Abstract
Introduction
The purpose of this study was to investigate coplanar and non‐coplanar volumetric modulated arc therapy (VMAT) delivery techniques for stereotactic ablative radiation therapy (SABR) to the lung.
Methods
For ten patients who had already completed a course of radiation therapy for early stage lung cancer, three new SABR treatment plans were created using (1) a coplanar full arc (FA) technique, (2) a coplanar partial arc technique (PA) and (3) a non‐coplanar technique utilising three partial arcs (NCA). These plans were evaluated using planning target volume (PTV) coverage, dose to organs at risk, and high and intermediate dose constraints as incorporated by radiation therapy oncology group (RTOG) 1021.
Results
When the FA and PA techniques were compared to the NCA technique, on average the PTV coverage (V 54Gy) was similar (P = 0.15); FA (95.1%), PA (95.11%) and NCA (95.71%). The NCA resulted in a better conformity index (CI) of the prescription dose (0.89) when compared to the FA technique (0.88, P = 0.23) and the PA technique (0.83, P = 0.06). The NCA technique improved the intermediate dose constraints with a statistically significant difference for the D 2cm and R 50% when compared with the FA (P < 0.03 and <0.0001) and PA (P < 0.04 and <0.0001) techniques. The NCA technique reduced the maximum spinal cord dose by 2.72 and 4.2 Gy when compared to the PA and FA techniques respectively. Mean lung doses were 4.09, 4.31 and 3.98 Gy for the FA, PA and NCA techniques respectively.
Conclusion
The NCA VMAT technique provided the highest compliance to RTOG 1021 when compared to coplanar techniques for lung SABR. However, single FA coplanar VMAT was suitable for 70% of patients when minor deviations to both the intermediate dose and organ at risk (OAR) constraints were accepted.
Keywords: Dosimetry, lung cancer, stereotactic ablative radiation therapy, treatment planning, volumetric modulated arc therapy
Introduction
Stereotactic ablative radiation therapy (SABR) is the delivery of a highly ablative radiation dose in a few fractions. It was originally introduced for early stage lung cancer patients who were deemed medically unfit for surgery.1 SABR is commonly delivered using a high number of coplanar and non‐coplanar three‐dimensional conformal radiation therapy (3DCRT) beams. In a previous single centre dosimetry comparison, the authors demonstrated that a predominantly non‐coplanar, 10 beam technique had the most favourable compliance with the radiation therapy oncology group (RTOG) 1021 protocol.2, 3 A highly non‐coplanar beam arrangement allowed for improved intermediate dose conformity and organ at risk (OAR) sparing. However, the engagement of a high number of couch rotations can extend the treatment times to potentially unfavourable lengths.4 It has previously been reported that for treatment times extending over 34 min that a baseline shift in tumour position of up to 5 mm can occur.5 Delivery times for lung SABR can vary depending on the equipment used, fractional dose, patient compliance and the delivery technique itself.
Volumetric modulated arc therapy (VMAT) is a novel technique that delivers the dose whereas the linear accelerator rotates continuously around the patient.6, 7 The dose rate, gantry rotation speed and multileaf collimator (MLC) positions are all variables that can be altered whereas the machine is delivering the dose. Single coplanar arcs have already been shown to reduce treatment times for SABR to the lung when compared to 3DCRT, whereas achieving highly conformal dose distributions.4 However, non‐coplanar beam arrangements improve the intermediate dose conformity, which is one of the key dosimetry metrics for SABR. Therefore, this study was designed to quantify any benefits arising from non‐coplanar VMAT when compared to coplanar VMAT for SABR to the lung.
Methods
Institutional ethics approval was granted for this retrospective study. Ten patients who were eligible for SABR and had completed their course of radiation therapy were identified from our local radiation oncology information system. Inclusion criteria was limited to early stage disease (Ia/b or IIa) measuring <5 cm in the largest dimension. Furthermore, the gross tumour volume (GTV) was required to be >2 cm away from the proximal bronchial tree.
Patients were simulated as previously reported.2 A four‐dimensional computed tomography (4DCT) scan was acquired at the time of simulation along with a free‐breathing CT scan. Both scans were exported to Pinnacle v9.4 (Philips Medical Systems, Fitchburg, WI) with the 4DCT used to generate an internal target volume (ITV). The planning target volume (PTV) was created by expanding the ITV 5 mm isotropically. The free‐breathing simulation CT scan was used for all OAR (Table 1) contouring and treatment planning. All maximum doses reported were to a clinically significant and measurable volume of 0.03 cm3. The chest wall contour was defined as a 2‐cm expansion on the ipsilateral lung, excluding the vertebral body, sternum and mediastinal structures. A structure was also created for reporting the maximum dose at any point 2 cm from the PTV (D 2cm).
Table 1.
Organ at risk dose constraints
| Organ | Constraint(s) |
|---|---|
| Spinal cord | 18 Gy < 0.35 cm3 |
| 12.3 Gy < 1.2 cm3 | |
| MPD < 21.9 Gy | |
| Brachial plexus | 20.4 Gy < 3 cm3 |
| MPD < 24 Gy | |
| Aorta, SVC and IVC | 39 Gy < 10 cm3 |
| MPD < 49 Gy | |
| Pericardium | 24 Gy < 15 cm3 |
| MPD < 30 Gy | |
| Trachea | 15 Gy < 4 cm3 |
| MPD < 30 Gy | |
| Combined lungs – ITV | 11.4 Gy < 1000 cm3 |
| 10.5 Gy < 1500 cm3 | |
| Oesophagus | 17.7 Gy < 5 cm3 |
| MPD < 25.2 Gy | |
| Rib | 40 Gy < 5 cm3 |
| MPD < 50 Gy | |
| Chestwall | 30 Gy < 30 cm3 (<70 cm3 for tumours on the CW) |
| Skin | 30 Gy < 10 cm3 |
| MPD < 33 Gy |
IVC, inferior vena cava; SVC, superior vena cava; ITV, internal target volume; MPD, maximum point dose (defined as ≥0.03 cm3).
Treatment planning was carried out with Pinnacle v9.4 and the SmartArc™ algorithm. The final gantry spacing option in Pinnacle allows for the treatment planner to select the angular separation (in degrees) of the arc segments. It has previously been reported that a gantry spacing of 4° (new segment every 4°) is optimal, with no benefit in reducing the spacing any further.8 In this study, a full 360° arc will always have 91 segments with 0°, or the starting angle, being included as a segment. The plans were computed using an Elekta Axesse beam model with the beam modulator collimator system with 4‐mm MLC leaves. A dose grid resolution of 0.25 cm3 was used for all plans. All plans were calculated using the collapsed cone convolution algorithm (CCC). The CCC algorithm is a type B algorithm and accounts for changes in lateral electron transport and should therefore be used for lung tumour treatments. Treatment planning was performed by a single planner and the machine quality assurance was performed by a medical physicist to ensure the plans were clinically deliverable with a gamma analysis passing rate of 3 mm/3%.
Unlike 3DCRT where the isocentre is placed in the centre of the PTV, the isocentre for the arc plans was placed on the patient's midline. This was to avoid any further complications that could cause a collision, such as when the bed is shifted laterally and is coupled with a rotating gantry and non‐coplanar floor angles. The isocentre could have been placed in the PTV for the coplanar arcs but was left on the patient's midline to avoid any bias. All fields used 6 MV photons delivered with a collimator angle of zero.
The single full arc (FA) technique started at 181°, and travelled in a clockwise (CW) direction for 359° to stop at 180°. The partial arc (PA) technique started at either 181° or 180° and travelled an arc length of 180–200° around the ipsilateral side of the patient either in a CW or counter‐clockwise (CCW) direction. The non‐coplanar partial arc technique (NCA) used three partial arcs, one with a couch angle of 0° and two using non‐coplanar couch angles. For left‐sided tumours, the couch angles were 0°, 15° and 340° and for right‐sided tumours they were 0°, 20° and 345°. These angles were chosen as they were the greatest possible couch rotations away from zero (allowing for less overlapping of beams, and reducing the intermediate dose wash) without the gantry head and couch colliding. The arc angles for the NCA technique were the same as used for the PA technique. Multileaf collimator speed was constrained to 0.46 cm/degree as per department protocol.
A total dose of 54 Gy in 3 fractions was prescribed to the periphery of the PTV ensuring that >95% of the PTV received the prescription dose (PTV54Gy) and that 99% of the PTV received 90% of the prescription dose (PTV48.6Gy). The 54 Gy isodose (prescription isodose) was planned to fall between 59% and 90% of the maximum dose in the plan, resulting in a maximum dose of no more than 91.5 Gy. Organ at risk tolerances used were those reported in RTOG 1021 (Table 1). The constraints to limit the intermediate doses, D 2cm, the dose at any point 2 cm from the PTV and the ratio of the volume of half the prescription dose to the volume of the PTV (R 50%) are also shown in Table 2. To quantify the conformity of the prescription isodose, the conformity index (CI) was used2
where TVPTV is defined as the total volume of PTV covered by the covering isodose (54 Gy), TV is defined as the total volume of the PTV and PIV is defined as the total volume of the covering isodose in the patient. A CI value of ≥0.75 was no deviation, with ≥0.65 constituting an acceptable deviation and anything <0.65 was considered unacceptable. This CI formula was used instead of the RTOG formula as it is more robust and less prone to errors.
Table 2.
Acceptable dose spillage guidelines from RTOG 1021
| Ratio of prescription isodose volume to the PTV | Ratio of 27 Gy isodose volume to the PTV (R 50%) | Maximum dose at 2 cm from PTV in any direction as % of prescribed dose (PD). D 2cm (Gy) = % × PD | PTV volume (cc) | |||
|---|---|---|---|---|---|---|
| Deviation | Deviation | Deviation | ||||
| None | Acceptable | None | Acceptable | None | Acceptable | |
| <1.2 | <1.5 | <5.9 | <7.5 | <50.0 | <57.0 | 1.8 |
| <1.2 | <1.5 | <5.5 | <6.5 | <50.0 | <57.0 | 3.8 |
| <1.2 | <1.5 | <5.1 | <6.0 | <50.0 | <58.0 | 7.4 |
| <1.2 | <1.5 | <4.7 | <5.8 | <50.0 | <58.0 | 13.2 |
| <1.2 | <1.5 | <4.5 | <5.5 | <54.0 | <63.0 | 22.0 |
| <1.2 | <1.5 | <4.3 | <5.3 | <58.0 | <68.0 | 34.0 |
| <1.2 | <1.5 | <4.0 | <5.0 | <62.0 | <77.0 | 50.0 |
| <1.2 | <1.5 | <3.5 | <4.8 | <66.0 | <86.0 | 70.0 |
| <1.2 | <1.5 | <3.3 | <4.4 | <70.0 | <89.0 | 95.0 |
| <1.2 | <1.5 | <3.1 | <4.0 | <73.0 | <91.0 | 126.0 |
| <1.2 | <1.5 | <2.9 | <3.7 | <77.0 | <94.0 | 163.0 |
Each technique was created using the initial set of objectives outlined in Table 3. Other objectives such as the maximum dose to the spinal cord, pericardium, chest wall, trachea and oesophagus were used on an individual patient basis as necessary. As the dose being delivered to the PTV is of an ablative nature, limiting the dose to surrounding tissues directly adjacent to the PTV is extremely important. Therefore, unlike conventional intensity modulation, no expansion was made to the PTV for dose optimisation. The objective used on the PTV was a dose volume histogram (DVH) objective to cover a minimum of 100% of the PTV with 54 Gy. To promote a steep‐dose gradient, a minimum and maximum dose objective was used to control dose to the ITV. This would ensure that the maximum dose would be between 60 Gy and 91.5 Gy, and that the 54 Gy isodose would fall within 59–90% of the maximum dose. To control the prescription dose, a ring with a 1 mm gap to the PTV was created with a maximum dose objective equal to the prescription dose. This gave the optimisation algorithm a 1 mm gap to place the 54 Gy isodose, ensuring a tight and compact high‐ dose region. To limit the intermediate dose, two different objective functions were used. First, a structure constructed from the patients external contour minus the PTV plus a 2‐cm expansion was used to control the dose at D 2cm. This region of interest (ROI) was given a maximum dose objective as per the relevant values in the no deviation column for D 2cm in Table 2. Furthermore, to help meet the R 50% (the value of half the prescription dose divided by the volume of the PTV) constraint (Table 2), a structure constructed from the patients external contour minus an expansion on the PTV was used to control the 27 Gy isodose. This expansion was typically a 1‐cm isotropic expansion of the PTV based on the assumption that dose reduction of 5%/mm is achievable. The expansion on the PTV was reduced/increased but was altered on an individual patient basis as needed to better control the 27 Gy isodose volume. The weights in the objective list were chosen to first cover the entire PTV with 54 Gy isodose, and then use the ring structures to control the intermediate dose to meet the dose conformity constraints. For PTVs adjacent to or overlapping the chest wall, the allowable maximum dose to the rib was increased to 105% of the prescription dose and recorded as an acceptable deviation.
Table 3.
List of starting objectives for all techniques
| ROI | Objective type | Target dose (Gy) | Volume (%)a | Weight |
|---|---|---|---|---|
| PTV | Minimum DVH | 54 | 100 | 5 |
| ITV | Minimum dose | 60 | 1 | |
| ITV | Maximum dose | 91.5 | 5 | |
| D 2cm | Maximum dose | Table 2 | 5 | |
| 27 Gy ring | Maximum dose | 27 | 5 | |
| 54 Gy ring | Maximum dose | 54 | 1 |
ROI, region of interest; DVH, dose volume histogram.
Only applicable for Maximum or Minimum DVH objective types.
Statistical analyses were performed using R statistical software (http://www.r-project.org). The three new SABR treatment plans were compared using a repeated measures ANOVA (parametric test) for normally distributed data and the Friedman test (non‐parametric test) for non‐normally distributed data. Normality of the data has been tested using the Shapiro–Wilk test. When an overall significant difference between treatment plans was demonstrated, post hoc tests (paired t‐tests for normally distributed data or Wilcoxon signed‐rank tests for non‐normally distributed data) were performed to confirm where the differences occurred between treatment plans. The P‐values have been adjusted for multiple comparisons using false discovery rate (FDR) correction to control the expected proportion of incorrectly rejected null hypotheses. The P‐values obtained have been adjusted for multiple comparisons with a Bonferroni correction. Statistical significance was defined as P ≤ 0.05.
Results
Mean PTV size was 32.3 cm3 with five of the ten patients having tumours adjacent to the chest wall. Patient characteristics are detailed in Table 4. Acceptable plans, defined as having no major protocol deviations as outlined in RTOG 1021 (Table 2), were obtained for 70%, 40% and 100% of the FA, PA and NCA techniques respectively. A summary of the dosimetry parameters for each technique are reported in Table 5.
Table 4.
Patient characteristics
| Gender (n) | |
| Male | 7 |
| Female | 3 |
| Age (years) | |
| Range | 61–83 |
| Median | 76 |
| Mean | 74.8 |
| Staging | |
| T1aN0M0 | 5 |
| T1bN0M0 | 2 |
| T1NOSN0M0 | 3 |
| Location | |
| RUL | 5 |
| RML | 1 |
| RLL | 2 |
| LUL | 1 |
| LLL | 1 |
| Overlapping with CW (n) | |
| Yes | 5 |
| No | 5 |
| ITV size (cm3) | |
| Range | 4.43–29.9 |
| Median | 8.3 |
| Mean | 10.4 |
| PTV size (cm3) | |
| Range | 22.8–79.12 |
| Median | 27.49 |
| Mean | 32.26 |
NOS, not specified; CW, chest wall; ITV, internal target volume; PTV, planned target volume; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Table 5.
Mean dose statistics for each technique with associated P‐values
| Metric | Parameter | PA | FA | NCA | P‐valuea (PA‐NCA) | P‐valuea (FA‐NCA) |
|---|---|---|---|---|---|---|
| PTV54Gy | (%) | 95.11 | 95.09 | 95.71 | 0.15 | 0.15 |
| PTV48.6Gy | (%) | 99.92 | 99.97 | 99.99 | 0.04 | |
| D 2cm | Absolute difference | −0.46 | −3.71 | −5.33 | <0.0001 | <0.0001 |
| R 50% | Absolute difference | 1.04 | 0.52 | 0.12 | 0.04 | 0.03 |
| CI | 0.83 | 0.88 | 0.89 | 0.06 | 0.23 | |
| MLD | (Gy) | 4.09 | 4.31 | 3.98 | 0.05 | <0.0001 |
| Spinal cord | D max (Gy) | 9.52 | 11.0 | 6.80 | ||
| V 18Gy (cm3) | 0.0 | 0.0 | 0.0 | |||
| V 12.3Gy (cm3) | 0.02 | 0.11 | 0.0 | |||
| Rib | D max (Gy) | 45.45 | 43.3 | 42.75 | ||
| V 40Gy (cm3) | 1.55 | 1.40 | 1.47 | |||
| Chest wall | V 30Gy (cm3) | 20.12 | 16.95 | 16.44 | ||
| Combined Lung ‐ ITV | V 10.5Gy (cm3) | 434.79 | 414.79 | 390.93 | ||
| V 11.4Gy (cm3) | 400.01 | 380.41 | 355.59 | |||
| Pericardium | D max (Gy) | 19.53 | 19.91 | 18.46 | ||
| V 24Gy (cm3) | 1.93 | 1.49 | 0.52 | |||
| Skin | D max (Gy) | 23.62 | 21.39 | 20.0 | ||
| V 30Gy (cm3) | 0.5 | 0.49 | 0.49 | |||
| Oesophagus | D max (Gy) | 9.82 | 12.47 | 6.64 | ||
| V 17.7Gy (cm3) | 0.0 | 0.0 | 0.0 | |||
| Aorta | D max (Gy) | 11.97 | 14.60 | 9.94 | ||
| V 39Gy (cm3) | 0.0 | 0.0 | 0.0 | |||
| Trachea | D max (Gy) | 6.05 | 7.2 | 5.6 | ||
| V 15Gy (cm3) | 0.02 | 0.47 | 0.0 |
PA, coplanar partial arc technique; FA, coplanar full arc technique; NCA, non‐coplanar technique utilising three partial arcs; ITV, internal target volume; PTV, planned target volume; MLD, mean lung dose; CI, conformity index.
Adjusted P value of post hoc tests.
PTV54Gy coverage was similar for the FA, PA and NCA techniques achieving 95.09%, 95.11% and 95.71% respectively. Coverage for the PTV48.6Gy objective was 99.97%, 99.92% and 99.99% for the FA, PA and NCA respectively with a statistically significant difference (P = 0.04) between the PA and NCA. The NCA technique provided the best high dose conformity with a value of 0.89, when compared to the FA (0.88) and PA (0.82) techniques.
The NCA technique resulted in the highest compliance with the no deviation criteria in Table 2. A previously used scoring system for measuring the absolute difference of the D 2cm and R 50% was used to evaluate the deviations from an acceptable value.2 The mean absolute difference from the R 50% no deviation value (Table 2) was 1.04, 0.52 and 0.12 for the PA, FA and NCA techniques respectively. The mean absolute difference from the D 2cm no deviation was of −5.33, −3.71 and −0.46 for the NCA, FA and PA respectively. This resulted in a statistically significant difference of 0.03 when comparing the FA and PA, and P = 0.04 when comparing the NCA and PA for the R 50% constraint and <0.0001 for the D 2cm between all techniques. Figure 1 plots the achieved R 50% values against the PTV size for each of the ten patients. Organ at risk sparing was similar among techniques (Table 5). For doses to the combined lung minus the ITV volume, the NCA reduced the 10.5 Gy wash by a mean volume of 44 and 24 cm3 for the PA and FA technique, respectively, and the 11.4 Gy dose wash by a mean volume of 44 and 25 cm3 respectively. Spinal cord maximum doses were lower with the NCA technique (Table 5). The FA had 1 plan and the PA technique had 3 plans where the maximum dose to the ribs could not be achieved. Conversely, the NCA technique was able to achieve maximum rib doses for all 10 patients. The chest wall volume receiving 30 Gy (V 30Gy) ranged from 0 to 62.07, 0 to 52.36 and 0 to 53.30 cm3 for the PA, FA and NCA techniques, respectively, for all ten patients. Where the PTV was overlapping the chest wall, the mean V 30Gy was 35.79, 33.18 and 32.46 cm3 for the PA, FA and NCA techniques respectively.
Figure 1.
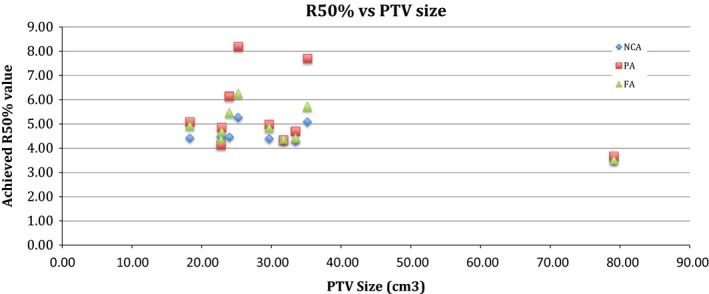
The R50% value achieved in the non‐coplanar arc (NCA), partial arc (PA) and full arc (FA) techniques plotted against PTV size.
Discussion
This study presents an expansion on previous work where the effects of non‐coplanar beam arrangements for lung SABR using 3DCRT were reported. Similar to those findings with 3DCRT, the non‐coplanar VMAT technique tested in this study also provides greater compliance with the RTOG 1021 protocol when compared to single arc coplanar techniques.
The NCA technique provided the most optimal plan with greater adherence to the RTOG 1021 guidelines than the other two techniques. All 10 of the NCA treatment plans adhered to RTOG 1021 protocol guidelines. The technique that had the least compliance with the planning objectives was the PA technique. Only 40% of the plans were acceptable with a majority of the deviations being associated with the intermediate dose constraints. Having an arc only enter through a 180–200° sector did not allow for enough low dose spread throughout the normal tissue, resulting in higher than favourable intermediate doses. The FA technique had 7 out of 10 plans which were clinically acceptable. Of the three not acceptable, the R 50% was above an acceptable deviation in 2 plans and the rib maximum dose was over tolerance in the other.
Similarly to Holt et al. , we also report that the CI for the prescription isodose were within acceptable limits for all techniques8. Holt et al. report a few exceptions to achieving an optimal CI for the prescription dose. In this study, we report no deviations to the CI regardless of delivery technique. This could be due to a number of different factors including the different CI equations used, different treatment machine and contrasting intensity modulation objectives. Furthermore, there was also an improvement in the CI with the FA and NCA techniques, which is largely due to the greater number of segments, and therefore ‘individual beams’ used with the FA and NCA techniques.
The OAR sparing was similar between each technique. There was improved spinal cord sparing with the NCA and PA technique which is due to the fact that neither of these techniques had beams entering through the spinal cord, an unavoidable consequence of the FA technique. Furthermore, the NCA was able to improve both the maximum dose and specific volumetric dose constraint for all midline structures such as the aorta, trachea and oesophagus. The NCA technique was able to either achieve the constraint or limit the maximum rib dose to acceptable deviation for all 10 patients. Furthermore, the maximum V 30Gy (62.07 cm3) to the chest wall for the PA technique was able to be reduced by almost 10 cm3 (52.36 and 53.33 cm3 for the FA and NCA, respectively) by the other two techniques. Although still within the 70 cm3 constraint, this reduction in chest wall dose is likely to be clinically significant, as reported by both Ong et al. and Dunlap et al. where a V 30Gy < 30 cm3 could reduce the risk of toxicity, especially if SABR offers an improvement in long‐term survival.4 , 9 The improvement to OAR sparing could be due to the larger number of control points for the NCA and FA techniques, and therefore larger number of opportunities to shield out OAR.
A universal issue arising from arc‐based techniques is the increased dose wash to the lungs, especially the contralateral lung. Pre‐established 3DCRT non‐coplanar techniques enter through the contralateral lung, however, they are generally only from one or two static angles. The FA technique used in this study enters through the entire contralateral lung, exposing more volume to a lower dose. The effect of this can be seen with the increase in the mean lung dose (MLD). Holt et al. report a MLD of 4.2 Gy for single coplanar VMAT which is on par with our result of an average MLD of 4.3 Gy).8 The reduction in MLD for the PA technique is because a smaller volume of lung is receiving low dose. Furthermore, dose is being deposited through non‐coplanar angles with the NCA technique, further reducing the MLD. In a matched analysis study, Palma et al. investigated radiobiological and clinical pneumonitis after both VMAT (RapidArc) and 3DCRT and concluded that there was no difference in the severity of clinical or radiobiological sequelae after treatment.10 Figure 2 displays the reduced dose wash to the contralateral lung with both the PA and NCA techniques when compared to the FA technique.
Figure 2.
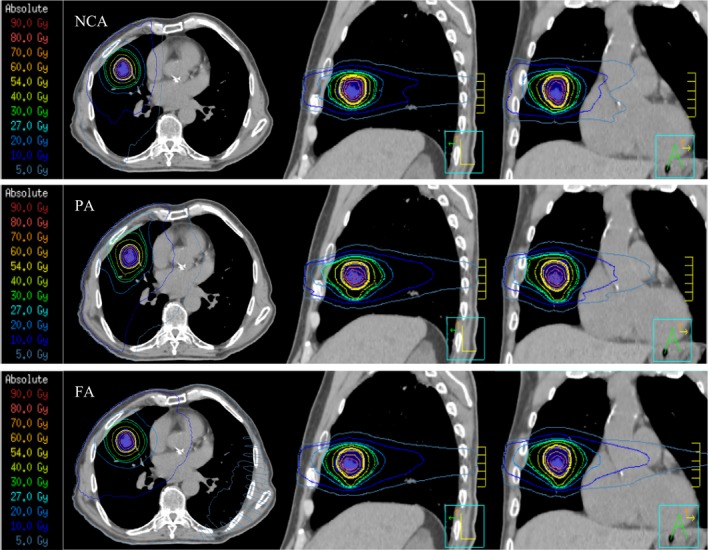
Isodose distribution for the non‐coplanar arc (NCA), partial arc (PA) and fall arc (FA) techniques viewing in the transverse, sagittal and coronal projections (from left to right).
A general concern with intensity modulated treatments for lung cancer is the interplay effect, which is the potential difference in the planned dose to the delivered dose. This is caused by differences in the MLC movements to tumour motion when comparing the static respiratory phase the planning CT captured with the breathing cycle during treatment.11 Several groups have investigated this phenomenon (VMAT or RapidArc) and report that for a single fraction split over two arcs, or >1 treatment fractions, the interplay effect is negligible and the actual delivered dose is within reasonable tolerance to the planned dose.11, 12, 13
Although the NCA provides improved plan quality, these small gains in intermediate dose reduction may be of little importance in the current clinical setting. With R 50% and D 2cm values achieved by the FA technique within acceptable protocol deviations, the advantage of a single coplanar arc may outweigh the improved performance of non‐coplanar techniques. In a clinical setting where patients are generally from an older population and may not tolerate long treatment times and a high emphasis is placed on departmental efficiency, the FA technique provides acceptable treatment plans in a majority of cases and can be delivered in a shorter treatment time. However, if the delivery of highly ablative doses is beneficial to a younger cohort of patients diagnosed with early stage lung cancer, increased reduction in intermediate doses available with the NCA may be of benefit. Furthermore, a coplanar arc technique may better lead to advanced treatment techniques such as dynamic MLC tracking or breath hold techniques where quicker treatment times are a necessity.
Conclusion
The non‐coplanar (NCA) VMAT technique utilising three non‐coplanar partial arcs produced optimal plans that demonstrated better compliance with the dose constraints in the RTOG 1021 protocol when compared to the single arc coplanar techniques. For those tumours entirely encapsulated in lung parenchyma, full single arc coplanar VMAT provided acceptable plans when accepting small deviations to the intermediate dose constraints. Single FA coplanar VMAT is a suitable treatment option for lung SABR when intermediate and OAR doses are within acceptable limits.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
Rhys Fitzgerald would like to acknowledge that this study was undertaken at the Department of Radiation Oncology, Princess Alexandra Hospital between May 2012 and June 2015 and was employed by the institution during this time.
J Med Radiat Sci 63 (2016) 23–30
References
- 1. Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014; 32: 2847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzgerald R, Owen R, Barry T, et al. The effect of beam arrangements and the impact of non‐coplanar beams on the treatment planning of stereotactic ablative radiation therapy for early stage lung cancer. The effect of beam arrangements and the impact of non‐coplanar beams on the treatment planning of stereotactic ablative radiation therapy for early stage lung cancer. J Med Radiat Sci, doi: 10.1002/jmrs.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RTOG . 2011. RTOG 1021: a randomized phase III Study of Sublobar Resection (+/‐ Brachytherapy) versus Stereotactic Body Radiation Therapy in High Risk Patients with Stage I Non‐Small Cell Lung Cancer (NSCLC). Available at http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1021 (accessed 1 May 2015).
- 4. Ong CL, Verbakel WFAR, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: A comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010; 97: 437–42. [DOI] [PubMed] [Google Scholar]
- 5. Purdie TG, Bissonnette J‐P, Franks K, et al. Cone‐beam computed tomography for on‐line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007; 68: 243–52. [DOI] [PubMed] [Google Scholar]
- 6. Elith C, Dempsey SE, Findlay N, Warren‐Forward HM. 2011. An introduction to the intensity‐modulated radiation therapy (IMRT) techniques, tomotherapy, and VMAT. [DOI] [PubMed]
- 7. Verbakel WFAR, Senan S, Cuijpers JP, Slotman BJ, Lagerwaard FJ. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity‐modulated arcs. Radiother Oncol 2009; 93: 122–4. [DOI] [PubMed] [Google Scholar]
- 8. Holt A, van Vliet‐Vroegindeweij C, Mans A, Belderbos JS, Damen EMF. Volumetric‐modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity‐modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011; 81: 1560–7. [DOI] [PubMed] [Google Scholar]
- 9. Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 796–801. [DOI] [PubMed] [Google Scholar]
- 10. Palma DA, Senan S, Haasbeek CJA, Verbakel WFAR, Vincent A, Lagerwaard F. Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three‐dimensional conformal and volumetric‐modulated arc therapy techniques. Int J Radiat Oncol Biol Phys 2011; 80: 506–13. [DOI] [PubMed] [Google Scholar]
- 11. Ong C, Verbakel WFAR, Cuijpers JP, Slotman BJ, Senan S. Dosimetric impact of interplay effect on rapidarc lung stereotactic treatment delivery. Int J Radiat Oncol Biol Phys 2011; 79: 305–11. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen D, Josserand Pietri F, Zinutti M, Khodri M. 2013. Dosimetric impact of interplay effect on a volumetric modulated arc therapy (VMAT) stereotactic lung (SBRT) delivery: Validation using a 6D motion platform and 3D dosimeter. Physica Med 29 (Suppl.1):e29–30. [Google Scholar]
- 13. Stambaugh C, Nelms BE, Dilling T, et al. Experimentally studied dynamic dose interplay does not meaningfully affect target dose in VMAT SBRT lung treatments. Med Phys 2013; 40: 091710. [DOI] [PubMed] [Google Scholar]


