Abstract
Aim:
To investigate the potential effects of Y-QA31, a novel dopamine D3 receptor antagonist, as an antipsychotic drug.
Methods:
A panel of radioligand-receptor binding assays was performed to identify the affinities of Y-QA31 for different G protein-coupled receptors. [35S]GTPγS-binding assays and Ca2+ imaging were used to assess its intrinsic activities. The antipsychotic profile of Y-QA31 was characterized in mouse models for the positive symptoms and cognitive deficits of schizophrenia and extrapyramidal side effects with haloperidol and clozapine as positive controls.
Results:
In vitro, Y-QA31 is a dopamine D3 receptor antagonist that is 186-fold more potent at the D3 receptor than at the D2 receptor. Y-QA31 also exhibits 5-HT1A receptor partial agonist and α1A adrenoceptor antagonist activities with medium affinity, whereas it exhibits very little affinity for other receptors (100-fold lower than for the D3 receptor). In vivo, Y-QA31 (10–40 mg/kg, po) significantly inhibited MK-801-induced hyperlocomotion and methamphetamine-induced prepulse inhibition disruption in a dose-dependent manner. Y-QA31 also inhibited the avoidance response and methamphetamine-induced hyperlocomotion with potency lower than haloperidol. Y-QA31 was effective in alleviating the MK-801-induced disruption of novel object recognition at a low dose (1 mg/kg, po). Moreover, Y-QA31 itself did not affect spontaneous locomotion or induce cataleptic response until its dose reached 120 mg/kg.
Conclusion:
Y-QA31 is a selective D3R antagonist that exhibits antipsychotic effects in some animal models with positive symptoms and cognitive disorder and less extrapyramidal side effects.
Keywords: schizophrenia, D3 receptor antagonist, positive symptoms, cognitive deficits, haloperidol, clozapine
Introduction
Schizophrenia is an intractable neuropsychiatric disorder characterized by positive (delusions and hallucinations) and negative (social withdrawal, blunted affect, and mutism) symptoms, as well as cognitive deficits (deficits in attention, working and verbal memory, and executive function). Dopaminergic systems play a dominant role in regulating the development of schizophrenia, and therefore, dopamine receptors have been the primary target for antipsychotic research1. Five types of dopamine receptor subtypes have been cloned, including D1–D5 receptors. Dopamine D1 and D5 receptors are classified as D1-like receptors that activate adenylyl cyclase through coupling with the Gs-protein. Dopamine D2, D3 and D4 receptors are classified as D2-like receptors that inhibit adenylyl cyclase through coupling with the Gi/o-protein2.
Blocking dopamine D2 receptors (D2R) with haloperidol successfully alleviates positive symptoms. However, this treatment shows limited effectiveness on negative and cognitive symptoms3,4. Moreover, haloperidol causes severe side effects, such as extrapyramidal reactions and tardive dyskinesia, which lead to patients' non-compliance to these medications3,4.
In contrast to D2R, dopamine D3 receptors (D3R) are found important for psychotic disorders in the brain regions such as the nucleus accumbens (NAc), the thalamus, and the cortex5, while D3R distribute less in the striatum, the brain region associated with movement function. Therefore, the anti-psychotic compounds targeting D3R cause less extrapyramidal side effects than those targeting D2R. Previous studies have suggested that the prefrontal cortex was associated with the occurrence of negative symptoms as well as the cognitive impairment of schizophrenia. In the D3R-KO mice, cognitive performance was enhanced6,7, suggesting that D3R has a regulating effect in schizophrenia, especially in its cognitive and negative symptoms. In addition, Shaikh and colleagues reported that in a study of 133 Caucasian subjects the Ser9 D3 allele was associated with susceptibility to schizophrenia8. The autopsy study found that the density of D3R in the ventral striatum increased significantly in patients with schizophrenia but remained relatively normal in patients treated with antipsychotic drugs, further suggesting the important role of D3R in the development of schizophrenia. Therefore, D3R has been considered as a potential target for antipsychotic agents.
Several D3R antagonists have been developed. Among them, some selective D3R antagonists, such as S330849 and SB-277011A (D3/D2 selectivity=112)10, are not as effective as the D2R antagonist L741,62611 in reducing positive symptoms in animal models, such as amphetamine-induced hyperlocomotion and the impaired prepulse inhibition (PPI) induced by apomorphine. The preferential D3R antagonist S33138 (D3/D2 selectivity=25) potently alleviates the positive, negative and cognitive symptoms of schizophrenia12. Because of the pharmacological properties of these compounds, however, their effects cannot be attributed solely to D3R, and a selective D3R antagonist is still needed to identify the effects of D3R on the symptoms of schizophrenia.
In the current study, we evaluated Y-QA31, a novel and selective D3R antagonist (D3/D2 selectivity=186) with medium affinity to the 5-HT1A receptor and the α1A adrenoceptor. Because the blocking of D3 versus D2 receptors is debated in the treatment of various symptoms of schizophrenia, we investigated the effects of Y-QA31 in animal models with positive and cognitive symptoms and analyzed its ability to induce catalepsy in mice to assess the potential of Y-QA31 as an antipsychotic drug.
Materials and methods
Materials
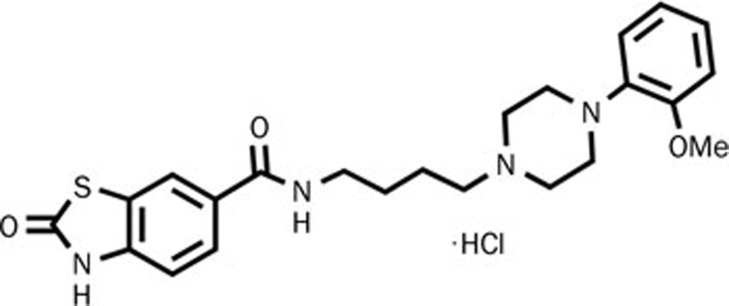
All radioligands used in our experiments were purchased from PerkinElmer Life Sciences (NEN, Boston, MA, USA). Other drugs used in the experiments were obtained from Sigma (St Louis, MO, USA). Y-QA31 (N-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-2-benzothiazolinone-6-carboxamide hydrochloride), the chemical structure as shown in Figure 1, was synthesized at the Beijing Institute of Pharmacology and Toxicology and was dissolved in 25% 2-hydroxypropyl-cyclodextrin (Sigma, St Louis, MO, USA). hD4R-, hD5R-, h5-HT1AR-, h5-HT1BR-, h5-HT1DR-, h5-HT2AR-, h5-HT2CR-, h5-HT5AR-, h5-HT6R-, hA1R-, hA2AR-, hH1R-, hH2R-, hH3R-, hCB1R-, hCB2R-, hα2A-, and hM2R-pcDNA3.1(+) plasmids were purchased from Missouri S&T cDNA Resource Center (Rolla, MO, USA).
Figure 1.
The chemical structure of Y-QA31.
Animals
Male CD1 mice (weighing 18–22 g) and SD rats (weighing 180–220 g) were obtained from the Beijing Animal Center. The mice were maintained in cages with unrestricted access to food and water at 21±1 °C, 60%±5% humidity, and under a 12-h light/dark cycle. All protocols in this study were approved by the Committee of Animal Use and Protection of the Beijing Institute of Pharmacology and Toxicology.
In vitro studies on Y-QA31
Radioligand-receptor binding assays were performed to identify the affinity of Y-QA31 for G protein-coupled receptors, [35S]GTPγS-binding assays and intracellular calcium assays were performed to identify the intrinsic activity of Y-QA31. The protocols were performed according to previous reports3,13,14,15, with some modifications.
Radioligand-receptor binding assays
Radioligand-receptor binding assays were used to identify the affinity of Y-QA31 for G protein-coupled receptors, and the protocols were performed according to previous reports3,13 with some modifications. The reactions were initiated by adding diluted membranes and were incubated at 25 °C, 30 °C or 37 °C for 30–60 min, depending on the individual assay, until binding reached equilibrium (see Table 1 for details). Non-specific binding was defined in the presence of saturating concentrations of the unlabeled compounds listed in Table 1. The concentrations of Y-QA31 ranged from 10−12 to 10−5 mol/L. Radioactivity bound to filters was quantified by liquid scintillation in a MicroScint-20 (PerkinElmer LAS Ltd, Beaconsfield, UK). IC50 values were analyzed by nonlinear regression using Prism 5 (GraphPad Software, San Diego, CA, USA). Ki values were calculated according to Cheng-Pruss of: Ki=IC50/(1+L/Kd), where L is the radioligand concentration, and Kd is the dissociation constant.
Table 1. Details of radioligand-receptor binding assays.
| Receptor | Species | Host cell line or tissue | Radioligand | Unlabeled ligand | Assay buffer | Reaction tempereture/time |
|---|---|---|---|---|---|---|
| D1 | Human | HEK293 | 3H-SCH23390 (1 nmol/L) | (+)-Butaclamol (10 μmol/L) | A | 25 °C/60 min |
| D2 | Human | HEK293 | 3H-Spiperone (1 nmol/L) | Haloperidol (10 μmol/L) | A | 25 °C/60 min |
| D3 | Human | CHO | 3H-Spiperone (1.5 nmol/L) | Haloperidol (10 μmol/L) | A | 25 °C/60 min |
| D4 | Human | HEK293 | 3H-Spiperone (1 nmol/L) | Haloperidol (10 μmol/L) | A | 25 °C/60 min |
| D5 | Human | HEK293 | 3H-SCH23390 (1 nmol/L) | (+)-Butaclamol (10 μmol/L) | A | 25 °C/60 min |
| 5-HT1A | Human | HEK293 | 3H-8-OH-DPAT (1 nmol/L) | Way100635 (10 μmol/L) | H | 25 °C/60 min |
| 5-HT1B | Human | HEK293 | 3H-LSD (6 nmol/L) | 5-HT (10 μmol/L) | B | 25 °C/60 min |
| 5-HT1D | Human | HEK293 | 3H-LSD (10 nmol/L) | 5-HT (10 μmol/L) | B | 25 °C/60 min |
| 5-HT2A | Human | HEK293 | 3H-Spiperone (1 nmol/L) | Clozapine (10 μmol/L) | A | 25 °C/60 min |
| 5-HT2C | Human | HEK293 | 3H-LSD (4 nmol/L) | 5-HT (10 μmol/L) | B | 25 °C/60 min |
| 5-HT5A | Human | HEK293 | 3H-LSD (1 nmol/L) | 5-HT (10 μmol/L) | B | 25 °C/60 min |
| 5-HT6 | Human | HEK293 | 3H-LSD (1 nmol/L) | 5-HT (10 μmol/L) | B | 25 °C/60 min |
| 5-HT7 | Rat | Rat thalamus | 3H-LSD (4 nmol/L) | 5-HT (10 μmol/L) | B | 37 °C/30 min |
| α1A | Human | HEK293 | 3H-Parzosin (1 nmol/L) | Chloropromazine (10 μmol/L) | C | 25 °C/60 min |
| α1B | Human | HEK293 | 3H-Parzosin (1 nmol/L) | Phentolamine (10 μmol/L) | C | 25 °C/60 min |
| α2 | Rat | Brain | 3H-Clonidine (2 nmol/L) | Yohimbine (10 μmol/L) | C | 37 °C/30 min |
| β1 | Human | HEK293 | 3H-DHA (1 nmol/L) | Isopropyl noradrenalin (10 μmol/L) | B | 25 °C/60 min |
| M1 | Human | CHO | 3H-NMS (1 nmol/L) | Atropine (10 μmol/L) | D | 25 °C/60 min |
| M2 | Human | HEK293 | 3H-NMS (1 nmol/L) | Atropine (10 μmol/L) | D | 25 °C/60 min |
| M3 | Human | CHO | 3H-NMS (1 nmol/L) | Atropine (10 μmol/L) | D | 25 °C/60 min |
| M4 | Human | CHO | 3H-NMS (1 nmol/L) | Atropine (10 μmol/L) | D | 25 °C/60 min |
| M5 | Human | CHO | 3H-NMS (1 nmol/L) | Atropine (10 μmol/L) | D | 25 °C/60 min |
| μ | Human | CHO | 3H-Dipnorphine (1 nmol/L) | Naloxone (10 μmol/L) | E | 37 °C/30 min |
| δ | Human | CHO | 3H-Dipnorphine (1 nmol/L) | Naloxone (10 μmol/L) | E | 37 °C/30 min |
| κ | Human | CHO | 3H-Dipnorphine (1 nmol/L) | Naloxone (10 μmol/L) | E | 37 °C/30 min |
| A1 | Human | CHO | 3H-DPCPX (1 nmol/L) | R-PIA (10 μmol/L) | F | 37 °C/30 min |
| A2A | Human | HEK293 | 3H-CGS21680 (10 nmol/L) | NECA (10 μmol/L) | F | 25 °C/60 min |
| H1 | Human | HEK293 | 3H-Pyrilamine (1 nmol/L) | Promethazine (10 μmol/L) | B | 37 °C/30 min |
| H2 | Human | HEK293 | 3H-Tiotidine (3 nmol/L) | Cimetidine (10 μmol/L) | B | 37 °C/30 min |
| H3 | Human | HEK293 | 3H-Histamine (10 nmol/L) | Promethazine (10 μmol/L) | B | 25 °C/60 min |
| CB1 | Human | HEK293 | 3H-CP55940 (1 nmol/L) | WIN 55,212-2 (10 μmol/L) | G | 30 °C/60 min |
| CB2 | Human | HEK293 | 3H-CP55940 (1 nmol/L) | WIN 55,212-2 (10 μmol/L) | G | 30 °C/60 min |
Buffer A (in mmol/L, pH7.4): 50 Tris-HCl, 120 NaCl, 5 KCl, 5 EDTA-Na2·2H2O, 5 MgCl2, 1.5 CaCl2;
Buffer B (in mmol/L, pH7.4): 50 Tris-HCl, 10 MgCl2, 1 EDTA-Na2·2H2O;
Buffer C (in mmol/L, pH7.4): 20 Tris-HCl, 145 NaCl;
Buffer D (in mmol/L, pH7.4): 10 HEPES, 0.1 EDTA-Na2·2H2O;
Buffer E (in mmol/L, pH7.4): 50 Tris-HCl;
Buffer F (in mmol/L, pH7.4): 50 Tris-HCl, 2 MgCl2, 1 ADA;
Buffer G (pH7.4): 10 mmol/L HEPES, 10 mmol/L MgCl2, 0.3 g/L BSA;
Buffer H (in mmol/L, pH7.4): 130 NaCl, 4.8 KCl, 1.2 Na2HPO4, 1.3 CaCl2, 1.2 MgSO4, 10 glucose, 25 HEPES.
[35S]GTPγS-binding assay
To test the intrinsic activity of Y-QA31 at the dopamine D2 and D3 receptors13, cellular membrane protein (35–50 μg) was pre-incubated with unlabeled quinpirole in a reaction buffer containing 3 μmol/L GTP at 30 °C for 30 min. Then, 1.0 nmol/L [35S]GTPγS was added for incubation for another 30 min. Basal [35S]GTPγS binding was measured in the absence of quinpirole. Non-specific binding was measured in the presence of 0.2 nmol/L [35S]GTPγS and 40 μmol/L unlabeled GTPγS. To test the activity of Y-QA31 at 5-HT1A receptors, the procedures for the [35S]GTPγS-binding assay were slightly modified from the reported method16. The intrinsic activity (agonist or antagonist) of Y-QA31 on [35S]GTPγS binding was measured in the absence or presence of quinpirole (10 μmol/L) or 8-OH-DPAT (10 μmol/L, a 5-HT1A receptor agonist). The concentrations of Y-QA31 ranged from 10−10 to 10−5 mol/L. IC50 values were determined as described above.
Ca2+ imaging
The cell line used for Ca2+ imaging was HEK293 cells stably expressing α1A adrenoceptor (HEK293-α1A cells). The cells were washed with HBSS buffer (145 mmol/L NaCl, 5 mmol/L KCl, 1.2 mmol/L NaH2PO4, 1.2 mmol/L CaCl2, 1.3 mmol/L MgCl2, 10 mmol/L glucose, 20 mmol/L HEPES, and 1 mmol/L probenecid; pH 7.4) and co-incubated with 10 μmol/L Fluo-3/AM in extracellular medium for 30 min at 37 °C. A laser confocal microscope was used to measure intracellular calcium. The intrinsic activity (agonist or antagonist) of Y-QA31 (10 μmol/L) for α1A adrenoceptor was tested in the absence or presence of norepinephrine (10 μmol/L).
In vivo studies of Y-QA31
Methamphetamine- or MK-801-induced hyperlocomotion and spontaneous locomotion in mice
The day before testing, mice were placed in spontaneous activity chambers (40 cm×40 cm×40 cm) for a 30 min habituation period. On the testing day, the animals were administered either the vehicle or drug 20 min prior to the injection of methamphetamine (0.5 mg/kg, ip) or MK-801 (0.25 mg/kg, ip). A video system was used to track the movement for 1 h and record the horizontal distance traveled.
Disruption of prepulse inhibition (PPI) by methamphetamine in mice
The procedures used were previously reported by Millan12, with some modifications. Male CD1 mice were administered the drug or vehicle 20 min before the injection of methamphetamine (6 mg/kg, ip) or vehicle and then placed individually in the startle chambers connected via an interface to a computer that controlled auditory stimuli and monitored startle responses. The prepulse parameter test session consisted of 4 trial types: startle stimulus trials (pulse-alone), two different prepulse-alone trial types (prepulse-alone), and two different prepulse trial types (prepulse+pulse). The pulse-alone trial type consisted of the presentation of a 20-ms, 115-dB pulse. The two prepulse-alone trial types consisted of 4-ms, 80-dB or 85-dB prepulse stimuli that were randomly administered. The two prepulse+pulse trial types consisted of 80-dB and 85-dB prepulses, followed 30 ms later (the interstimulus interval; the time between prepulse offset and pulse onset) by the 20-ms, 115-dB startle pulse. After a 5 min acclimation period with 65-dB background noise, 10 of the 4 trial types (a total of 40 trials) were presented in a pseudo-random order with an average inter-trial interval of 15 s for a total of 40 trials. PPI was defined as the percentage of reduction in startle amplitude in the presence of prepulse versus the absence of prepulse. %PPI=100–[(startle amplitude for prepulse+pluse)/(startle amplitude for pluse alone)]×100.
Conditioned avoidance responses (CAR) in mice
Mice were trained to avoid an electric shock (0.16 mA, 5 s) by switching compartments of a shuttle box upon the appearance of a light. The mice were trained for 30 trials per day, and the intertrial interval was 15–30 s, randomly administered. Each trial consisted of a 10-s “light on” period. The mouse received a shock if it did not change compartments, or it avoided a shock by switching during the “light on” period. Testing was performed when each mouse learned to avoid the shock in over 24 trials per day. The drug or vehicle was administered 30 min before testing.
Induction of catalepsy in mice
The procedure was performed according to Reavill10 with some modifications. The tails of the animals were lifted so that the forelimbs could touch the 3-cm-high wooden block top edge. Then, the hind limbs were placed on the desktop gently, and the duration spent in this position was determined. The animals that did not move for more than 30 s were categorized as positive animals. Three independent measurements were obtained, separated by 15-min intervals. The drug or vehicle was administered 30 min before testing.
Novel object recognition (NOR) in mice
The procedure was performed according to Karasawa14 with some modifications. The task was performed in a 40 cm×50 cm×50 cm chamber. All animals were given a habituation session during which they were left to freely explore the environment for 10 min. No objects were placed in the chamber during the habituation trial. 24 h after habituation, training was conducted by placing individual mice into a chamber for 5 min, in which two identical objects (object A1 and A2) were positioned in two adjacent corners, 10 cm away from the walls. In a short-term memory (STM) test given 1.5 h after training, the mice explored the chamber for 5 min in the presence of one familiar and one novel object. All objects presented had similar textures, colors, and sizes, but distinctive shapes. A recognition index calculated for each animal was expressed by the ratio TB/(TA+TB) (TA=time spent exploring the familiar object A; TB= time spent exploring the novel object B). Between the trials, the objects were washed with a 10% ethanol solution. Exploration was defined as sniffing or touching the object with the nose and/or forepaws. The mice were administered Y-QA31, haloperidol, or clozapine at the indicated doses (po, once daily) for four days prior to the testing day. On the testing day (5th day), the mice were injected with MK-801 (0.2 mg/kg, ip) with or without one of the abovemention drugs 30 min before the training session.
Statistical analysis
The data were expressed as the mean±SEM. All statistical analyses were performed using Prism 5 (GraphPad Software Incorporate, CA, USA). The statistical significance of the catalepsy data was calculated by Fisher's exact test. Other data were tested by one-way ANOVA or two-way ANOVA to compare the differences among multiple groups. P<0.05 was considered statistically significant.
Results
Y-QA31 selectively antagonized D3R over D2R
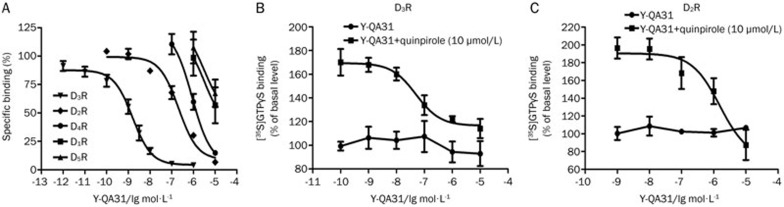
In radioligand-receptor binding assays, Y-QA31 bound to the recombinant dopamine D3R with an IC50 value of 1.74±0.47 nmol/L and a Ki value of 0.28±0.08 nmol/L, suggesting a high affinity for D3R (Figure 2A and Table 2). Y-QA31 also bound to the dopamine D2R; however, the affinity was much lower (IC50=220.2±48.4 nmol/L, Ki=52.1±11.1 nmol/L) (Figure 2A and Table 2). These results indicate that Y-QA31 had 186-fold selectivity for D3R over D2R. In contrast, the affinity of Y-QA31 for D4R was low, with an IC50 value of 877.3±150.1 nmol/L. Additionally, Y-QA31 rarely bound to dopamine D1 and D5 receptors, with IC50 values>10 μmol/L. In [35S]GTPγS-binding assays, Y-QA31 (0.1 nmol/L−10 μmol/L) did not activate D3R, but it antagonized the D3R activation stimulated by 10 μmol/L quinpirole, indicating that Y-QA31 is an antagonist for D3R (Figure 2B). Y-QA31 also inhibited D2R activation stimulated by 10 μmol/L quinpirole (Figure 2B). These results suggest that Y-QA31 selectively antagonized D3 over D1, D2, D4 and D5 receptors.
Figure 2.
Affinity and intrinsic activity of Y-QA31 for dopamine receptors. (A) Competitive binding curves of Y-QA31 to dopamine receptor subtypes determined from radioligand-receptor binding assays. Data are representative of three to five independent experiments. (B and C) Antagonism of Y-QA31 for dopamine D3 and D2 receptors in [35S]GTPγS-binding assays. The data are representative of three independent experiments.
Table 2. IC50 values of Y-QA31 for GPCRs.
| Receptor | IC50 | Receptor | IC50 | Receptor | IC50 |
|---|---|---|---|---|---|
| D1 | >10.0 μmol/L | 5-HT6 | >10.0 μmol/L | μ | >10.0 μmol/L |
| D2 | 220.2±48.4 nmol/L | 5-HT7 | 1.65±1.52 μmol/L | δ | >10.0 μmol/L |
| D3 | 1.74±0.47 nmol/L | α1A | 46.3±10.4 nmol/L | κ | >10.0 μmol/L |
| D4 | 877.3±150.1 μmol/L | α1B | 170.8±62.5 nmol/L | A1 | >10.0 μmol/L |
| D5 | >10.0 μmol/L | α2 | 2.17±2.38 μmol/L | A2A | >10.0 μmol/L |
| 5-HT1A | 11.70±3.87 nmol/L | β1 | >10.0 μmol/L | H1 | 568.5±95.7 nmol/L |
| 5-HT1B | 7.52±6.16 μmol/L | M1 | >10.0 μmol/L | H2 | >10.0 μmol/L |
| 5-HT1D | 677.9±254.1 nmol/L | M2 | >10.0 μmol/L | H3 | >10.0 μmol/L |
| 5-HT2A | 2.40±4.77 μmol/L | M3 | >10.0 μmol/L | CB1 | >10.0 μmol/L |
| 5-HT2C | 839.0±472.2 nmol/L | M4 | >10.0 μmol/L | CB2 | >10.0 μmol/L |
| 5-HT5A | 1.68±0.32 μmol/L | M5 | >10.0 μmol/L |
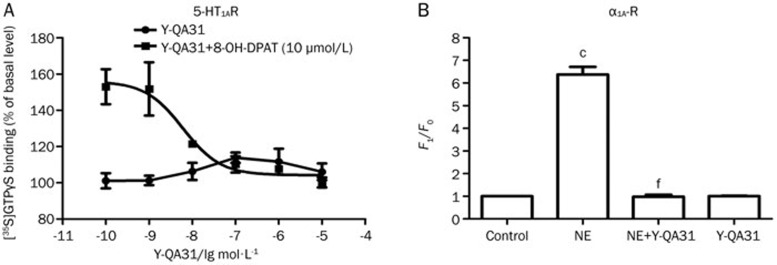
Y-QA31 rarely bound to cholinergic muscarinic receptors (M1, M2, M3, M4 and M5 subtypes), opioid receptors (μ, δ and κ subtypes), 5-HT6 receptor, adenosine A1 and A2A receptors, cannabinoid CB1 and CB2 receptors, histamine H2 and H3 receptors, or β1 adrenoceptors (IC50>10 μmol/L), and it weakly bound to α2 adrenergic receptors, histamine H1 receptors and some subtypes of 5-HT receptors, including 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2C, 5-HT5A and 5-HT7 subtypes (IC50>500 nmol/L, Table 2). Y-QA31 displayed significant affinity for 5-HT1A receptors (IC50=11.70±3.87 nmol/L, Ki=8.36±2.76 nmol/L) and α1A adrenoceptors (IC50=46.3±10.4 nmol/L, Ki=14.8±3.4 nmol/L), although its affinity to 5-HT1A receptors and α1A adrenoceptors was still lower than that to D3R (30-fold for D3R over 5-HT1A receptors and 53-fold for D3R over α1A adrenoceptors, Table 2). Y-QA31 showed a modest affinity for α1B adrenoceptors (IC50=170.8±62.5 nmol/L, Ki=56.9±21.0 nmol/L), but the affinity was much lower (203-fold for D3R over α1B adrenoceptors). Functional assays showed weak partial agonism of 5-HT1A receptors and antagonism of α1A adrenoceptors (Figure 3).
Figure 3.
Antagonism of Y-QA31 for α1A adrenoceptor and partial agonism for 5-HT1A receptor. (A) [35S]GTPγS-binding assay was used for the 5-HT1A receptor. Data are representative of three independent experiments. (B) An intracellular Ca2+ release assay was used for the α1A adrenoceptor. F0: the basis fluorescence intensity that did not receive drug treatment, F1: the peak fluorescence intensity after drug treatment. F1/F0: the ratio of the fluorescence intensity after drug treatment compared to that before drug treatment. The data are representative of five to six independent experiments. cP<0.01 vs control, fP<0.01 vs NE; one way ANOVA followed by Bonferroni's multiple comparisons test. NE, norepinephrine, 10 μmol/L; Y-QA31, 10 μmol/L.
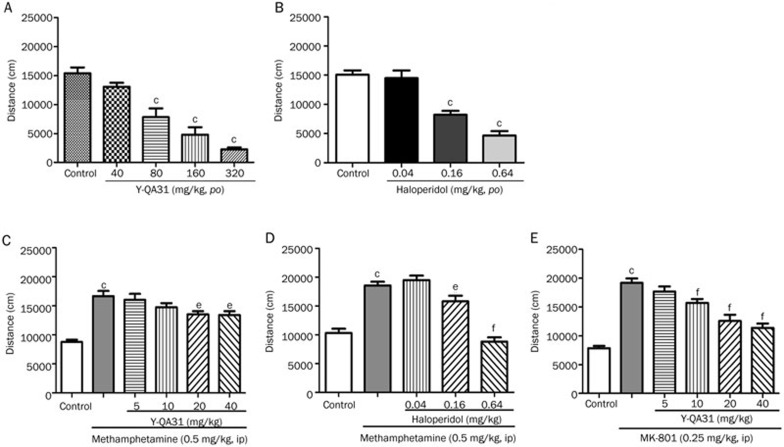
Effects of Y-QA31 on methamphetamine- or MK-801-induced locomotion activity in mice
Antagonism of hyperlocomotion induced by dopamine system agonists has traditionally been used to predict antipsychotic efficacy. Methamphetamine (0.5 mg/kg, ip) induced hyperlocomotion activity was used to test the effect of Y-QA31. Y-QA31 inhibited methamphetamine-induced hyperlocomotion at 20 mg/kg and 40 mg/kg [F(5,80)=14.79; P<0.0001] (Figure 4C). Y-QA31 alone did not influence spontaneous locomotion until its concentration was higher than 80 mg/kg [F(4,38)=28.86; P<0.0001] (Figure 4A). In comparison, haloperidol (0.16 and 0.64 mg/kg, po) significantly decreased methamphetamine-induced hyperlocomotion [F(4,44)=36.45; P<0.0001] (Figure 4D), and, by itself, also inhibited spontaneous locomotor activity [F(3,29)=28.07; P<0.0001] at these doses (Figure 4B).
Figure 4.
Effect of Y-QA31 on methamphetamine- or MK-801-induced hyperlocomotion in mice. (A) Effects of Y-QA31 alone on spontaneous locomotion. (B) Effects of haloperidol alone on spontaneous locomotion. (C) Inhibitory effect of Y-QA31 on methamphetamine-induced hyperlocomotion. (D) Inhibitory effect of haloperidol on methamphetamine-induced hyperlocomotion. (E) Inhibitory effect of Y-QA31 on MK-801-induced hyperlocomotion. n=10–15. cP<0.01 vs the control; eP<0.05, fP<0.01 vs methamphetamine treated group or MK-801 treated group; one-way ANOVA followed by Newman-Keuls test.
Hyperactivity induced by MK-801 was then used to assess the effect of Y-QA31. Figure 4E shows that Y-QA31 (10–40 mg/kg, po) significantly inhibited MK-801-induced hyperlocomotion (0.25 mg/kg, ip) in a dose-dependent manner [F(5,73)=30.98; P<0.0001].
Disruption by Y-QA31 of performance of CAR in mice
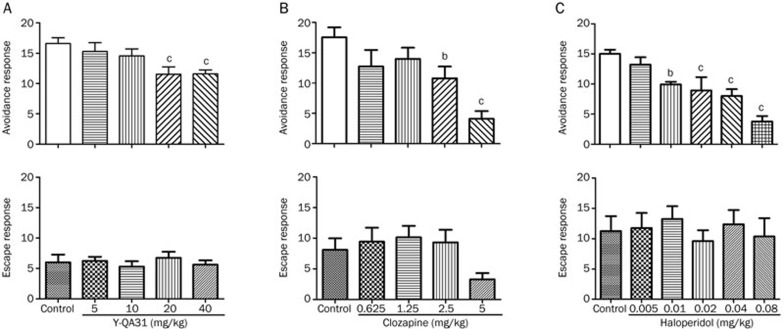
Y-QA31 (20 mg/kg and 40 mg/kg, po) inhibited the avoidance response in a dose-dependent manner [F(4,86)=4.233; P<0.01] without affecting the escape response (Figure 5A). Haloperidol (0.01–0.08 mg/kg, po) significantly inhibited the avoidance response [F(5,72)=10.24; P<0.0001] (Figure 5C) and did not affect the escape response. Clozapine also suppressed the avoidance response at 2.5 and 5 mg/kg [F(4,44)=6.05; P<0.001] (Figure 5B) but had the trend to produce the escape response failure at a high dose (5 mg/kg), probably because of its motor side effect. In this experiment, the effect of haloperidol was more potent, which was consistent with a previous report12.
Figure 5.
Inhibition of the conditioned avoidance response in mice by Y-QA31 (A), clozapine (B), and haloperidol (C). n=15–20; bP<0.05, cP<0.01 vs control; one-way ANOVA followed by Newman-Keuls test.
Effects of Y-QA31 on PPI in mice
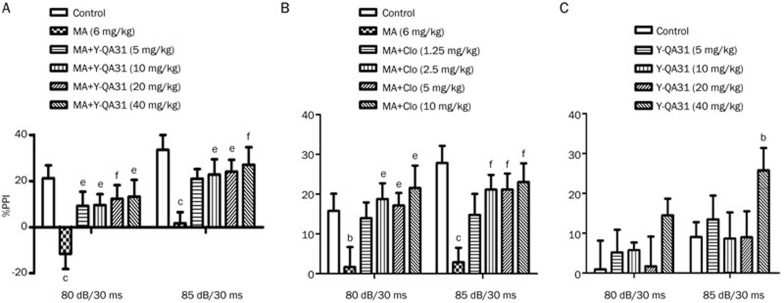
Y-QA31 alone had no influence on the basal %PPI at low doses; Y-QA31 at dosage of 40 mg/kg reduced the %PPI by 85-dB prepulse [dB: F(1,35)=10.666, P<0.01; treatment: F(4,35)=1.5, P<0.05; interaction: F(4,35)=0.333, P>0.05] (Figure 6C). Methamphetamine (6 mg/kg, ip) disrupted PPI, as expected, and Y-QA31 (5–40 mg/kg, po) significantly reversed this disruption of PPI in a dose-dependent manner [dB: F(1,45)=24.418, P<0.01; treatment: F(5,45)=5.121, P<0.01; interaction: F(5,45)=0.101, P>0.05] (Figure 6A). This perturbation of PPI was also abolished by clozapine (1.25–10 mg/kg, po) [dB: F(1,45)=1.741, P>0.05; treatment: F(5,45)=4.497, P<0.01; interaction: F(5,45)=1.416, P>0.05] (Figure 6B), which was consistent with a previous report12.
Figure 6.
Inhibition of the disruption of prepulse inhibition (PPI) elicited by methamphetamine in mice by Y-QA31 (A), clozapine (B), and the effect of Y-QA31 alone on prepulse inhibition (C). n=8–10. bP<0.05, cP<0.01 vs control; eP<0.05, fP<0.01 vs the methamphetamine treated group; two-way ANOVA with repeated measurement followed by Bonferroni post-tests. MA, methamphetamine. Clo, clozapine.
Effects of Y-QA31 on MK-801-induced NOR disruption in mice
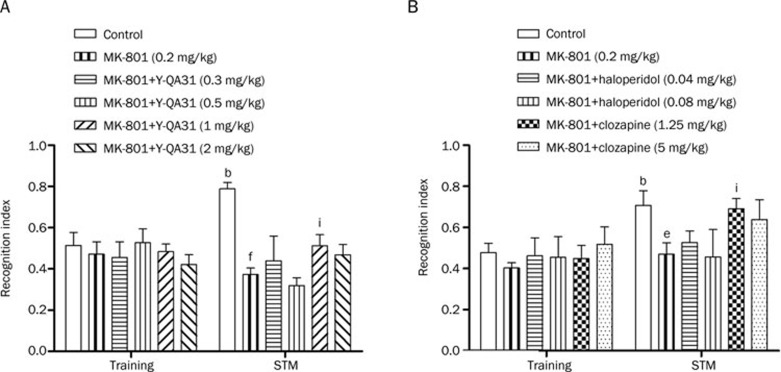
During the training trial, no difference was found among the vehicle, MK-801, or MK-801+drug groups in the total object exploration time (Figure 7A). In the testing session, vehicle-treated mice spent more time exploring the novel object than the familiar object. In contrast, throughout the experiment, MK-801 (0.2 mg/kg, ip)-treated mice spent less time exploring the novel object, indicating that MK-801 treatment resulted in cognitive deficit. The deficit in NOR induced by MK-801 was alleviated by Y-QA31 (1 mg/kg, po) [trial: F(1,54)=0.126, P>0.05; treatment: F(5,54)=5,565, P<0.01; interaction: F(5,54)=4.831, P<0.05] (Figure 7A). In comparison, the atypical antipsychotic clozapine (1.25 mg/kg, po), but not the classical agent haloperidol, reversed the NOR disruption [trial: F(1,28)=19.281, P<0.01; treatment: F(5,28)=1.377, P<0.05; interaction: F(5,28)=0.784, P>0.05] (Figure 7B), which was consistent with a previous report14.
Figure 7.
Effects of 4-d pretreatment with Y-QA31, haloperidol, or clozapine on MK-801-induced object recognition disruption in mice. n=8. bP<0.05 vs saline control in training. eP<0.05, fP<0.01, the MK-801 group vs the saline control in short-term memory (STM) testing; iP<0.05 vs MK-801; two-way ANOVA with repeated measurement followed by Bonferroni post-tests.
Y-QA31 induced catalepsy in mice
Within the effective doses of 5–40 mg/kg (po), Y-QA31 did not elicit catalepsy. Induction of catalepsy by Y-QA31 was observed at 120 mg/kg (Table 3). Clozapine did not induce catalepsy until its dose was over 7.5 mg/kg (Table 3). Haloperidol elicited potent catalepsy at doses up to 0.64 mg/kg (Table 3).
Table 3. Induction of catalepsy in mice by Y-QA31, clozapine, or haloperidol.
| Drug | Dose (mg/kg) | n | Catalepsy (n) |
|---|---|---|---|
| Control | 10 | 0 | |
| Y-QA31 | 40 | 10 | 0 |
| 80 | 10 | 3 | |
| 120 | 15 | 7b | |
| 160 | 10 | 7c | |
| 240 | 10 | 10c | |
| 320 | 10 | 10c | |
| Drug | Dose (mg/kg) | n | Catalepsy (n) |
| Control | 5 | 0 | |
| Clozapine | 2.5 | 5 | 0 |
| 5 | 5 | 0 | |
| 7.5 | 10 | 7c | |
| 10 | 10 | 8c | |
| 20 | 10 | 7c | |
| 40 | 5 | 5c | |
| 80 | 5 | 5c | |
| Drug | Dose (mg/kg) | n | Catalepsy (n) |
| Control | 10 | 0 | |
| Haloperidol | 0.16 | 10 | 0 |
| 0.32 | 10 | 3 | |
| 0.64 | 10 | 8c | |
| 1.28 | 10 | 10c | |
| 2.56 | 10 | 10c |
n=5–15 per value.
bP<0.05,
cP<0.01 vs control; Fisher's exact test.
Discussion
This study demonstrates that Y-QA31 binds with a high affinity to human D3R with 186-fold selectivity for D3R over D2R and over 1000-fold selectivity over D1, D4 and D5 receptor subtypes. Y-QA31 also exhibited 5-HT1A receptor partial agonism (D3/5-HT1A receptor selectivity=30) and α1A adrenoceptor antagonism (D3/α1A-R selectivity=53) with medium affinity, and had very little affinity for other G protein-coupled receptors, such as other subtypes of 5-HT receptors, cholinergic muscarinic receptors (M1–M5), opioid receptors (μ, δ and κ subtypes) and adenosine A1 and A2A receptors. Therefore, the in vitro results showed that Y-QA31 is a highly selective antagonist for D3R and a fairly selective partial agonist for 5-HT1A receptor.
Excess dopamine in the brain caused by dysfunction in the dopamine system is considered to be an important mechanism for positive symptoms of schizophrenia. Methamphetamine induces hyperactivity by facilitating the release of dopamine from presynaptic nerve terminals and inhibiting reuptake, which increases extracellular dopamine levels in the brain. For this reason, methamphetamine-induced hyperlocomotion is used as a classic dopaminergic model for the identification of antipsychotics17. The current data showed that Y-QA31 inhibited methamphetamine-induced hyperlocomotion at doses lower than those used to inhibit spontaneous locomotion. Thus far, most of the evidence indicates that blocking D3R may not inhibit methamphetamine-induced hyperactivity. For example, the selective D3R antagonist S33084 did not alter methamphetamine-induced hyperactivity10,11. Furthermore, knocking down D3R with mRNA antisense oligodeoxynucleotides18 or knocking out the receptor entirely results in increased locomotion19. By comparison, D2R knockout mice do not exhibit the hypolocomotor effects of 7-OH-DPAT, a D2/D3R agonist, which produced decreased locomotion, while D3R knockout and wild-type mice showed similar decreases of locomotor activity response to 7-OH-DPAT20. Y-QA31 exerted low potency in inhibiting hyperlocomotion induced by methamphetamine, whereas the D2R-selective antagonist haloperidol markedly inhibited hyperlocomotion. One possible explanation for the weak effect of Y-QA31 against methamphetamine might be the low D2R occupancy present even at high doses of Y-QA31 (20 mg/kg and 40 mg/kg). In addition, because Y-QA31 binds the α1A adrenoceptor with medium affinity and the α1A adrenoceptor may play a role in hyperactivity21, another explanation for Y-QA31's weak inhibitory action may due to its α1A adrenoceptor antagonism.
In comparison with methamphetamine-induced hyperlocomotion, MK-801-induced hyperlocomotion was significantly inhibited by Y-QA31. Previous studies have also found that MK-801-induced hyperactivity is blocked by inhibiting D3R22, such as using the D3R selective antagonist F1714123. The NMDA receptor antagonists PCP, ketamine, and MK-801 produce similar hyperlocomotion as methamphetamine does24. However, the mechanism underlying MK-801-induced hyperlocomotion is different from methamphetamine-induced hyperlocomotion, possibly because of an increased release of dopamine and associated activation of the dopamine system in the mesolimbic zone caused by the influence of glutamate25. Nakajima et al proposed that D3R may control NMDA receptor signaling by acting on pyramidal cells, either directly at postsynaptic levels in the NAc or indirectly at presynaptic levels in the PFC26.
PPI of the startle reflex is a currently accepted sensorimotor gating model in mammals. Clinical studies have confirmed the phenomenon of disrupted PPI in schizophrenia patients and found that this disruption is related to cognitive deficits such as disordered thought and poor attention27. In animal models, PPI disruption can be induced by dopamine agonists, NMDA receptor blockers and 5-HT2A receptor agonists; pharmacological reversal of PPI disruption indicates antipsychotic potential. Similar to other selective D3R antagonists, SB-277011A and A-43720328, Y-QA31 alone did not affect the basal PPI at low doses but increased the PPI at high doses. In the model of methamphetamine-induced PPI disruption, the inhibitory effect of Y-QA31 (5–40 mg/kg) on PPI deficit indicated its antipsychotic effect and also suggested a role for D3R in PPI dysfunction. Previous findings showed that PPI dysfunction induced by PD128907, a D3R agonist, was reversed by two selective D3R antagonists, SB-277011A and A-691990, but not by raclopride or haloperidol, both of which are D2R antagonists29,30. SB-277011A also reversed PPI disruption induced by social isolation10,30. However, research in genetically modified mice was not consistent with the pharmacological findings. Amphetamine-induced PPI disruption was unaffected in D3R-KO mice but absent in D2R-KO mice31. Therefore, more research is needed to address the involvement of D3R in PPI. Many other receptors are involved in the development of PPI, such as 5-HT1A receptors and α1A adrenoceptor21. Previous studies showed that 5-HT1A receptor antagonists attenuate or abolish the PPI disruption induced by MK-80132, 8-OH-DPAT, or apomorphine33. Considering Y-QA31's modest affinity for the 5-HT1A receptor and α1A adrenoceptor, these receptors may mediate the effects of Y-QA31 on PPI disruption21. Additionally, D2R antagonism may also contribute to the effect of Y-QA31 on PPI at high doses.
Y-QA31 produced weak suppression of CAR compared with the D2R antagonist haloperidol. The behavioral mechanisms of antipsychotic-induced suppression of CAR are still not fully understood, but some evidence suggests that CAR is mediated primarily by D2R in the ventral striatum34. Generally, 50% D2R occupancy is required for CAR suppression35. Considering the low D2R occupancy of Y-QA31, we speculate that this weak effect may occur because the D2R antagonism of Y-QA31 is not strong enough for potent inhibition of CAR. Some evidence suggests that CAR may be at least partially dissociated from D2R blockade. The 5-HT1A and 5-HT2A receptors, the α1A adrenoceptor and NMDA receptor may participate in this process36. For example, the 5-HT1A receptor agonist 8-OH-DPAT produced a moderate suppression of CAR37. We propose that the moderate 5-HT1A receptor partial agonism and α1A adrenoceptor antagonism of Y-QA31 may also contribute to CAR. Thus far, the role of D3R in CAR remains unclear.
Cognitive deficit is considered as a core feature of schizophrenia, and it lacks responsiveness to many current antipsychotic drugs. NOR is a form of visual-recognition memory that is dependent on animals' innate preference to investigate novel objects, which is decreased in schizophrenia patients because of their visual-recognition memory impairments38. The current study found that NOR was disrupted by MK-801, consistent with previous reports39,40. Y-QA31 (1 mg/kg) significantly alleviated the NOR disruption induced by MK-801, suggesting that blocking D3R improved the cognitive and learning dysfunction. It has recently been confirmed that D3R plays an important role in cognitive dysfunctions41,42. D3R knockout mice exhibit increased cognitive flexibility in the attentional set-shifting task and improved retention in a passive-avoidance test43. Moreover, the pharmacological findings also support the potential role of D3R in treating cognitive dysfunctions and negative symptoms. For example, D3R antagonist S33084 enhances cognitive performance in wild-type mice but not in D3R knockout mice44. Other D3 antagonists, such as the highly selective SB-277011A and RGH-1756 and the moderately selective U-99194A, significantly attenuated the learning deficit caused by FG-7142 and scopolamine-induced amnesia41. D2R antagonists, however, produced deleterious effects or no effect on cognition. For example, L741,626 had no pro-cognitive effect in models on social isolation rearing and the social recognition paradigm45,46. Notably, Y-QA31 was effective in improving cognitive symptoms in the NOR model at 10-fold lower doses than the doses used to attenuate positive symptoms. This phenomenon was also observed in the dual D2R/D3R antagonist, amisulpride, which controls both cognitive deficit and positive symptoms in the clinic. The effective dose of amisulpride is 50–100 mg per day when cognitive deficit predominate but 400–800 mg per day when positive symptoms predominate47,48. The mechanism of amisulpride in treating cognitive and positive symptoms with different doses possibly involves its preferential blockade effects of presynaptic or postsynaptic mechanisms and limbic structures. Blocking presynaptic dopamine D2/D3 autoreceptors with low doses of amisulpride induces increased dopaminergic neurotransmission, whereas high doses may block postsynaptic dopaminergic activity. The reason that Y-QA31 treats cognitive deficit and positive symptoms at different doses remains unknown, but preferential binding to presynaptic or postsynaptic receptors is a possible mechanism.
Blockade of D2R in the striatum can induce extrapyramidal motor side effects such as catalepsy, whereas D3R blockade is unlikely to provoke extrapyramidal side effects. Consistent with this idea, Y-QA31 did not induce catalepsy until dosage reached 120 mg/kg, whereas haloperidol (0.64 mg/kg) and clozapine (7.5 mg/kg) significantly induced catalepsy in therapeutic doses. These results further support the observation that D3R blockade is associated with very low incidence of catalepsy. In addition, D3R antagonists such as SB-277011A and S33084 were found to inhibit haloperidol-induced catalepsy49,50, which may predict the therapeutic value of D3R blockade in the treatment of schizophrenia.
Taken together, Y-QA31 exhibits anti-psychotic effects in some animal models of positive symptoms without inducing the extrapyramidal side effects and the inhibition of spontaneous locomotion compared with haloperidol and clozapine. Moreover, similar to clozapine, Y-QA31 alleviates cognitive dysfunction in the NOR model. Given the high affinity of Y-QA31 to D3R, the mechanism of its antipsychotic effect might be associated with D3R blockade, but D2R, as well as α1A adrenoceptor antagonism and 5-HT1A receptor partial agonism, should also be considered. Further pharmacological profiling studies with Y-QA31 could contribute to a more precise understanding of the function of D3R or the 5-HT1A receptor, as well as the α1A adrenoceptor relevant to schizophrenia.
Conclusions
Y-QA31 is a dopamine D3 receptor antagonist that also exhibits partial agonism of the 5-HT1A receptor and antagonism to the α1A adrenoceptor with medium affinity. Y-QA31 produced antipsychotic effects in some animal models of positive symptoms at high doses and pro-cognitive effects at low doses, with low extrapyramidal syndrome. The results of this study further suggest that D3 receptor antagonism is still of therapeutic value for treating symptoms of schizophrenia.
Author contribution
Jin LI, Rui-bin SU and Fei LI designed the research; Xue SUN, Hong-yan GOU, Guan-yi LU and Rui SONG performed the experiments; Ri-fang YANG synthesized the compound; Xue SUN, Fei LI and Ning WU analyzed the data and wrote the draft; Jin LI, Rui-bin SU and Bin CONG revised and edited the manuscript. All authors read and approved the manuscript.
Abbreviations
D3R, dopamine D3 receptor; D2R, dopamine D2 receptor; PPI, prepulse inhibition; CAR, conditioned avoidance responses; NOR, novel object recognition; NMDA, N-methyl-D-aspartate; PCP, phencyclidine; STM, short-term memory.
Acknowledgments
This research was supported by grants from the Innovation of Major New Drug Program, National Science and Technology Major Project of China (No 2011ZX09102-005-01 and 2012ZX09301-003).
References
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci 1994; 15: 264–70. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 2004; 24: 165–205. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Mannoury la Cour C, Novi F, Maggio R, Audinot V, Newman-Tancredi A, et al. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: I. Receptor-binding profile and functional actions at G-protein-coupled receptors. J Pharmacol Exp Ther 2008; 324: 587–99. [DOI] [PubMed] [Google Scholar]
- Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005; 79: 145–55. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 2006; 5: 25–43. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D3 receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev 2000; 31: 277–87. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex 2005; 15: 1016–24. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Collier DA, Sham PC, Ball D, Aitchison K, Vallada H, et al. Allelic association between a Ser-9-Gly polymorphism in the dopamine D3 receptor gene and schizophrenia. Hum Genet 1996; 97: 714–9. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Newman-Tancredi A, Lejeune F, Cussac D, Rivet JM, et al. S33084, a novel, potent, selective, and competitive antagonist at dopamine D3-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther 2000; 293: 1048–62. [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 2000; 294: 1154–65. [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D3-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther 2000; 293: 1063–73. [PubMed] [Google Scholar]
- Millan MJ, Loiseau F, Dekeyne A, Gobert A, Flik G, Cremers TI, et al. S33138 (N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl-acetamide), a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: III. Actions in models of therapeutic activity and induction of side effects. J Pharmacol Exp Ther 2008; 324: 1212–26. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, et al. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol 2012; 17: 259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res 2008; 186: 78–83. [DOI] [PubMed] [Google Scholar]
- Luttgen M, Elvander E, Madjid N, Ogren SO. Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology 2005; 48: 830–52. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Rivet JM, Cussac D, Touzard M, Chaput C, Marini L, et al. Comparison of hippocampal G protein activation by 5-HT1A receptor agonists and the atypical antipsychotics clozapine and S16924. Naunyn Schmiedebergs Arch Pharmacol 2003; 368: 188–99. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996; 379: 606–12. [DOI] [PubMed] [Google Scholar]
- Ekman A, Nissbrandt H, Heilig M, Dijkstra D, Eriksson E. Central administration of dopamine D3 receptor antisense to rat: effects on locomotion, dopamine release and [3H]spiperone binding. Naunyn Schmiedebergs Arch Pharmacol 1998; 358: 342–50. [DOI] [PubMed] [Google Scholar]
- Yarkov AV, Der TC, Joyce JN. Locomotor activity induced by MK-801 is enhanced in dopamine D3 receptor knockout mice but suppression by dopamine D3/D2 antagonists does not occur through the dopamine D3 receptor. Eur J Pharmacol 2010; 627: 167–72. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology 1999; 38: 1389–96. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 1145–58. [DOI] [PubMed] [Google Scholar]
- Leriche L, Schwartz JC, Sokoloff P. The dopamine D receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology 2003; 45: 174–81. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Leriche L, Diaz J, Louvel J, Pumain R. Direct and indirect interactions of the dopamine D3 receptor with glutamate pathways: implications for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol 2013; 386: 107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Levenson S, Ceci A. Characterization of the actions of phencyclidine on midbrain dopamine neurons. NIDA Res Monogr 1989; 95: 255–63. [PubMed] [Google Scholar]
- Adell A, Jimenez-Sanchez L, Lopez-Gil X, Romon T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr Bull 2012; 38: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, et al. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol 2013; 23: 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001; 156: 194–215. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Kohlhaas KL, Browman KE, Jongen-Relo AL, Unger LV, et al. Effect of dopamine D3 antagonists on PPI in DBA/2J mice or PPI deficit induced by neonatal ventral hippocampal lesions in rats. Neuropsychopharmacology 2006; 31: 1382–92. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Drescher K, Gross G, Decker MW, LE R. Evaluation of D3 receptor involvement in psychosis: studies in preclinical animal models. 33th Annual Meeting 2003; Society of Neuroscience.
- Zhang M, Ballard ME, Unger LV, Haupt A, Gross G, Decker MW, et al. Effects of antipsychotics and selective D3 antagonists on PPI deficits induced by PD 128907 and apomorphine. Behav Brain Res 2007; 182: 1–11. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci 1999; 19: 4627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Mackowiak M, Zajaczkowski W, Fijal K, Chocyk A, Czyrak A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology 2000; 23: 547–59. [DOI] [PubMed] [Google Scholar]
- Gogos A, Kwek P, Chavez C, van den Buuse M. Estrogen treatment blocks 8-hydroxy-2-dipropylaminotetralin- and apomorphine-induced disruptions of prepulse inhibition: involvement of dopamine D1 or D2 or serotonin 5-HT1A, 5-HT2A, or 5-HT7 receptors. J Pharmacol Exp Ther 2010; 333: 218–27. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Ericson E, Magnusson O, Ahlenius S. Suppression of conditioned avoidance behavior by the local application of (-)sulpiride into the ventral, but not the dorsal, striatum of the rat. Biol Psychiatry 1990; 28: 297–307. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology 2001; 25: 633–41. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev 1999; 23: 851–62. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Ahlenius S. Antipsychotic-like profile of combined treatment with raclopride and 8-OH-DPAT in the rat: enhancement of antipsychotic-like effects without catalepsy. J Neural Transm Gen Sect 1991; 83: 43–53. [DOI] [PubMed] [Google Scholar]
- Young ME, Sutherland S. The spatiotemporal distinctiveness of direct causation. Psychon Bull Rev 2009; 16: 729–35. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 2007; 184: 31–8. [DOI] [PubMed] [Google Scholar]
- Ahlander M, Misane I, Schott PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology 1999; 21: 414–26. [DOI] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyan I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl) 2005; 179: 567–75. [DOI] [PubMed] [Google Scholar]
- Zimnisky R, Chang G, Gyertyan I, Kiss B, Adham N, Schmauss C. Cariprazine, a dopamine D3-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl) 2013; 226: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Di Marzo V, et al. Enhanced cognitive performance of dopamine D3 receptor "knock-out" mice in the step-through passive-avoidance test: assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol Res 2010; 61: 531–6. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 2012; 37: 770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau F, Millan MJ. Blockade of dopamine D3 receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: similar actions of D1 receptor agonists, but not of D2 antagonists. Eur Neuropsychopharmacol 2009; 19: 23–33. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Di Cara B, Dekeyne A, Panayi F, De Groote L, Sicard D, et al. Selective blockade of dopamine D3 versus D2 receptors enhances frontocortical cholinergic transmission and social memory in rats: a parallel neurochemical and behavioural analysis. J Neurochem 2007; 100: 1047–61. [DOI] [PubMed] [Google Scholar]
- Muller-Spahn F. Current use of atypical antipsychotics. Eur Psychiatry 2002; 17: 377s–84s. [DOI] [PubMed] [Google Scholar]
- Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psycho-pharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997; 280: 73–82. [PubMed] [Google Scholar]
- Millan MJ, Gressier H, Brocco M. The dopamine D3 receptor antagonist, (+)-S 14297, blocks the cataleptic properties of haloperidol in rats. Eur J Pharmacol 1997; 321: R7–9. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Saghy K. The selective dopamine D3 receptor antagonists, SB 277011-A and S 33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol 2007; 572: 171–4. [DOI] [PubMed] [Google Scholar]