Abstract
Introduction
Accurate localisation is an essential component for the delivery of intracranial stereotactic treatment. For fractionated stereotactic radiotherapy, we compared the daily localisation accuracy of a standard thermoplastic mask with a new maxillary fixation device (MFD).
Methods
Daily pre‐treatment kV cone‐beam computed tomography (CBCT) scans of 23 patients (12 localised in the MFD and 11 in the mask) with benign skull‐based lesions were reviewed retrospectively. The set up accuracy was measured in 6° of freedom, to ascertain both individual and population random and systematic errors. The appropriate clinical target volume to planning target volume margin was computed from set up error data.
Results
A total of 682 CBCT scans were evaluated. Systematic (Σ) and random (σ) population errors were Σ = 0.8 mm, 0.2 mm and 0.2 mm and σ = 0.3 mm, 0.3 mm and 0.2 mm, respectively, for the standard mask in the left/right (LR), superior/inferior (SI), and anterior/posterior (AP) translational planes, and Σ = 0.2 mm, 0.1 mm and 0.2 mm and σ = 0.2 mm, 0.3 mm and 0.2 mm, respectively, for the MFD. There was a reduction in rotation errors in the MFD compared to the mask. Margin calculations suggested an isotropic margin could be safely reduced to 2 mm for the MFD.
Conclusion
The two devices demonstrate similar daily positional accuracy for fractionated stereotactic treatment of intracranial lesions. Combined with daily image guidance and couch correction, either of these devices is a viable frameless option for fractionated stereotactic radiation therapy.
Keywords: Intracranial, radiotherapy, stereotactic
Introduction
The management of skull base tumours is complex with lesions frequently abutting or surrounding optic structures and other cranial nerves. Gross surgical removal is often not possible without significant patient morbidity.1, 2
Fractionated stereotactic radiotherapy, hypo‐fractionated stereotactic radiotherapy and radiosurgery are established treatments in the management of skull base tumours.1, 2, 3, 4 Treatment with radiation is complex due to the proximity of critical organs at risk (OAR), mandating a steep dose gradient between the treatment volume and OAR. Accurate localisation is essential to ensure safe dose delivery to the target while sparing surrounding OAR and preserving functional outcome. These restrictions necessitate reliable patient set up and a small imaging action level.
Frameless stabilisation devices are becoming increasingly common in the treatment of stereotactic radiation therapy, especially for hypo‐fractionated and fully fractionated treatment regimes, due to the invasive nature of the frame‐based system. Thermoplastic masks, alone or in combination with bite blocks have been used successfully for intracranial lesions.5, 6 On‐board imaging and couch correction is also employed to assist patient localisation.7
The aim of this study was to evaluate the difference between a current and a new stabilisation device for patients receiving intracranial fractionated stereotactic radiotherapy. A new relocatable headframe with a maxillary fixation device (MFD), which differs from the standard bite‐block systems, was assessed for its accuracy in reproducible daily patient set up, in comparison with the department's standard immobilisation mask. These results were then used to determine if current planning margins were appropriate for the two devices.
Methods
Subject population
Patients were selected for inclusion in this study if they were treated using the MFD and had a benign intracranial tumour diagnosis. A minimum of one viable maxillary tooth was required for eligibility with the MFD within our department, to provide an anchor for the mouthpiece prior to the vacuum suction being applied. The first 12 patients positioned using the MFD that met the inclusion criteria were included in this evaluation. Data were also collected on 11 control patients with similar benign tumour characteristics treated with the standard mask in order to compare the accuracy and reproducibility of the two systems within similar patient cohorts. Ethics exemption was obtained through the Institutional Human Research Ethics Committee.
The standard immobilisation device at this institution for intracranial treatments is a Type‐S Head‐Only thermoplastic mask (Civco Medical Solutions, Rotterdam, The Netherlands), using either the Posifix® Supine Headrests or the Silverman Headrests (Civco Medical Solutions).
MFD system description
Figure 1 illustrates the primary components of the Fraxion system (Elekta, Stockholm, Sweden) (referred to as the MFD). The MFD is a similar device to the eXtend system for Elekta Perfexion Gamma Knife treatment.8, 9 The MFD consists of a table top adapter fastened to a repositioning carbon fibre headframe, to which a frontpiece and a mouthpiece are attached. A vacuum head cushion inserts into the base plate of the headframe. A patient control unit (PCU) is connected to the mouthpiece with tubing, providing a vacuum that correctly positions the mouthpiece and removes saliva from the patient's mouth. A stereotactic frame and templates are used for daily localisation.
Figure 1.
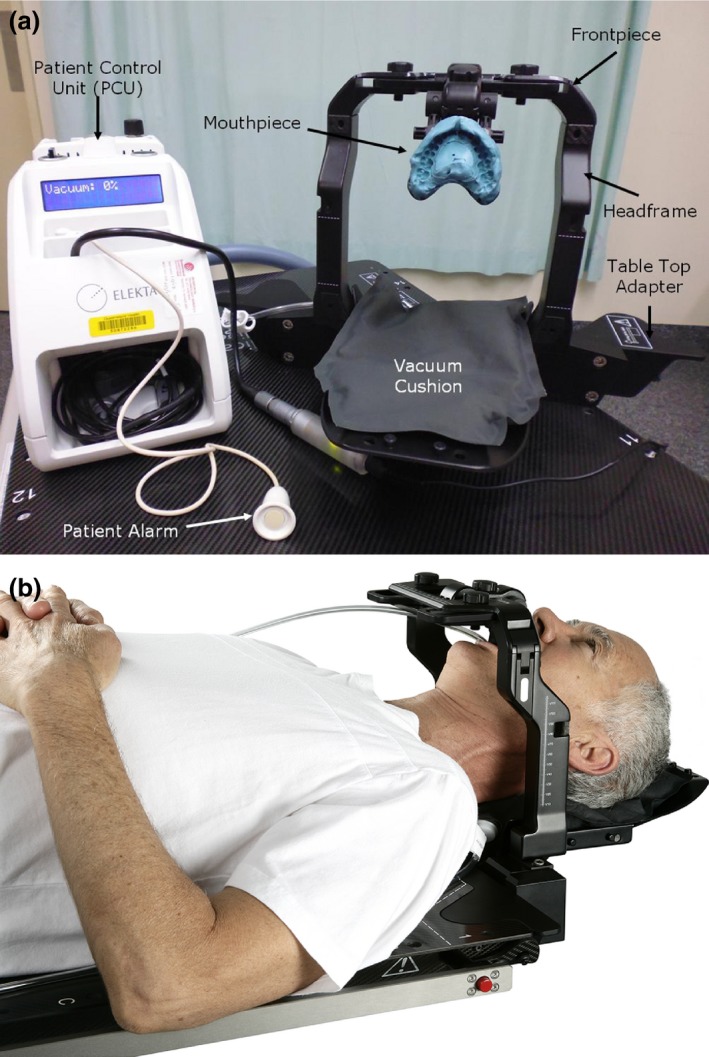
(A) Components of the Elekta Fraxion stabilisation system. PCU, headframe and frontpiece with mouthpiece and inflated vacuum headrest (image courtesy of Princess Alexandra Hospital Radiation Oncology Department). (B) Elekta Fraxion system in place for patient set up (image courtesy of Elekta, Elekta catalogue).
Pre‐simulation
Patients were required to attend a dental appointment to ascertain suitability for the new system. A dental mould was taken and converted into a plaster positive, which was made into the requisite mouthpiece prior to the patient presenting to the department.
Simulation
Patients attended a planning session for set up simulation and a computed tomography (CT) scan. The mouthpiece, attached to the loosely assembled frontpiece, was inserted into the patient's mouth with the vacuum applied to a level of 40% (400 mbar at sea level) prior to the patient being directed onto the head cushion. The frontpiece was placed tension‐free onto the headframe before being clamped into place. The head cushion was evacuated before tightening the frontpiece securing screws with a torque wrench, which was not adjusted for the duration of the patient's treatment. The PCU provided surveillance and alarmed if the vacuum fell below 90% of the original level. MFD patients had an hour allocated for their simulation session, compared to 30 min for the standard device.
Staff training in the production of mouthpieces and use of the equipment was originally provided by the vendor, and then as an in‐house training programme. The dentist involved provided additional support.
A CT scan was performed for all patients using the Aquilion (Toshiba Medical Systems, Europe BV) scanner with 2 mm slices.
Planning
The gross tumour volume (GTV) or clinical target volume (CTV) were marked, with a 2–3 mm isotropic margin to create the planning tumour volume (PTV) at the physician's discretion. Critical OAR, especially optic nerves and optic chiasm, were also given a 2–3 mm expanded planning risk volume to help shape the rapid dose falloff and also to provide a small safety margin whereby high dose did not abut the OAR. Patients were planned using intensity modulated radiation therapy and volumetric modulated arc therapy.
Treatment
Treatment was delivered using 6 MV on the Elekta Axesse linear accelerator, which operates with 4 mm multi‐leaf collimator leaves. Each day a pre‐treatment cone‐beam computed tomography (CBCT) scan was taken and a bone match (translation and rotation) was performed using the Elekta XVI imaging system, within a clipbox defined by a single radiation oncologist. Observed errors were recorded in the left/right (LR), superior/inferior (SI) and anterior/posterior (AP) translation and the pitch, roll and yaw rotation directions for each CBCT scan. Patients were scanned using F0 filter/S10 collimation insert, rotating 100° at a speed of 360° per minute. About 183 frames were captured, and the dose per CBCT scan was 0.5 mGy. All images were independently checked by two radiation therapists prior to treatment, and the match results recorded from the initial bone match without adjustment to eliminate inter‐user error.
Patients had a 2 mm/2° action level, whereby correction was not applied if errors fell below this level. Hexapod table correction was made using the IGuide tracking system (Elekta), which corrects for all 6° of freedom, for all treatment fractions that fell outside of this action level. Any movement using Hexapod required a post‐shift verification CBCT scan prior to the commencement of treatment. All CBCT scans were sent to the radiation oncologist for offline review and approval. For this study, data were collected without influence of couch correction to assess daily stabilisation of the devices alone. Data were also collected at 1 mm/1° action levels to assess errors if margin reduction was applied.
Statistical analysis
Systematic set up errors (individual mean set up error, overall population mean set up error and population systematic error) and random set up errors (individual random error and population random error) were computed following the formula described by the Royal College of Radiologists10 (chapter 4). To assess the significance of the difference of the individual mean set up error and individual random error between standard and new conditions, independent t‐tests were performed. Statistical significance was defined as P ≤ 0.05. Statistical analyses were performed using R statistical software (http://www.r-project.org/).
Random and systematic errors for both the individual and population (translation and rotation) were calculated and recorded using previously published methods.10 Population random (σ) and systematic (Σ) errors were calculated as the mean of the individual random errors and the SD of the individual systematic errors respectively. For calculating set up error and resultant margins,11 both Van Herk's (2.5Σ + 0.7σ) and Stroom's (2Σ + 0.7σ) margin calculations12, 13 were selected.
Results
Patient demographics
Between October 2012 and December 2013, 13 patients that met the inclusion criteria were treated using the MFD, and 11 patients with benign skull‐based lesions were treated using the mask on the Elekta Axesse machine. One patient treated with the MFD device was excluded from the study due to poor moulding of the MFD mouthpiece requiring re‐simulation. Consequently, a total of 23 patients were included in this evaluation. Twenty (87%) patients had a skull base meningioma.
Patients were planned using between four and seven intensity modulated radiation therapy fields or one to two volumetric modulated arc therapy arcs. Non‐coplanar angles were utilised in 18 (78%) of the 23 patients. Patients were treated with a median dose of 54 Gy in 30 fractions (range 50.4–60 Gy in 28–30 fractions).
A total of 682 pre‐treatment CBCT scans were analysed, 356 in the test group and 326 in the standard group.
Errors
Table 1 describes the daily set up error and separates the CBCT images into groups exceeding the action levels listed (1 mm/1°, 2 mm/2°). There is a notable difference in the number of images falling outside the rotational thresholds for the standard device compared to the MFD. The comparison of the mean systematic and random errors between individuals in both devices is displayed in Table 2. Significantly different results for the MFD were found in the AP direction (P = 0.02) and the mean random rotation errors in the roll (P = 0) and yaw (P = 0) values against the mask.
Table 1.
Proportion of CBCT images outside imaging action thresholds for the standard mask and new MFD conditions
| Imaging action threshold | Condition | Number of images | |||||
|---|---|---|---|---|---|---|---|
| Translation | Rotation | ||||||
| Left/right | Superior/inferior | Anterior/posterior | Left/right | Superior/inferior | Anterior/posterior | ||
| ≥1 mm/1° | Standard | 128/326 | 180/326 | 171/326 | 121/326 | 138/326 | 105/326 |
| (%) | 39.26 | 55.21 | 52.45 | 37.12 | 42.33 | 32.21 | |
| New | 201/356 | 167/356 | 143/356 | 50/356 | 65/356 | 67/356 | |
| (%) | 56.46 | 46.91 | 40.17 | 14.04 | 18.26 | 18.82 | |
| ≥2 mm/2° | Standard | 67/326 | 71/326 | 64/326 | 22/326 | 33/326 | 15/326 |
| (%) | 20.55 | 21.78 | 19.63 | 6.75 | 10.12 | 4.6 | |
| New | 61/356 | 49/356 | 71/356 | 5/356 | 0/356 | 0/356 | |
| (%) | 17.13 | 13.76 | 19.94 | 1.4 | 0 | 0 | |
MFD, maxillary fixation device; CBCT, cone‐beam computed tomography.
Table 2.
Errors for individuals in standard mask versus new MFD conditions
| Mean systematic error (Σ) (mm) | Mean random error (σ) (mm) | |||||
|---|---|---|---|---|---|---|
| Direction | Standard mask | MFD | P‐value | Standard mask | MFD | P‐value |
| Trans (L/R) | 0.5 | −0.3 | 0.43 | 0.3 | 0.2 | 0.52 |
| Trans (S/I) | 0.5 | 0.5 | 0.91 | 0.3 | 0.3 | 0.55 |
| Trans (A/P) | −0.8 | 0.6 | 0.02a | 0.2 | 0.2 | 0.16 |
| (degrees) | (degrees) | |||||
|---|---|---|---|---|---|---|
| Rot (pitch) | −0.09 | −0.09 | 1 | 0.21 | 0.16 | 0.12 |
| Rot (roll) | −0.36 | −0.59 | 0.35 | 0.30 | 0.07 | 0a |
| Rot (yaw) | −0.57 | −0.49 | 0.74 | 0.18 | 0.09 | 0a |
Indicates statistical significance.
MFD, maxillary fixation device; L/R, left/right; S/I, superior/inferior; A/P, anterior/posterior; trans, translational planes; rot, rotation.
Differences in the individual mean set up errors for rotation are shown in Figure 2. The mask showed a maximum error of 1.02° and the MFD had a maximum error of 0.21°.
Figure 2.
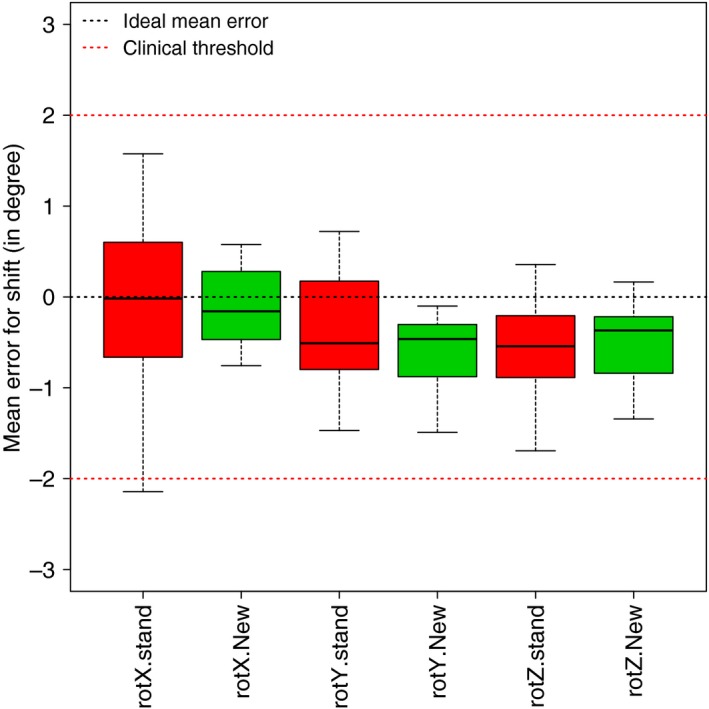
Individual mean set up errors for rotation in the standard mask and new MFD conditions. rot, rotation; stand, standard; X rotation, pitch; Y rotation, roll; Z rotation, yaw.
Margin selection
Table 3 shows the summarised data for the population random and systematic errors, and the resultant margins populated from the above‐mentioned formulae. A margin of up to 2.2 mm for the mask and less than 1 mm for the MFD was calculated using Van Herk's and Stroom's formulae.
Table 3.
Summarised data of errors and margin calculations in the standard mask and new MFD devices
| Condition | Direction | Population set up errors | Van Herk's (2.5Σ + 0.7σ) | Stroom's (2Σ + 0.7σ) | |
|---|---|---|---|---|---|
| Systematic (Σ) | Random (σ) | ||||
| Standard | Trans L/R (mm) | 0.8 | 0.3 | 2.2 | 1.8 |
| Trans S/I (mm) | 0.2 | 0.3 | 0.7 | 0.6 | |
| Trans A/P (mm) | 0.2 | 0.2 | 0.6 | 0.5 | |
| Rot pitch (degrees) | 1.02 | 0.21 | |||
| Rot roll (degrees) | 0.47 | 0.30 | |||
| Rot yaw (degrees) | 0.39 | 0.18 | |||
| New | Trans L/R (mm) | 0.2 | 0.2 | 0.6 | 0.5 |
| Trans S/I (mm) | 0.1 | 0.3 | 0.5 | 0.4 | |
| Trans A/P (mm) | 0.2 | 0.2 | 0.6 | 0.5 | |
| Rot pitch (degrees) | 0.21 | 0.16 | |||
| Rot roll (degrees) | 0.19 | 0.07 | |||
| Rot yaw (degrees) | 0.21 | 0.09 | |||
MFD, maxillary fixation device; L/R, left/right; S/I, superior/inferior; A/P, anterior/posterior; trans, translational planes; rot, rotation.
Discussion
The MFD was evaluated as an alternative device to the mask‐based immobilisation system for the treatment of fractionated stereotactic radiotherapy. Differences between the two devices were most noticeable for rotations, in both the 1 mm/1° and 2 mm/2° action level thresholds. The translation errors were comparable for both conditions.
The findings of this study are consistent with similar published studies. Rosenfelder et al.14 showed errors of 0.3–0.7 mm/° and 0.6–1.5 mm/° for the Gill–Thomas–Cosman frame and three‐point thermoplastic shell respectively. An overall error of 1.2 mm was noted in Peng et al.'s15 study of a bite plate and thermoplastic mask. Ruschin et al.9 reported that the Perfexion repositioning headframe system, which is most similar to the Fraxion system, had a 3D mean positioning displacement of 1.1 ± 0.8 mm.
Safely reducing planning margins, while adequately treating tumour volumes, can decrease dose to normal brain tissue and critical OAR, leading to better functional outcomes for patients with brain tumours.16 Decreasing the tumour volume may also potentially increase tumour control or reduce radiation‐induced side effects for patients with a meningioma diagnosis.17 Although the findings suggest the MFD provides better daily reproducibility statistically, there is not enough clinical significance to suggest one device gives superior daily positioning than the other. However, the margin formulae indicate that, based on the daily random and systematic set up errors alone, our current practice of using a 3 mm margin is still feasible for the mask, while a 2 mm margin could be safely considered for the MFD. The results do also suggest that while the two devices are similar clinically, the current institutional practices are of a high standard. While not assessed in this study, it is also important to note the significant roles that daily image guidance and couch correction play in their ability to safely reduce margins for these patients.
The potential benefits of margin reduction with the MFD need to be considered in the context of changes to departmental workflow. Our department was reliant on a dentist to create the plaster positive for the mouthpiece, and required additional resources and training for staff. The MFD was a more labour intensive process than the mask, and additional time was required to fit and clean the mouthpiece daily. Patient compliance was essential to the mouthpiece fitting and working well.
A limitation of this study was that intra‐fraction motion was not assessed. Further research is warranted to determine if the MFD is better able to stabilise patients who are having stereotactic radiation therapy by including an intra‐fractional component, which may show a more clinically relevant difference between the two systems.
Another limitation of this study was our inability with this current practice to assess isocentric movement when treating non‐coplanar fields. It is important to note that other potential sources of error, such as imaging resolution and fusion accuracy, mechanical and treatment isocentre size and positional accuracy, are not included in the selected margin formulae. However, any potential mechanical errors such as differences between the kV and MV isocentres or accurate laser position would be equally present for both devices.
Conclusion
Equipment capable of precise and accurate immobilisation and reproducibility, in combination with image guidance and couch correction, is vital to the delivery of stereotactic radiotherapy. The results of this study have shown that the MFD provides comparable positioning accuracy and reproducibility to a standard thermoplastic mask and has the potential to enable margin reduction when used in conjunction with daily image guidance and couch correction. Either device is a valid frameless option for intracranial stereotactic radiation therapy.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the staff members and the management at the Princess Alexandra Hospital, and the Private Practice Trust Fund that contributed to time in the research position. Special thanks to Eric Hew, Elizabeth Brown, Ryan Lusk and Brock Lamprecht.
J Med Radiat Sci 63 (2016) 41–47
References
- 1. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Factors affecting outcome following treatment of patients with cavernous sinus meningiomas. J Neurosurg 2010; 113: 1087–92. [DOI] [PubMed] [Google Scholar]
- 2. Dufour H, Muracciole X, Métellus P, Regis J, Chinot O, Grisoli F. Long‐term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: Is there an alternative to aggressive tumor removal? Neurosurgery 2001; 48: 285–96. [DOI] [PubMed] [Google Scholar]
- 3. Metellus P, Batra S, Karkar S, et al. Fractionated conformal radiotherapy in the management of cavernous sinus meningiomas: Long‐term functional outcome and tumor control at a single institution. Int J Radiat Oncol Biol Phys 2010; 78: 836–43. [DOI] [PubMed] [Google Scholar]
- 4. Elia AE, Shih HA, Loeffler JS. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus 2007; 23: 1–7. [DOI] [PubMed] [Google Scholar]
- 5. Minniti G, Scaringi C, Clarke E, Valeriani M, Osti M, Enrici RM. Frameless linac‐based stereotactic radiosurgery (SRS) for brain metastases: Analysis of patient repositioning using a mask fixation system and clinical outcomes. Radiat Oncol 2011; 6: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nath SK, Lawson JD, Wang JZ, et al. Optically‐guided frameless linac‐based radiosurgery for brain metastases: Clinical experience. J Neurooncol 2010; 97: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tryggestad E, Christian M, Ford E, et al. Inter‐ and intrafraction patient positioning uncertainties for intracranial radiotherapy: A study of four frameless, thermoplastic mask‐based immobilization strategies using daily Cone‐Beam CT. Int J Radiat Oncol Biol Phys 2011; 80: 281–90. [DOI] [PubMed] [Google Scholar]
- 8. Sayer FT, Sherman JH, Yen CP, Schlesinger DJ, Kersh R, Sheehan JP. Initial experience with the extend system: A relocatable frame system for multiple‐session gamma knife radiosurgery. World Neurosurg 2011; 75: 665–72. [DOI] [PubMed] [Google Scholar]
- 9. Ruschin M, Nayebi N, Carlsson P, et al. Performance of a novel repositioning head frame for Gamma Knife Perfexion and image‐guided linac‐based intracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2010; 78: 306–13. [DOI] [PubMed] [Google Scholar]
- 10. The Royal College of Radiologists SoR, Institute of Physics and Engineering in Medicine . On Target: Ensuring Geometric Accuracy in Radiotherapy. The Royal College of Radiologists, London: 2008. [Google Scholar]
- 11. van Herk M. Errors and margins in radiotherapy. Seminars in Radiat Oncol 2004; 14: 52–64. [DOI] [PubMed] [Google Scholar]
- 12. Das S, Isiah R, Rajesh B. Accuracy of relocation, evaluation of geometric uncertainties and clinical target volume (CTV) to planning target volume (PTV) margin in fractionated stereotactic radiotherapy for intracranial tumors using relocatable Gill‐Thomas‐Cosman (GTC) frame. J Appl Clin Med Phys 2011; 12: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dionisi F, Palazzi MF, Bracco F, et al. Set‐up errors and planning target volume margins in head and neck cancer radiotherapy: A clinical study of image guidance with on‐line cone‐beam computed tomography. Int J Clin Oncol 2013; 18: 418–27. [DOI] [PubMed] [Google Scholar]
- 14. Rosenfelder N, Corsini L, McNair H, et al. Achieving the relocation accuracy of stereotactic frame‐based cranial radiotherapy in a three‐point thermoplastic shell. Clin Oncol 2013; 25: 66–73. [DOI] [PubMed] [Google Scholar]
- 15. Peng LC, Kahler D, Samant S, et al. Quality assessment of frameless fractionated stereotactic radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys 2010; 78: 1586–93. [DOI] [PubMed] [Google Scholar]
- 16. Shields LBE, Coons JM, Dedich C, et al. Improvement of therapeutic index for brain tumors with daily image guidance. Radiat Oncol 2013; 8: 283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SR, Yang KA, Kim SK, Kim SH. Radiation‐induced intratumoral necrosis and peritumoral edema after gamma knife radiosurgery for intracranial meningiomas. J Korean Neurosurg 2012; 52: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


