Abstract
Introduction
The aim of this study was to compare various coplanar and non‐coplanar 3‐dimensional conformal radiation therapy (3DCRT) beam arrangements for the delivery of stereotactic ablative radiation therapy (SABR) to patients with early stage lung cancer, based on the dosimetric criteria from the Radiation Therapy Oncology Group (RTOG) 1021 protocol.
Methods
Ten medically inoperable lung cancer patients eligible for SABR were re‐planned using three different coplanar and three different non‐coplanar beam arrangements. The plans were compared by assessing planning target volume (PTV) coverage, doses to normal tissues, the high‐dose conformity (conformity index) and intermediate dose spillage as defined by the D2cm, (the dose at any point 2 cm away from the PTV), and the R50% (the ratio of the volume of half the prescription dose to the volume of the PTV).
Results
Sixty plans in total were assessed. Mean PTV coverage with the prescription isodose was similar between coplanar (95.14%) and non‐coplanar (95.26%) techniques (P = 0.47). There was significant difference between all coplanar and all non‐coplanar fields for the R50% (P < 0.0001) but none for the D2cm (P = 0.19). The seven and nine field beam arrangements with two non‐coplanar fields had less unacceptable protocol deviations (10 and 7) than the seven and nine field plans with only coplanar fields (13 and 8). The 13 field coplanar fields did not improve protocol compliance with eight unacceptable deviations. The 10 field non‐coplanar beam arrangement achieved best compliance with the RTOG 1021 dose criteria with only one unacceptable deviation (maximum rib dose).
Conclusion
A 3DCRT planning technique using 10 fields with ≥6 non‐coplanar beams best satisfied high and intermediate dose constraints stipulated in the RTOG 1021 trial. Further investigations are required to determine if minor protocol deviations should be balanced against efficiency with the extended treatment times required to deliver non‐coplanar fields and if treatment times can be improved using novel intensity modulated techniques.
Keywords: 3‐Dimensional conformal radiation therapy, dosimetry, lung cancer, stereotactic ablative radiation therapy, treatment planning
Introduction
Lung cancer is the fifth most common cancer in Australia but the most common cause of cancer‐related deaths.1 The majority of patients diagnosed with early stage (I/IIa) non‐small cell lung cancer are able to undergo surgical resection. However, 33% of patients present with co‐morbidities that make them unfit for surgery.2 For these patients, local failure following conventionally fractionated external beam radiation therapy is in the order of 40% and treatment involves 20–30 attendances over 4–6 weeks.3 Stereotactic ablative radiation therapy (SABR) has emerged as an alternative treatment option capable of delivering a higher biologically effective dose (BED) resulting in higher local control rates in excess of 85%.2, 4, 5, 6 SABR involves the delivery of hypofractionated schedules of >7.5 Gy per day in 1–5 fractions, typically to a BED10 of >100 Gy.7 SABR utilises advanced immobilisation, motion management and image guidance systems and utilises complex planning techniques to achieve highly conformal, ablative doses with rapid dose fall off outside the planning target volume (PTV).8, 9, 10
Dosimetric parameters such as high‐ and intermediate dose constraints have been established in an attempt to quantitatively describe the quality of SABR plans in regards to dose fall off and conformity. The high‐dose constraint refers to the conformity of the prescription isodose to the PTV, measured using the conformity index (CI). The intermediate dose constraints refer to both the maximum dose to any point 2 cm from the edge of the PTV (D2cm) and the ratio of the volume encompassed by the 50% isodose line (relative to the prescription dose, PD) to the volume of the PTV (R50%). These planning quality metrics have been integrated into Radiation Therapy Oncology Group (RTOG) trial protocols evaluating lung SABR.11, 12, 13 The high‐ and intermediate dose constraints are important dose metrics as a rapid dose fall‐off minimises toxicity.14
Several groups have reported on their experiences with SABR beam arrangements that were required to meet the high‐ and intermediate dose constraints.15, 16, 17 Both Lim et al.15 and Fakiris et al.17 report on using multiple non‐coplanar beams to achieve SABR constraints. However, Richmond et al.16 report that in 17 of 19 cases, seven equidistant coplanar fields produced no more than two minor deviations. This group, however, used high‐ and intermediate dose constraints from the ROSEL study,18 which are more relaxed than those of RTOG. Furthermore, both Lim et al.15 and Fakiris et al.17 did not apply tissue heterogeneity corrections, which when reported by Xiao et al.19 is shown to change the value of an acceptable and unacceptable protocol deviation. This study was therefore designed to compare different beam arrangements (coplanar and non‐coplanar) for lung SABR taking into account heterogeneity correction to determine which best satisfies RTOG 1021 dosimetric criteria.
Methods and Materials
Patient selection
Institutional ethical approval was granted for ten patients that had previously received treatment for lung cancer at the Princess Alexandra Hospital and who met the SABR eligibility criteria to be randomly identified from our local radiation oncology information system database. Patient eligibility was defined as early stage (IA/B or IIA), with the PTV <5 cm in the largest dimension and the gross tumour volume (GTV) >2 cm away from the proximal bronchial tree.
Simulation
All patients had been positioned in the supine position with their forearms above head in a Civco® (Coralville, Iowa) Vac‐lok cushion. All patients had a 4‐dimensional computed tomography (4DCT) scan with 10 respiratory phase bins created. A free breathing scan with a 2 mm slice thickness was obtained with the length including the entire lung volume and exported and registered to the 4DCT in Pinnacle v9.4 (Philips Medical Systems, Stockholm, Sweden). The free breathing scan was nominated as the primary data set for planning purposes. The GTV was contoured on each of the respiratory phases and then combined to create an internal target volume (ITV). The PTV was created by expanding the ITV 5 mm isotropically. In addition, the organs at risk (OAR) were contoured and their constraints are listed in Table 1. The chest wall (CW) was defined as a 2 cm expansion anteriorly, posteriorly and laterally on the ipsilateral lung, excluding the mediastinum, vertebral body and sternum. A 2 cm expansion of the PTV was used to create the D2cm. All reported doses are to a minimum clinically relevant measurable volume of 0.03 cm3.
Table 1.
Organ at risk dose constraints
| Organ | Constraint(s) |
|---|---|
| Spinal cord | 18 Gy < 0.35 cm3 |
| 12.3 Gy < 1.2 cm3 | |
| MPD < 21.9 Gy | |
| Brachial plexus | 20.4 Gy < 3 cm3 |
| MPD < 24 Gy | |
| IVC | 39 Gy < 10 cm3 |
| MPD < 49 Gy | |
| SVC | 39 Gy < 10 cm3 |
| MPD < 49 Gy | |
| Aorta | 39 Gy < 10 cm3 |
| MPD < 49 Gy | |
| Pericardium | 24 Gy < 15 cm3 |
| MPD < 30 Gy | |
| Trachea | 15 Gy < 4 cm3 |
| MPD < 30 Gy | |
| Combined lungs – ITV | 11.4 Gy < 1000 cm3 |
| 10.5 Gy < 1500 cm3 | |
| Oesophagus | 17.7 Gy < 5 cm3 |
| MPD < 25.2 Gy | |
| Rib | 40 Gy < 5 cm3 |
| MPD < 50 Gy | |
| CW | 30 Gy < 30 cm3 (<70 cm3 for tumours on the CW) |
| Skin | 30 Gy < 10 cm3 |
| MPD < 33 Gy |
IVC, inferior vena cava; SVC, superior vena cava; ITV, internal target volume; CW, chest wall; MPD, maximum point dose (defined as ≥0.03 cm3).
Dose prescribing
Patients were planned to receive a PD of 54 Gy in three fractions at the periphery of the PTV. Dose was prescribed so the covering (prescription) isodose fell between 59% and 90% of the absolute maximum dose in the plan as recommended by RTOG.11, 12, 13, 20 PTV coverage was required to be >95% for the PD (PTV54Gy), and >99% for 90% of the PD (PTV48.6Gy). All D2cm and R50% constraints (RTOG 1021) are relative to PTV size (Table 2) and were interpolated as required for each patient. In this study, the CI was calculated using the following equation:
where TVPTV is defined as the total volume of PTV covered by the covering isodose (54 Gy), TV is defined as the total volume of the PTV and PIV is defined as the total volume of the covering isodose in the patient.21 A CI value of ≥0.75 was desirable, with ≥0.65 constituting an acceptable deviation and anything <0.65 was considered unacceptable.
Table 2.
Acceptable dose spillage guidelines from RTOG 1021
| Ratio of prescription isodose volume to the PTV | Ratio of 27 Gy isodose volume to the PTV R50% | Maximum dose at 2 cm from PTV in any direction as % of prescribed dose (PD). D2cm (gy) = % × PD | Percent of lung receiving 20 Gy total of more V20 (%) | PTV volume (cc) | ||||
|---|---|---|---|---|---|---|---|---|
| Deviation | Deviation | Deviation | Deviation | |||||
| None | Acceptable | None | Acceptable | None | Acceptable | None | Acceptable | |
| <1.2 | <1.5 | <5.9 | <7.5 | <50.0 | <57.0 | <10 | <15 | 1.8 |
| <1.2 | <1.5 | <5.5 | <6.5 | <50.0 | <57.0 | <10 | <15 | 3.8 |
| <1.2 | <1.5 | <5.1 | <6.0 | <50.0 | <58.0 | <10 | <15 | 7.4 |
| <1.2 | <1.5 | <4.7 | <5.8 | <50.0 | <58.0 | <10 | <15 | 13.2 |
| <1.2 | <1.5 | <4.5 | <5.5 | <54.0 | <63.0 | <10 | <15 | 22.0 |
| <1.2 | <1.5 | <4.3 | <5.3 | <58.0 | <68.0 | <10 | <15 | 34.0 |
| <1.2 | <1.5 | <4.0 | <5.0 | <62.0 | <77.0 | <10 | <15 | 50.0 |
| <1.2 | <1.5 | <3.5 | <4.8 | <66.0 | <86.0 | <10 | <15 | 70.0 |
| <1.2 | <1.5 | <3.3 | <4.4 | <70.0 | <89.0 | <10 | <15 | 95.0 |
| <1.2 | <1.5 | <3.1 | <4.0 | <73.0 | <91.0 | <10 | <15 | 126.0 |
| <1.2 | <1.5 | <2.9 | <3.7 | <77.0 | <94.0 | <10 | <15 | 163.0 |
Deviation values can be interpolated as required.
Treatment planning
All plans were constructed by a single planner and calculated with Pinnacle v9.4 using the collapsed cone convolution (CCC) algorithm with a grid spacing of 0.25 cm3. The CCC algorithm is a type B algorithm and accounts for changes in lateral electron transport and should therefore be used for lung tumour treatments. As large differences are noted in calculations, the dose prescription and spillage guidelines (Table 2) that were calculated using a type A or water based algorithm (0236) should not be used when using a type B algorithm.8, 19 As a consensus does not exist in the literature, beam arrangements were derived from multiple sources to account for a wide range of recommendations. RTOG recommends the use of seven beams as a minimum, where as a retrospective review of local departmental preference showed nine beams, including two non‐coplanar beams was typical. Furthermore, the use of 10 beams, with six being non‐coplanar is recommended by Ding et al. while the European Organisation for the Research and Treatment of Cancer (EORTC) recommend against non‐coplanar beams due to the associated increase in treatment times.8, 20 Lastly, a 13 field evenly spaced all coplanar arrangement was also investigated to assess if number of coplanar beams, or non‐coplanar beams improves plan quality. Treatment plans investigated in this study therefore included the following: 7 coplanar beams (7C), 9 coplanar beams (9C), 13 coplanar beams (13C), 7 beams including 2 non‐coplanar beams (7NC), 9 beams including 2 non‐coplanar beams (9NC) and 10 beams with 6 or more non‐coplanar beams (10NC). Starting beam angles for a right‐sided tumour where G represents gantry and angle and F represents floor angle were: 7C technique, G180, G210, G240, G270, G300, G330 and G10, all with a floor of 0 (F0), the 9C technique, G180, G210, G240, G270, G300, G330 and G10, G40, G100 all with a floor of 0 (F0), the 7NC technique, G210F0, G330F90, G240F0, G270F0, G300, G330 and G30F90, the 9NC technique G210F0, G330F90, G240F0, G270F0, G300, G330 and G30F90, G40F0 and G100F0. The 10NC used beam angles as referenced by Ding et al.20 and the 13C technique used 13 evenly spaced beams around 360° with a floor of 0. Beam angles were adjusted as necessary to achieve protocol compliance. Angles were mirrored for a left‐sided tumour. All beam angles were checked for clearance on the treatment machine.
Every attempt was made to ensure the 7C field techniques used beam angles entering only through the ipsilateral lung to avoid unnecessary exposure of the contra‐lateral lung to radiation. However, the 9C field technique required beams entering through the contra‐lateral to avoid overlapping beams and consequently increasing the low‐ and intermediate dose wash. Two coplanar beams in the 7C and 9C techniques were made non‐coplanar for the 7NC and 9NC techniques. These were typically superior anterior and superior posterior oblique fields. The 10NC technique used 6 non‐coplanar beams and only introduced more non‐coplanar beams if the D2cm or R50% values were unachievable. Beam weights were manipulated by the planner to achieve isotropic dose fall off in accordance with criteria listed in Table 2, aside from the 13C technique, where each beam was given equal weighting and only adjusted if OAR were over tolerance. PTV coverage below 95% was only allowed if the spinal cord or brachial plexus constraints could not be met.
A structure for creating a block margin was created by shrinking the PTV 2 mm laterally, anteriorly and posteriorly and expanding 2 mm superiorly and inferiorly. The multi‐leaf collimator (MLC) shielding of each beam was then shaped to the structure. This structure was adjusted as necessary to achieve PTV coverage and a prescription isodose between 59% and 90%. The block margin structure results in the MLC shielding the periphery of the PTV such that the prescription isodose falls into the beam penumbra, allowing for a steep dose gradient beyond the PTV. An expansion superior and inferior to the block margin structure was needed to account for limitations of non‐coplanar beams. Even though non‐coplanar beams are used, most of the dose is still delivered across the transverse plane. Dose in all plans was normalised to the maximum dose in the plan which was generally located in the centre of the PTV.
Planning priorities and protocol deviations
Planning priorities were first and foremost, to adhere to the spinal cord and brachial plexus constraint, secondly to meet the high‐ and intermediate dose constraints and lastly to meet the remaining OAR constraints.22 The RTOG 1021 protocol defines plan deviations as either being none or acceptable, with an unacceptable deviation for plans that exceeds the acceptable deviation. With all plans, every attempt was made to achieve the no deviation values (Table 2). However, some situations resulted in unavoidable digression from the priorities. For instance, if an OAR is immediately adjacent to the PTV, then adhering to the maximum dose constraint could be challenging. In this instance the maximum dose to the adjacent OAR can be 105% of the PD and registering as an acceptable deviation. However, all volumetric dose constraints to the structure must still be respected. Furthermore, to avoid clinical toxicity due to overdosing OAR, the dose fall off may be weighted so it is not isotropic, but still falls within the acceptable deviation. Plans were considered clinically suitable if there were no unacceptable deviations from protocol.
To represent protocol deviations with respect to the D2cm and R50% constraints, a scoring system was devised. As intermediate dose constraints are dependent on PTV size, mean D2cm and R50% for each planning technique would not best represent the cohort. Therefore, the absolute difference (if any), from the no deviation constraint was calculated. For example, if the no deviation constraint for D2cm was 30 Gy, and the technique achieved 32 Gy, then this would result in a value of 2. Conversely, if another technique achieved 29.5 Gy at D2cm, this would give a value of −0.5. Therefore, a D2cm or R50% value of 0 represents compliance with a no deviation.
Statistical methodology
Statistical analyses was performed using R statistical software (http://www.r-project.org). To compare coplanar and non‐coplanar arrangements statistical tests for paired data were performed with the normality of the data tested using the Shapiro–Wilk test. The paired Student parametric test has been used for normally distributed data and Wilcoxon signed‐rank non parametric test for non‐normally distributed data. Statistical significance was defined as P ≤ 0.05.
Results
Median patient age was 76, with 70% being male. Median PTV size was 27.5 cm3 (22.8–79.1 cm3). In 50% of cases, the PTV was overlapping the CW. There were between 6 and 8 non‐coplanar beams for the 10NC technique. The 10NC beam arrangement was the only technique where the number of non‐coplanar beams was varied and the resultant plans met the dosimetric criteria. Across all techniques, only 19 of the 60 plans had no more than 2 minor protocol deviations. Total mean monitor units (MU) for the 7C, 9C, 7NC, 9NC, 10NC and 13C were 2867.65, 3019.54, 2919.23, 2996.87, 3225.36 and 3088.51 respectively. The largest difference in MUs was between the 7C and 10NC with a total of 357.71 MU. Plans were delivered at 600 MU/min.
Protocol deviations
A summary of the plan deviations, relative to RTOG 1021 (Table 2) is presented in Table 3. Overall, as the number of beams increased, there were fewer protocol deviations. Only on one occasion was the 10NC beam arrangement unable to produce an acceptable plan. In this case the PTV was overlapping the CW by 6.7 cm3 and the maximum dose could not be lowered to 56.7 Gy (105%), while maintaining PTV coverage.
Table 3.
Beam arrangements and protocol deviations following RTOG 1021 criteria
| 7C | 9C | 13C | 7NC | 9NC | 10NC | |
|---|---|---|---|---|---|---|
| D2cm | ||||||
| None | 1 | 2 | 6 | 2 | 4 | 10 |
| Acceptable | 6 | 8 | 4 | 6 | 6 | 0 |
| Unacceptable | 3 | 0 | 0 | 2 | 0 | 0 |
| R50% | ||||||
| None | 0 | 1 | 1 | 1 | 1 | 5 |
| Acceptable | 5 | 6 | 6 | 6 | 7 | 5 |
| Unacceptable | 5 | 3 | 3 | 3 | 2 | 0 |
| CI | ||||||
| None | 9 | 10 | 10 | 10 | 9 | 9 |
| Acceptable | 1 | 0 | 0 | 0 | 1 | 1 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 | 0 |
| OAR | ||||||
| None | 4 | 4 | 3 | 4 | 4 | 5 |
| Acceptable | 1 | 1 | 1 | 1 | 1 | 4 |
| Unacceptable | 5 | 5 | 5 | 5 | 5 | 1 |
| Total deviations | ||||||
| None | 14 | 17 | 20 | 17 | 18 | 29 |
| Acceptable | 13 | 15 | 12 | 13 | 15 | 10 |
| Unacceptable | 13 | 8 | 8 | 10 | 7 | 1 |
| Clinically suitable plans | 1 | 2 | 2 | 3 | 3 | 9 |
D2cm, dose at any point 2 cm from the PTV; R50%, ratio of the volume of half the PD to the volume of the PTV; CI, conformity index; OAR, organ(s) at risk.
PTV coverage
Table 5 reports the PTV coverage for all beam arrangements. No statistically significant difference was found between PTV coverage for coplanar versus non‐coplanar techniques (P = 0.47 and P = 0.87 for PTV54Gy and PTV48.6Gy respectively). The median prescription isodose, independent of technique was 68% (60.9–87%). Median prescription isodoses values and ranges were 68.4% (63.8–85.7%), 67.9% (62.2–86.9%), 66.8% (63.2–85.8%), 68.2% (63.3–86.4%), 62.6% (60.9–87%) and 68.8% (63.3–87%) for the 7C, 9C, 7NC, 9NC, 10NC and 13C plans respectively. Across all techniques, median prescription isodoses were 65.8% for PTVs not overlapping the CW, and 74.6% for PTVs overlapping the CW.
Table 5.
Mean values for recorded OAR doses for selected OAR categorised by technique and all coplanar and all non‐coplanar techniques combined
| SC | Pericardium | Combined lung – ITV | Ribs | CW | Oesophagus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technique/Constraint (Aim) | 18 Gy (<0.35 cm3) | 12.3 Gy (<1.2 cm3) | MPD (<21.9 Gy) | 24 Gy (<5 cm3) | 30 Gy (<30 cm3) | 10.5 Gy (<1500 cm3) | 11.4 Gy (<1000 cm3) | 40 Gy (<5 cm3) | MPD (<50 Gy) | 30 Gy (<30 cm3) | 17.7 Gy (<5 cm3) | MPD (<25.2 Gy) |
| Coplanar | ||||||||||||
| 7 Fields | 0 | 0.1 | 10.2 | 0.4 | 18.3 | 450.1 | 422.0 | 1.4 | 46.0 | 19.9 | 0.3 | 9.4 |
| 9 Fields | 0 | 0.2 | 10.6 | 0.7 | 17.5 | 472.9 | 438.9 | 1.4 | 45.6 | 19.1 | 0.1 | 12.3 |
| 13 Fields | 0 | 0.3 | 12.3 | 2.0 | 18.3 | 465.5 | 419.1 | 1.5 | 45.5 | 18.8 | 0.0 | 14.0 |
| Non‐coplanar | ||||||||||||
| 7 Fields | 0 | 0.1 | 8.3 | 0.2 | 15.9 | 453.9 | 420.4 | 1.5 | 45.2 | 18.0 | 0.2 | 9.8 |
| 9 Fields | 0 | 0.1 | 8.8 | 0.4 | 15.8 | 451.3 | 416.5 | 1.5 | 45.6 | 17.4 | 0.0 | 12.9 |
| 10 Fields | 0 | 0.2 | 9.9 | 0.5 | 13.6 | 508.9 | 461.7 | 1.2 | 42.6 | 15.4 | 0.0 | 12.4 |
| Mean values | ||||||||||||
| All coplanar | 0 | 0.2 | 11.0 | 1.0 | 18 | 462.8 | 426.7 | 1.4 | 45.7 | 19.3 | 0.0 | 11.9 |
| All non‐coplanar | 0 | 0.1 | 9.0 | 0.4 | 15.1 | 471.4 | 432.9 | 1.4 | 44.5 | 16.9 | 0.0 | 11.7 |
SC, spinal canal; ITV, internal target volume; CW, chest wall; MPD, maximum point dose.
High and intermediate constraints
The recorded D2cm and R50% deviations are reported in Table 4. The D2cm values for combined techniques were 1.14 and 0.66 for all coplanar and all non‐coplanar arrangements respectively with a non‐significant P value of 0.19. Combined techniques recorded a R50% value of 1.06 for coplanar, and 0.62 for non‐coplanar with a significant P value of <0.0001. CI values, different to D2cm and R50% constraints are independent of PTV size and can be reported as the actual value. The mean CI values are reported in Table 5 with no statistically significant difference (P = 0.71).
Table 4.
Mean dose statistics for each technique, categorised into coplanar and non‐coplanar and mean values for all coplanar and non‐coplanar techniques combined with associated P‐values
| PTV54Gy (%) | PTV48.6Gy (%) | Mean lung dose (Gy) | R50% (Deviation) | D2 cm (Deviation) | CI | |
|---|---|---|---|---|---|---|
| Coplanar | ||||||
| 7 Fields | 95.2 | 99.48 | 3.91 | 1.22 | 3.12 | 0.79 |
| 9 Fields | 95.12 | 99.59 | 4.12 | 1.05 | 1.16 | 0.80 |
| 13 Fields | 95.09 | 99.63 | 4.26 | 0.91 | −0.86 | 0.82 |
| Non‐coplanar | ||||||
| 7 Fields | 95.07 | 99.58 | 4.10 | 0.79 | 2.06 | 0.80 |
| 9 Fields | 95.12 | 99.57 | 4.13 | 0.71 | 0.70 | 0.81 |
| 10 Fields | 95.6 | 99.58 | 4.38 | 0.35 | −0.76 | 0.81 |
| Mean values | ||||||
| All coplanar | 95.14 | 99.57 | 4.01 | 1.10 | 1.14 | 0.80 |
| All non‐coplanar | 95.26 | 99.58 | 4.12 | 0.62 | 0.66 | 0.80 |
| P‐value | 0.47 | 0.87 | 0.09 | <0.0001 | 0.19 | 0.71 |
PTV54Gy, percentage of the PTV receiving 54 Gy; PTV48.6Gy, percentage of the PTV receiving 48.6 Gy; D2cm, dose at any point 2 cm from the PTV; R50%, ratio of the volume of half the prescription dose to the volume of the PTV; CI, conformity index.
Organs at risk
Forty‐three percent (n = 26) of plans had OAR tolerance dose violations, independent of technique. The maximum rib dose was responsible for the majority of protocol deviations. Five patients had PTVs overlapping the CW, limiting the rib to a maximum dose of 56.7 Gy. For the 7C, 9C, 7NC, 9NC and 13C, the mean maximum rib dose was on average for these plans, 60.5 Gy, 60.4 Gy, 60.5 Gy, 59.7 Gy and 60.4 Gy respectively, all of which are over the allowed tolerance. When removing patient 8 from the mean, the values for the aforementioned techniques are 61.0 Gy, 60.7 Gy, 60.8 Gy, 60.0 Gy and 60.8 Gy respectively. The 10NC technique had a mean maximum rib dose of 56.7 Gy (excluding patient 8), the allowable tolerance of 105% of the PD. There was however an increase in the lung volume receiving 10.5 Gy and 11.4 Gy for the 10NC compared to other techniques. The results in Table 5 show there was no statistically significant difference in mean lung dose between the coplanar and non‐coplanar techniques (P = 0.68).
Discussion
This study investigated dosimetric factors of various coplanar and non‐coplanar beam arrangements for the treatment of patients eligible for lung SABR with heterogeneity corrections applied.
There were no significant differences in PTV coverage (PTV54Gy or PTV48.6Gy) between beam arrangements, given that they are compulsory protocol requirements and coverage beyond 95% was only improved if the D2cm and R50% constraints maintained a no deviation. The 10NC technique had the greatest PTV54Gy coverage of 95.37%, which is on average 0.5% higher than any other technique.
The prescription isodoses for the plans were kept to between 59% and 90% as recommended by RTOG11, 12, 13 and Ding et al.11, 12, 13, 20 The median prescription isodose value in this study was 68% (62.6–68.7%), which is consistent with previous reports on optimal prescription isodoses for peripheral lung SABR.20 There was a difference in prescription isodoses for plans where the PTV was overlapping the CW. For those plans where PTV was overlapping the CW the prescription isodose could be increased from a median value of 65.8–74.6%. This is due to the more dense soft tissue adjacent to one side of the PTV and the reduced secondary electron range in the tissue.
Furthermore, without comprehensive rib maximum and dose volume constraints, it is considered best practice to minimise the dose where possible.23, 24, 25, 26, 27 Because plans are prescribed in a way so the maximum dose typically falls within the centre of the PTV, those patients whose PTV overlaps a rib could have a maximum dose that is well beyond the 105% of the PD. Only the 10NC technique was able to consistently achieve the maximum rib dose constraint while still achieving 95% coverage of the PTV (with the exception of patient 8). This is likely due to a greater ability to improve shielding of the rib by increasing the number of non‐coplanar beams coupled with a higher prescription isodose attainable on the CW.
The dosimetric study by Lim et al.15 performed without a tissue heterogeneity correction found that increasing the number of non‐coplanar beams increased the possibility of achieving intermediate dose constraints. Our study accounted for tissue heterogeneity and confirmed that an increase in non‐coplanar beams resulted in less protocol deviations. The greatest protocol deviations were found for the 7 and 9 field all coplanar beam arrangements. There were 13 and 8 instances respectively, where these techniques had major protocol deviations (Table 3). The 10NC was found to produce the best plan with only one protocol deviation for the maximum rib dose on patient 8 (59.1 Gy).
The dosimetric advantages of using non‐coplanar beams has previously been reported, however, the results of this study illustrate that non‐coplanar beams are necessary to meet the intermediate and OAR dosimetric constraints for any single plan.28 We demonstrated that there is a statistically significant difference in the volume of the 27 Gy isodose (R50%), for coplanar versus non‐coplanar techniques. Furthermore, there was no case where any technique aside from the 10NC was able to produce a plan with no protocol deviations. Increasing the number of fields (13C) resulted in a slight improvement when compared to either of the coplanar and non‐coplanar 7 and 9 field techniques with fewer protocol deviations in regards to the intermediate dose. However, it was not able to replicate the intermediate dose sparing achievable with the 10NC technique. Figure 1 demonstrates a dose wash of all 6 techniques through the isocentre highlighting the reduction in intermediate dose achievable with the 10NC technique. In theory, increasing the number of beams reduces the dose delivered through each beam, spreading out the low dose and overlapping beams, resulting in a lower intermediate dose. This is the philosophy of SABR and allows for a steep isotropic fall off and is shown clearly in our study.
Figure 1.
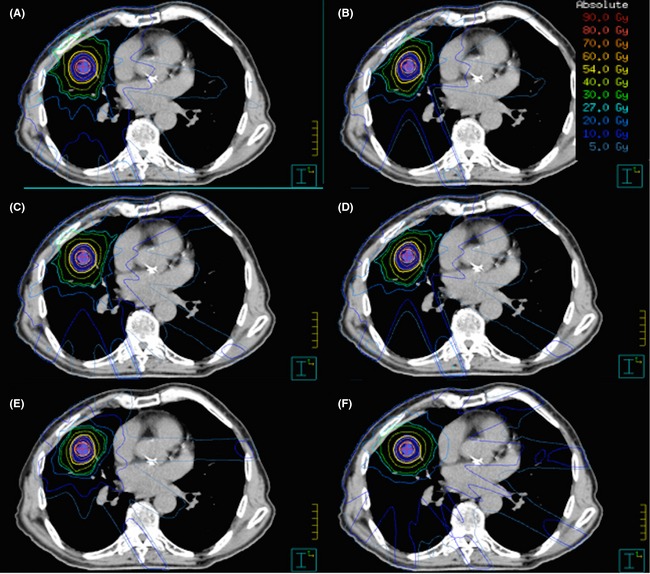
Dose wash through a transverse slice for each of the six techniques. (A) 7C. (B) 7NC. (C) 9C. (D) 9NC. (E) 10NC. (F) 13C.
Our findings differ from those reported by Richmond et al. where in 89.5% of cases a 7 field, all coplanar technique had 2 or less minor deviations.16 They too accounted for heterogeneity correction, but used the less stringent intermediate dose constraints from the ROSEL trial.18 The difference in intermediate dose constraints between RTOG 1021 and ROSEL significantly alters which beam arrangements are deemed acceptable. All the techniques tested in this study had intermediate doses that were acceptable following the ROSEL guidelines. However, following RTOG 1021 criteria we report that a 7 field technique, with beams entering through only the ipsilateral lung produced no plans with equal to, or less than 2 minor deviations. The difference in acceptable D2cm and R50% constraints is believed to be the main reason for disparity.
The EORTC recommend avoiding the use of non‐coplanar beams as this increases the treatment time, and chance of intra‐fraction motion.8 Furthermore, Purdie et al.29 suggest that for treatment times >34 minutes intra‐fraction motion can increase by up to 5 mm. The trade off between small gains in intermediate dose sparing versus increased treatment time and the potential for intra‐fractional error with increasing non‐coplanar beams should be critiqued on an individual patient basis.
Conclusion
Increased use of non‐coplanar beams for 3‐dimensional conformal radiation therapy lung SABR allows for improved control of intermediate dose objectives and produces fewer protocol deviations when correcting for tissue heterogeneity and following RTOG 1021 guidelines. A technique using 10 beams, six or more of which were non‐coplanar provided the greatest compliance to high‐ and intermediate dose constrains, while lowering doses to some critical structures. However, increased non‐coplanar beams results in an increased treatment time that needs to evaluated for each individual patient. The ability to deliver adequate PTV coverage and acceptable intermediate doses using coplanar techniques may be possible with novel techniques such as intensity modulated radiation therapy or volumetric modulated arc therapy and will be the subject of future work.
Conflict of Interest
The authors declare no conflict of interest.
J Med Radiat Sci 63 (2016) 31–40
References
- 1. AIHW, AACR . Cancer in Australia: An overview 2012. AIHW, Canberra, 2012. [Google Scholar]
- 2. Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: 348–53. [DOI] [PubMed] [Google Scholar]
- 3. Qiao X, Tullgren O, Lax I, Sirzén F, Lewensohn R. The role of radiotherapy in treatment of stage I non‐small cell lung cancer. Lung Cancer 2003; 41: 1–11. [DOI] [PubMed] [Google Scholar]
- 4. Takeda A, Sanuki N, Kunieda E, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys 2009; 73: 442–8. [DOI] [PubMed] [Google Scholar]
- 5. Senan S, Lagerwaard F. Stereotactic radiotherapy for stage I lung cancer: Current results and new developments. Cancer/Radiothérapie 2010; 14: 115–18. [DOI] [PubMed] [Google Scholar]
- 6. Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non‐small‐cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009; 27: 3290–6. [DOI] [PubMed] [Google Scholar]
- 7. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high‐dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004; 101: 1623–31. [DOI] [PubMed] [Google Scholar]
- 8. De Ruysscher D, Faivre‐Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high‐dose, high‐precision radiotherapy for lung cancer. J Clin Oncol 2010; 28: 5301–10. [DOI] [PubMed] [Google Scholar]
- 9. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM task group 101. Med Phys 2010; 68: 4078–101. [DOI] [PubMed] [Google Scholar]
- 10. Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non‐small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol 2013; 109: 1–7. [DOI] [PubMed] [Google Scholar]
- 11. RTOG . RTOG 0915 A randomized phase II study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage 1 peripheral non‐small cell lung cancer. 2012. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0915. (accessed 1 January 2015). [DOI] [PMC free article] [PubMed]
- 12. RTOG . RTOG 0813 Seamless phase I/II study of stereotactic lung radiotherapy (SBRT) for early stage, centrally located, non‐small cell lung cancer (NSCLC) in medically inoperable patients. 2012. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813. (accessed 1 January 2015).
- 13. RTOG . RTOG 0236 A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I/II Non‐Small Cell Lung Cancer. 2009. [cited 2014]. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0236. (accessed 1 January 2015).
- 14. Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 2008; 18: 215–22. [DOI] [PubMed] [Google Scholar]
- 15. Lim D, Yi B, Mirmiran A, Dhople A, Suntharalingam M, D'Souza W. Optimal beam arrangement for stereotactic body radiation therapy delivery in lung tumors. Acta Oncol 2010;49: 219–24. [DOI] [PubMed] [Google Scholar]
- 16. Richmond N, Green J, Peedell C, Shakespeare D, Walker C. Dosimetric evaluation of a conformal seven‐field coplanar technique for planning lung stereotactic body radiotherapy. Clin Oncol 2012; 24: e24–30. [DOI] [PubMed] [Google Scholar]
- 17. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early‐stage non‐small‐cell lung carcinoma: Four‐year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009; 75: 677–82. [DOI] [PubMed] [Google Scholar]
- 18. Hurkmans CW, Cuijpers JP, Lagerwaard FJ, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non‐small cell lung cancer: Report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol 2009; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: Stereotactic body radiotherapy of inoperable stage I‐II non–small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2009; 73: 1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding C, Solberg T, Hrycushko B, Xing L, Heinzerling J, Timmerman R. Optimization of normalized prescription isodose selection for stereotactic body radiation therapy: Conventional vs robotic linac. Med Phys 2013; 40: 051705. [DOI] [PubMed] [Google Scholar]
- 21. Gorayski P, Fitzgerald R, Barry T, Burmeister E, Foote M. Volumetric modulated arc therapy versus step‐and‐shoot intensity modulated radiation therapy in the treatment of large nerve perineural spread to the skull base: A comparative dosimetric planning study. J Med Radiat Sci 2014; 61: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RTOG . RTOG 1021: A Randomized Phase III Study of Sublobar Resection (+/‐ Brachytherapy) versus Stereotactic Body Radiation Therapy in High Risk Patients with Stage I Non‐Small Cell Lung Cancer (NSCLC). 2011. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1021. (accessed 1 January 2015).
- 23. Asai K, Shioyama Y, Nakamura K, et al. Radiation‐induced rib fractures after hypofractionated stereotactic body radiation therapy: Risk factors and dose‐volume relationship. Int J Radiat Oncol Biol Phys 2012; 84: 768–73. [DOI] [PubMed] [Google Scholar]
- 24. Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving 30 Gy predicts risk of severe pain and/or Rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 796–801. [DOI] [PubMed] [Google Scholar]
- 25. Kim SS, Song SY, Kwak J, et al. Clinical prognostic factors and grading system for rib fracture following stereotactic body radiation therapy (SBRT) in patients with peripheral lung tumors. Lung Cancer 2013; 79: 161–6. [DOI] [PubMed] [Google Scholar]
- 26. Kong F‐M, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: Atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011; 81: 1442–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pettersson N, Nyman J, Johansson K‐A. Radiation‐induced rib fractures after hypofractionated stereotactic body radiation therapy of non‐small cell lung cancer: A dose– and volume–response analysis. Radiother Oncol 2009; 91: 360–8. [DOI] [PubMed] [Google Scholar]
- 28. Papież L, Timmerman R, Desrosiers C, Randall M. Extracranial stereotactic radioablation physical principles. Acta Oncol 2003; 42: 882–94. [DOI] [PubMed] [Google Scholar]
- 29. Purdie TG, Bissonnette J‐P, Franks K, et al. Cone‐beam computed tomography for on‐line image guidance of lung stereotactic radiotherapy: Localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007; 68: 243–52. [DOI] [PubMed] [Google Scholar]


