T.J. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol) Biophys. J. 1995;68:1921–1936. doi: 10.1016/S0006-3495(95)80369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaffe J.S., Glaeser R.M. Preparation of frozen-hydrated specimens for high resolution electron microscopy. Ultramicroscopy. 1984;13:373–377. doi: 10.1016/0304-3991(84)90003-2. [DOI] [PubMed] [Google Scholar]
- 19.Taylor K.A., Glaeser R.M. Hydrophilic support films of controlled thickness and composition. Rev. Sci. Instrum. 1973;44:1546–1547. doi: 10.1063/1.1685999. [DOI] [PubMed] [Google Scholar]
- 20.Chang C.F., Ohno T., Glaeser R.M. The fatty-acid monolayer technique for preparing frozen-hydrated specimens. J. Electron Microsc. Tech. 1985;2:59–65. [Google Scholar]
- 21.Hayward S.B., Grano D.A., Fisher K.A. Molecular orientation of bacteriorhodopsin within the purple membrane of Halobacterium halobium. Proc. Natl. Acad. Sci. USA. 1978;75:4320–4324. doi: 10.1073/pnas.75.9.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao E., Liao M., Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheres S.H. Beam-induced motion correction for sub-megaDalton cryo-EM particles. eLife. 2014;3:e03665. doi: 10.7554/eLife.03665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Mooney P., Cheng Y. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell M.G., Veesler D., Carragher B. 2.8 Å resolution reconstruction of the Thermoplasma acidophilum 20 S proteasome using cryo-electron microscopy. eLife Sci. 2015 doi: 10.7554/eLife.06380. 03/2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petty M.C. Cambridge University Press; Cambridge, UK: 1996. Langmuir-Blodgett Films: an Introduction. [Google Scholar]
- 27.Xu L., Lio A., Salmeron M. Wetting and capillary phenomena of water on mica. J. Phys. Chem. B. 1998;102:540–548. [Google Scholar]
- 28.Tribet C., Audebert R., Popot J.L. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. USA. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao M., Cao E., Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie shows that a permanently dry patch forms when a small amount of chloroform solution, containing 1 mg/mL of phospholipid, touches a thin puddle of water on the surface of freshly cleaved mica. As is shown in this movie, the dry patch is largely hydrophobic.
The movie shows that a 10 mL volume of water spreads over an area of about 10 cm when applied to the surface of freshly cleaved mica. When a “point source” of chloroform vapor approaches but does not touch this puddle, the water thins appreciably, giving rise to interference fringes (Newton’s rings). When the point source is removed, the water returns. When chloroform liquid touches the puddle of water, however, it leaves a dry spot, which nevertheless can be wetted again quite easily.