Abstract
Aim:
To explore the modulatory effect of desensitized α7-containing nicotinic receptors (α7-nAChRs) on excitatory and inhibitory amino acid receptors in cultured hippocampal neurons and to identify the mechanism underlying this effect.
Methods:
Whole-cell patch-clamp recordings were performed on cultured rat hippocampal neurons to measure α7-nAChR currents and to determine the role of desensitized α7-nAChRs on brain amino acid receptor activity.
Results:
Pulse and perfusion applications of the α7-nAChR agonist choline were applied to induce different types of α7-nAChR desensitization in cultured hippocampal neurons. After a brief choline pulse, α7-nAChR was desensitized as a result of receptor activation, which reduced the response of the A type γ-aminobutyric acid (GABAA) receptor to its agonist, muscimol, and enhanced the response of the NMDA receptor to its agonist NMDA. By contrast, the responses of glycine or AMPA receptors to their agonists, glycine or AMPA, respectively, were not affected. Pretreatment with the α7-nAChR antagonist methyllycaconitine (MLA, 10 nmol/L) blocked the choline-induced negative modulation of the GABAA receptor and the positive modulation of the NMDA receptor. The regulation of the GABAA and NMDA receptors was confirmed using another type of α7-nAChR desensitization, which was produced by a low concentration of choline perfusion. The negative modulation of the GABAA receptor was characterized by choline-duration dependency and intracellular calcium dependency, but the positive modulation of the NMDA receptor was not associated with cytoplasmic calcium.
Conclusion:
Brain GABAA and NMDA receptors are modulated negatively and positively, respectively, by desensitized α7-nAChR as a result of choline pretreatment in cultured hippocampal neurons.
Keywords: α7 nicotinic receptors, desensitization, GABA receptors, NMDA receptors, choline
Introduction
Cholinergic neurons are sparse in the brain, and their projection systems provide broad, diffuse innervation to wide areas of brain1. Nicotinic acetylcholine receptors (nAChRs) have been shown to play important roles in rhythmogenesis and cognitive processes and are implicated in a number of neurological and psychiatric disorders2. Homopentameric α7-containing nAChR (α7-nAChR), which is easily and rapidly desensitized and has high calcium permeability, is expressed abundantly in the brain3 and contributes to the pathogenesis of epilepsy, Alzheimer's disease (AD) and schizophrenia4,5,6. Notably, these brain diseases are also relevant to the major neurotransmitters, GABA and glutamate. Excessive neuronal excitability resulting from the dysfunction of inhibitory signaling mediated by GABA receptors or the redundant performance of NMDA receptors is believed to be the potential mechanism that underlies epileptogenesis7. For AD, the chronic exposure of extrasynaptic NMDA receptors to agonists induces amyloid-β release and promotes neurodegenerative diseases process. A benzodiazepine receptor partial inverse agonist can be applied in the treatment of AD8,9. Thus, it is necessary to explore the relationship between amino acid receptors and α7-nAChRs in the brain.
Brain nAChRs possess resting, activated and desensitized states. Desensitized nAChRs are involved in several biological functions, such as neuroprotection, modulation of synaptic plasticity, and regulation of the release of neurotransmitters10. Studies from our laboratory have shown that neural nAChR desensitization via repeated nicotine pretreatment or the prolonged application of choline can facilitate the activities of muscarinic receptors10; however, the effects of desensitized nAChRs on inhibitory and excitatory amino acid receptors remain unclear.
Therefore, we designed this study to verify whether desensitized α7-nAChRs can affect the functions of amino acid receptors to further understand the role of cholinergic modulation in the brain.
Materials and methods
Animals
Neonatal Sprague–Dawley rats (postnatal day 0–1, SPF grade) were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences (AMMS, Beijing, China). All animals were handled in accordance with the animal care and use guidelines established by the AMMS, which entail minimizing both the number and the suffering of the animals used for the experiments.
Reagents
Choline chloride, muscimol, glycine, N-methyl-D-aspartic acid (NMDA), (±)-α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA), methyllycaconitine (MLA), α-bungarotoxin (α-Bgt), atropine, bicuculline, strychnine, 6,7-dinitroquinoxaline-2,3[1H,4H]-dinoe (DNQX), (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5,10-imine maleate (MK-801), tetrodotoxin (TTX), and 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BATPA) were purchased from Sigma (St Louis, MO, USA). Dulbecco's modified Eagle's medium/Ham's F-12 medium (DMEM/F12), Neurobasal-A medium, B-27 supplement, and fetal bovine serum were purchased from GIBCO (Gaithersberg, MD, USA).
The drugs were dissolved in distilled deionized water (DNQX and MK-801 were dissolved in dimethyl sulfoxide) to prepare a stock solution.
Hippocampal neuron preparation
The brains of the rats were removed and placed in ice-cold phosphate buffer solution (PBS), where they remained throughout the dissection. The PBS contained the following components (in mmol/L): 137 NaCl, 2.7 KCl, 11.6 NaH2PO4, and 1.47 KH2PO4. The solution was adjusted to pH 7.4 using NaOH. The hippocampi were isolated rapidly and cut into small pieces and then incubated for 20 min at 37 °C in PBS containing 2 mg/mL papain. The papain solution was replaced with 5 mL of DMEM/F12 that was supplemented with 10% fetal bovine serum after incubation. Following washing, the digested tissue was gently triturated by suction using a glass pipette. The cells were resuspended in DMEM/F12 that was supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin and 100 IU/mL penicillin in 35-mm-diameter Petri dishes (2 mL/dish) coated with poly-D-lysine and were then maintained in a 5% CO2 incubator at 37 °C. One day later, the medium was replaced with a Neurobasal-A medium that was deprived of serum to limit fibroblast proliferation. This medium contained 2% B-27 supplement and 292 μg/mL glutamine to facilitate the survival of hippocampal neurons in vitro. Thereafter, half of the medium was changed every 3 days. The experiments were performed on neurons that had been cultured for 10–15 days.
Patch-clamp whole-cell current recordings
Whole-cell currents were recorded from the soma of cultured hippocampal neurons. A MultiClamp 700B amplifier and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA, USA) were used for data acquisition and analysis. Patch pipettes (borosilicate glass with a resistance of 2–4 MΩ) were pulled using a pipette puller (Narishige PP-830, Tokyo, Japan). Membrane currents were sampled at a frequency of 5 kHz and low-pass filtered at 2 kHz. The current capacitances of the pipette and the whole cell were minimized. Before series resistance compensation, a whole-cell access resistance of less than 20 MΩ was accepted. The standard extracellular bathing solution (ECS) contained the following components (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 D-glucose, and 10 HEPES. The solution was adjusted to pH 7.4 with NaOH. TTX (0.5 μmol/L) and atropine (0.5 μmol/L) were added to the extracellular solution to block action-potential firing and muscarinic receptors. When NMDA-induced currents were recorded, Mg2+-free ECS plus 3 μmol/L glycine was utilized. Neurons were continuously perfused with extracellular solution (2 mL/min) throughout the duration of the experiments. A gap-free model was used to record the currents after their decomposition into the whole-cell configuration. The pipette solution contained the following components (in mmol/L): 120 CsCH3SO3, 30 CsCl, 2 MgCl2, 4 MgATP, 2 EGTA, 10 HEPES, which was adjusted to pH 7.2 with CsOH. The agonists were focally applied onto the soma of neurons with a puff pipette that was connected to Picospritzer III (Parker Hannifin, Hollis, NH, USA). The puff pipette consisted of three microtubes, two of which were filled with different drugs that were dissolved in the ECS. Pressure was applied to every cell, which did not produce an electrical response. Bath-applied drugs were diluted at final concentrations in the ECS and were delivered to the cell through a perfusion system. All experiments were performed at room temperature (22–25 °C).
Data analysis
Statistical analyses were conducted using SPSS 13.0 (SPSS, Chicago, IL, USA). Averaged data are presented as the mean±the standard error of the mean (SEM), and statistical significance was tested using Student's t-test or an ANOVA, when appropriate. P values of <0.05 were considered to be statistically significant. The decay phases of the current tracings were analyzed with the following mono-exponential equation using the Clampfit 9.2: I=[(IP–IS)exp−t/τ]+IS, where I=current amplitude at time t, IP=peak current, IS=steady-state current, and τ=decay rate constant. Curves were fitted to the logistic equation using Origin 8.1 (Microcal, Northampton, MA, USA).
Results
Desensitization properties of α7-nAChRs
A rapid pulse of choline (10 mmol/L for 1 s) delivered onto the soma of hippocampal neurons clamped at −80 mV could elicit an inward current that rapidly returned to baseline levels before the end of the choline application. Choline-evoked currents could reversibly or irreversibly be blocked by two specific α7-nAChR antagonists, MLA (10 nmol/L) or α-Bgt (100 nmol/L), respectively (Figure 1A and 1B).
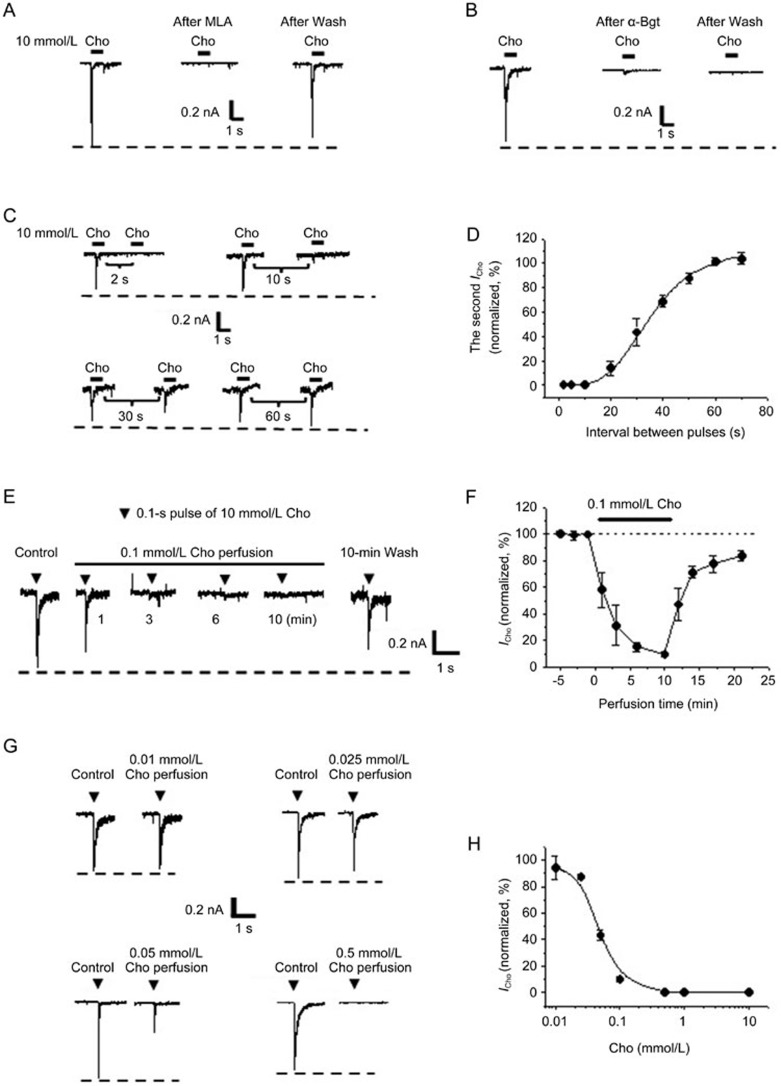
Figure 1.
Desensitization properties of the α7-nAChR in cultured hippocampal neurons. The choline-induced (10 mmol/L for 1 s) response was reversibly blocked by methyllycaconitine (MLA, 10 nmol/L) (A) or irreversibly blocked by α-bungarotoxin (α-Bgt, 100 nmol/L) (B). (C) The currents evoked by paired pulse of choline at different intervals. (D) Curve showing the recovery from desensitization of the α7-nAChR after a choline pulse (n=6 cells). (E) Test choline-induced currents (10 mmol/L for 0.1 s) obtained before, during 0.1 mol/L choline perfusion, and 10 min after agonist washout. (F) Time course plot showing the change in choline-induced currents before, during, and after 0.1 mol/L choline perfusion (n=6 cells). 0 min is the beginning of choline perfusion. (G) Test choline-induced currents obtained before and 10 min after the perfusion of varying concentrations of choline. (H) The plot showing concentration dependence of the desensitization of the α7-nAChR that is induced by choline perfusion. Cho, choline; ICho, choline-evoked current. Holding potential was −80 mV. Data are shown as the mean±SEM.
Here, two methods were used to produce α7-nAChR desensitization. First, we applied a fast pulse of a high concentration of choline (10 mmol/L) to produce classical desensitization. After current stimulation, which is referred to as receptor activation, the rapid decay was represented as receptor desensitization. The decay rate constant (τ) was determined to be approximately 91 ms. To measure recovery from desensitization, two 1-s pulses of choline were applied at different intervals. The intervals between each paired-pulse were longer than 120 s. As shown in Figure 1C, the second current was not observed (representing complete desensitization) when the interval was less than 10 s and partially recovered to the level of the first current (representing partial desensitization) when the intervals were between 20 s and 50 s. When the interpulse intervals were 20, 30, 40, and 50 s, the amplitudes of the second response were 14%±6%, 44%±11%, 69%±5%, and 88%±4%, respectively, of the first response. The second choline-evoked current exhibited a completely recovered response when the interval was 60 s (102%±3% of the first response). A plot of the normalized peak amplitude against the intervals between the paired-pulse was used to calculate the 50% recovery time (t1/2). The data fit well using a logistic regression (r2=0.97), and the values of t1/2 were calculated to be 34.4±4.2 s (Figure 1D).
Then, a low concentration of choline (0.1 mmol/L) was continuously perfused (10 min) to induce desensitization without apparent receptor activation, while the response of the receptor was evaluated with a 0.1-s pulse of 10 mmol/L choline. In this experiment, the perfusion of 0.1 mmol/L choline had little effect on the baseline current. When choline was perfused for 1, 3, 6, and 10 min, the amplitudes of the test currents decreased to 58%±13%, 32%±15%, 15%±3%, and 10%±2%, respectively, of the control. The rundown of the choline-induced response was gradually recovered after the removal of bath-applied agonists (Figure 1E). By varying the concentrations of choline from 0.01 to 10 mmol/L, the sustained perfusion of choline was used to study the concentration dependence of desensitization. After 10 min of bath application of choline, the response to the test pulse began to decrease from 0.025 mmol/L and disappeared completely at concentrations greater than 0.5 mmol/L (Figure 1G). The analysis of the concentration dependence with logistic regression (r2=0.99) yielded an IC50 for a desensitization of 46.6 μmol/L (95% confidence interval is 44.8 to 48.4 μmol/L; Figure 1H).
Desensitized α7-nAChR modulates amino acid receptors
The modulatory effects of the desensitized α7-nAChR on GABAA, glycine, AMPA, and NMDA receptors were investigated in this experiment. A puffer pulse of muscimol (10 μmol/L for 1 s), glycine (100 μmol/L for 1 s), AMPA (10 μmol/L for 5 s), and NMDA (100 μmol/L for 5 s) elicited four types of fast-activating, inward currents in these four receptors, respectively, and all could be reproducibly elicited at 120 s intervals. The blockade of these receptors by bicuculline (10 μmol/L), strychnine (1 μmol/L), DNQX (10 μmol/L), and MK-801 (10 μmol/L) confirmed that these currents were mediated by GABAA, glycine, AMPA, and NMDA receptors, respectively (Figure 2A and 2B).
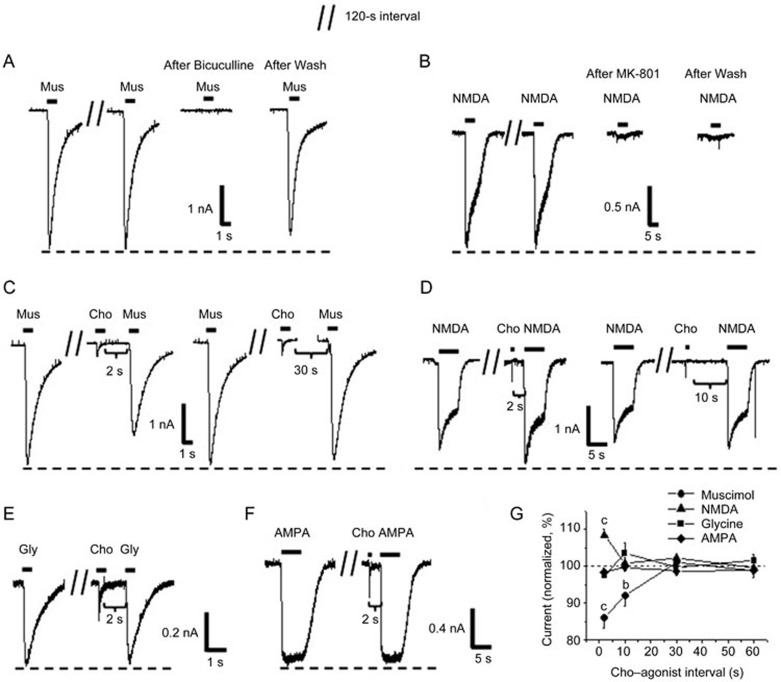
Figure 2.
Rapid choline pulse selectively modulates amino acid receptors in cultured hippocampal neurons. Stable muscimol-evoked (10 μmol/L for 1 s) currents or NMDA-induced currents (100 μmol/L for 5 s) were elicited at 120-s intervals and blocked by the GABAA receptor antagonist, bicuculline (A), or the NMDA receptor antagonist MK-801 (B), respectively. Sample responses to muscimol (C), NMDA (D), glycine (E) and AMPA (F) were obtained before and after a 1-s choline pulse (10 mmol/L) at different intervals. (G) Compiled results show the time course of recovery for muscimol- (n=8 cells), NMDA- (n=8 cells), glycine- (n=6 cells) or AMPA- (n=6 cells) induced responses after the choline pulse. Data are shown as the mean±SEM. bP<0.05, cP<0.01 vs control that was induced by a corresponding agonist, one-way ANOVA, LSD post hoc test. Cho, choline; Mus, muscimol; Gly, glycine. Holding potential was −80 mV.
We first studied the effects of classical desensitization that was induced by a high concentration of choline. In this experiment, a brief pulse of choline (10 mmol/L for 1 s) prior to the application of muscimol caused a significant reduction in the peak amplitudes of the muscimol-evoked currents when the intervals between the termination of the choline pulse and the initiation of muscimol administration were 2 s and 10 s. The reduction was cancelled when the interval was longer than 30 s (Figure 2C). The choline-evoked currents immediately returned to a baseline level before the application of muscimol. Thus, the inhibition was independent of whether there were any changes to the baseline current induced by choline. The opposite result was obtained in the experiment that was performed on the NMDA receptor. The amplitude of the NMDA response was increased after a choline pulse when the interval between choline and NMDA was 2 s, and the enhancement disappeared when the interval was longer than 10 s (Figure 2D). Finally, we failed to record any detectable changes in the amplitudes of glycine or AMPA-evoked currents using the same methods (Figure 2E and 2F).
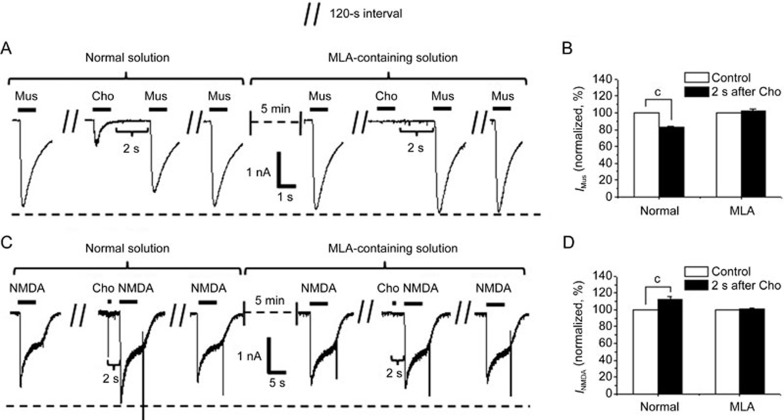
Then, MLA was applied to study whether these effects were mediated by the α7-nAChR. We confirmed that the interval between choline and muscimol or NMDA was 2 s. We then compared the effects of the choline pulse on the two receptors before and after MLA perfusion. Bath-applied MLA (10 nmol/L) abolished the reduction in the amplitudes of the muscimol-evoked currents (82%±2% of the control level in the normal solution vs 102%±5% of the control level in the MLA-containing solution) (Figure 3A and 3B) and the enhancement in the amplitudes of the NMDA-evoked currents (113%±3% of the control level in the normal solution vs 101%±1% of the control level in the MLA-containing solution) (Figure 3C and 3D). The evidence provided by the use of the antagonist MLA indicates a possible role for α7-nAChR in these effects.
Figure 3.
MLA abolishes the modulation of the GABAA or NMDA receptor by the choline pulse in cultured hippocampal neurons. Typical muscimol- (A) or NMDA- (C) evoked currents obtained before or after a choline pulse, as well as after a wash, in normal or 10 nmol/L MLA-containing solution. Corresponding bar graphs show the changes in muscimol- (B, n=5 cells) and NMDA- (D, n=5 cells) induced responses before and after a choline pulse in different bath solutions. Data are shown as the mean±SEM. cP<0.01 vs control in corresponding solution, Student's t-test. Cho, choline; Mus, muscimol; IMus, muscimol-evoked current; INMDA, NMDA-evoked current. Holding potential was −80 mV.
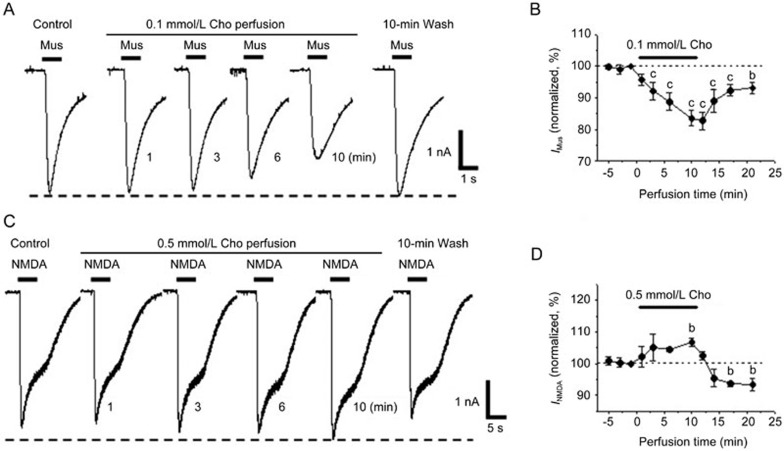
Finally, we perfused low concentrations of choline to test the effect of the α7-nAChR on the GABAA or NMDA receptor. When 0.1 mmol/L choline was perfused for 1, 3, 6, and 10 min, the amplitudes of the muscimol-induced currents decreased to 96%±2%, 92%±2%, 89%±3%, and 84%±3%, respectively, of the control. Furthermore, the reduction of the muscimol-evoked current was partially recovered after the removal of bath-applied choline (Figure 4A and 4B). For the NMDA receptor, 0.1 mmol/L choline perfusion could not induce significant changes in the NMDA-evoked current. Conversely, when the concentration of choline perfusion was greater than 0.5 mmol/L, we observed the reversible potentiation of the NMDA-evoked response (Figure 4C and 4D). Because low concentration choline perfusion induced desensitization without receptor activation, these data demonstrate that the effects on GABAA and NMDA receptors are associated with desensitized α7-nAChR.
Figure 4.
Chronic choline perfusion selectively modulates the GABAA or NMDA receptor in cultured hippocampal neurons. Muscimol- (A) and NMDA- (C) evoked currents obtained before and during 0.1 or 0.5 mmol/L choline perfusion, and 10 min after agonist washout. Time course plots indicate the changes in muscimol- (B, n=7 cells) and NMDA- (D, n=5 cells) induced currents before, during, and after choline perfusion. 0 min is the beginning of choline perfusion. Data are shown as the mean±SEM. bP<0.05, cP<0.01 vs control, one-way ANOVA, LSD post hoc test. Cho, choline; Mus, muscimol; IMus, muscimol-evoked current; INMDA, NMDA-evoked current. Holding potential was −80 mV.
Mechanisms underlying the modulation of the GABAA or NMDA receptor by the desensitized α7-nAChR
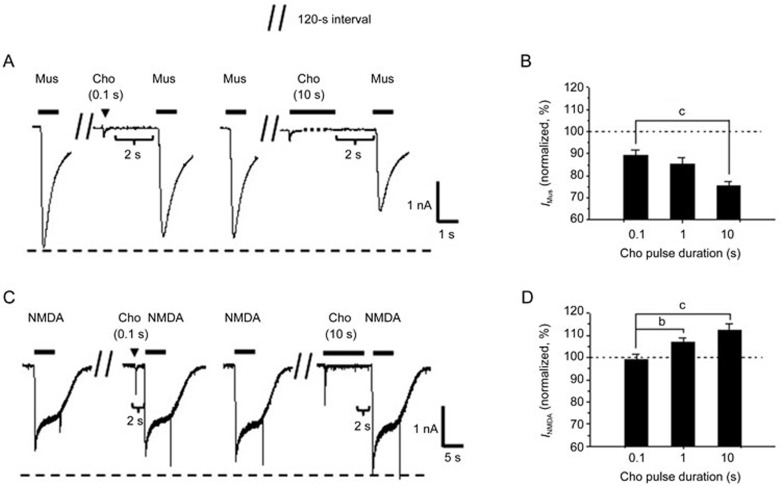
As the duration of agonist exposure influenced the recovery of the nAChRs from desensitization, we studied the effect of duration of the choline pulse on the above-mentioned modulatory actions. By varying the duration of the choline pulse at a similar interval of 2 s, we observed that both the inhibition of the GABAA receptor and the potentiation of the NMDA receptor increased in the presence of a prolonged duration of exposure to choline. At pulse durations of 0.1, 1, and 10 s in the same cell, the amplitudes of the muscimol-evoked current were 89%±2%, 85%±3% and 75%±2% of the control level, respectively (Figure 5A), and the amplitudes of the NMDA-evoked current were 99%±2%, 107%±2% and 112%±3%, respectively, of the control level (Figure 5C).
Figure 5.
Prolongation of choline duration increases the modulation of the GABAA or NMDA receptor by the desensitized α7-nAChR in cultured hippocampal neurons. Typical muscimol- (A) and NMDA- (C) evoked currents obtained before and after a 1-s or 10-s choline pulse. Corresponding bar graphs indicate the changes in muscimol- (B, n=5 cells) or NMDA- (D, n=5 cells) evoked responses after 0.1-, 1-, and 10-s choline pulses, respectively. Data are shown as the mean±SEM. bP<0.05, cP<0.01 vs the current after 0.1-s duration of choline, one-way ANOVA, LSD post hoc test. Cho, choline; Mus, muscimol; IMus, muscimol-evoked current; INMDA, NMDA-evoked current. Holding potential was −80 mV.
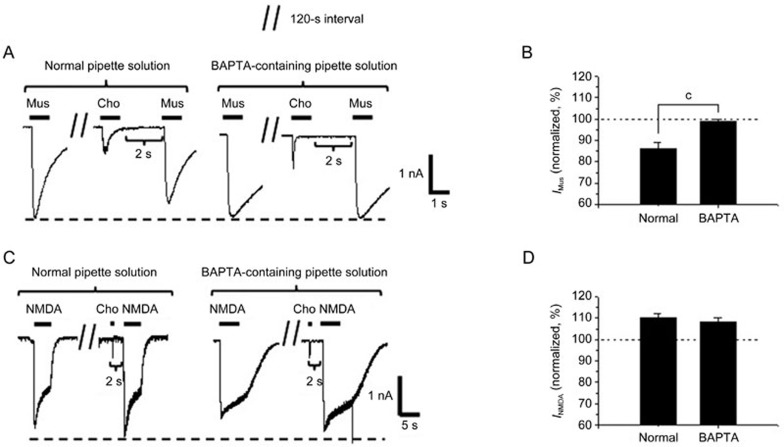
α7-nAChR is characterized as having a high Ca2+ permeability. Thus, cytoplasmic Ca2+ influx via the α7-nAChR may be involved in the modulatory action of choline on the GABAA or NMDA receptor. The Ca2+ chelator BAPTA (10 mmol/L) was injected into a pipette solution to test the role of intercellular Ca2+. Here, choline elicited an inward current in a BAPTA-containing pipette solution, which was similar to what was observed using the normal intracellular solution. The introduction of BAPTA into neurons through a pipette abolished the inhibition in the amplitudes of muscimol-induced responses (99%±1% of the control level in the BAPTA-containing pipette solution) (Figure 6A) but did not change the potentiation in the amplitudes of NMDA-induced responses (108%±2% of the control level in the BAPTA-containing pipette solution) (Figure 6C). These data demonstrate that cytoplasmic Ca2+ has a role in the choline's inhibition of the GABAA receptor but does not participate in potentiation of the NMDA receptor.
Figure 6.
Intracellular calcium participates in desensitized α7-nAChR-mediated depression of the GABAA receptor but is not involved in the potentiation of the NMDA receptor in cultured hippocampal neurons. Typical muscimol- (A) or NMDA- (C) evoked currents recorded before and after a choline pulse in normal or 10 mmol/L BAPTA-containing pipette solution. Corresponding bar graphs indicate the changes in muscimol- (B, n=5 cells) and NMDA- (D, n=5 cells) evoked currents after a choline pulse in varying pipette solutions. Data are shown as the mean±SEM. cP<0.01 vs the current after choline in normal pipette solution, Student's t-test. Cho, choline; Mus, muscimol; IMus, muscimol-evoked current; INMDA, NMDA-evoked current. Holding potential was −80 mV.
Discussion
The present study demonstrates that the desensitization of α7-nAChR by choline selectively modulates the functions of amino acid receptors (ie, α7-nAChR desensitization reversibly depresses the GABAA receptor and enhances the NMDA receptor but does not affect glycine or AMPA receptor). Moreover, the depression of GABAA is associated with the duration of choline administration and the level of intracellular Ca2+. The NMDA response is also enhanced, which depends on the duration of choline but is independent of cytoplasmic Ca2+. As GABAA and NMDA receptors in the hippocampus are important for network oscillations and cognitive processes, these actions may enlarge the effect of α7-nAChR desensitization on the regulation of the excitatory rhythm of local neuronal circuits and the modulation of synaptic plasticity.
Many studies have proven the idea that cholinergic neurotransmission in the brain is primarily neuromodulatory1. As one part of the cholinergic system, nAChRs act through their natural agonists: ACh and choline. Choline is a selective agonist of the α7-nAChR. In our study, the induction of a fast activating/desensitizing current, which is sensitive to MLA and α-Bgt, by choline confirmed that choline-evoked currents were generated through the α7-nAChR. The modulatory effects of choline depend mainly on its concentration. The high frequency of ACh release and its enzymolysis by nearby acetylcholine esterase would likely produce choline at relatively high concentrations. In this condition, α7-nAChR is first activated and then desensitized with a subsequent recovery after agonist removal. This process is regarded as “classical desensitization”11. Here, a paired pulse was used to study recovery from the desensitization of the α7-nAChR that was induced by a high concentration of choline. After the first pulse, the second choline-induced response was not observed when the interval was less than 10 s, which was partially recovered when the interval was between 20 s and 50 s and was completely recovered when the interval was 60 s. The three periods represent complete desensitization, partial desensitization and full recovery of the α7-nAChR. Conversely, free extracellular choline in the cerebrospinal fluid is approximately 5–10 μmol/L, whereas this level can be elevated (3–4 fold) under several conditions, such as ischemia, stroke, hypoxia, and substantial plasma membrane damage12. The basal level of choline is far lower than the efficacious concentration that is necessary to activate α7-nAChRs; however, it is sufficient to desensitize α7-nAChRs. This type of desensitization is a slow process, has a high affinity for agonists and can be produced by long term perfusion of low concentrations of choline11. Thus, choline has a central physiological action that mainly depends on desensitizing α7-nAChRs.
Brain nAChR desensitization protects cells against excessive stimulation and participates in several types of neural activities13. As a particular functional state, and according to our previous reports, the desensitization of nAChRs not only results in its loss of gating ability to open channels but also adjusts the activities of other receptors, (ie, it facilitates the function of muscarinic receptors14 or dopamine receptors15). This experiment suggests that desensitized α7-nAChRs depresses the response of the GABAA receptor to its agonist and facilitates the response of the NMDA receptor. The main finding was obtained via three experiments. We first examined the change in the muscimol or NMDA-induced response during recovery from desensitization after a 10 mmol/L choline pulse and then employed MLA to determine a possible role for α7-nAChR and finally perfused a low concentration of choline to study the role of receptor desensitization. Our conclusion concerning the depression of GABAA receptor activity in our report is similar to that of a previous study involving hippocampal slices and knockout mice16. They observed that the activation of cholinergic fibers by electrical stimulation could reduce the subsequent GABAA receptor-mediated currents. Furthermore, this reduction was absent in hippocampal slices that were obtained from α7-nAChR knockout mice. In contrast to their results, we presumed that the desensitization of the α7-nAChR was the cause of the downregulation of the GABAA receptor because 0.1 mmol/L choline perfusion did not evoke apparent receptor activation but was sufficient to induce a desensitized receptor. In addition, the GABAA receptor appeared to be more sensitive to α7-nAChR desensitization than the NMDA receptor. This was concluded because the depression of the muscimol response occurred within 10 s after a choline pulse and 10 min after 0.1 mmol/L choline perfusion, whereas potentiation of the NMDA response appeared within 2 s after agonist removal and 10 min after 0.5 mmol/L choline perfusion. Furthermore, a 0.1-s choline pulse significantly inhibited the muscimol-evoked current but did not affect the NMDA-induced current. According to previous studies, prolonging the duration of agonist exposure or increasing the agonist concentration can induce nAChRs to enter into greater depths of desensitized states17. Thus, a deeper desensitized state of the α7-nAChR is more important for regulating the function of the NMDA receptor than for regulating the function of the GABAA receptor.
Intracellular Ca2+ was a possible effector that was evolved in the depression of the GABAA receptor after the choline pulse because the Ca2+ chelator BAPTA abolished this action of choline, which is similar to the results that were obtained for induction by nicotine and ACh16. The influx of Ca2+ through nAChRs has been shown to directly or indirectly participate in regulating the desensitization of nAChRs18 through protein kinase activation, such as an increase in intracellular Ca2+, which enhanced the onset of nAChR desensitization, or a decrease in intracellular Ca2+ by chelation with BAPTA. This accelerates the recovery from desensitization of nAChRs and, according to our studies, also modulates the following inhibition of GABAA receptors by choline. Now, this effect could be regarded as one event of receptor-crosstalk through Ca2+-dependent signal transduction. Nevertheless, intracellular Ca2+ had no effect on potentiation of the NMDA receptor. The Ca2+-independent mechanism may result from the action of other minor ions through α7-nAChRs, such as Na+, or membrane lipid composition, such as the metabolites of arachidonic acid. These effectors also regulate the desensitizing processes through its allosteric effects19. Moreover, further studies will be required to understand the details of how the desensitization of α7-nAChR influences the function of GABAA and NMDA receptors.
The modulation of GABAA or NMDA receptors by α7-nAChR desensitization may be a new pathway of cholinergic innervation. The hippocampus, which is the center of cognitive and memory function, is sensitive to cholinergic modulation by the medial septum/diagonal band of Broca. Furthermore, the desensitization of α7-nAChR in the hippocampus is important for the regulation of neuronal excitability and synaptic communication because cholinergic receptors are frequently expressed in glutamatergic and GABAergic terminals20. Several reports have recently focused on the interactions between α7-nAChR and GABAA receptors. For example, α7-nAChR facilitates the TTX-sensitive release of GABA, which regulates the activities of pyramidal neurons21. Specific agonists of the α7-nAChR elicit an inward current in interneurons22. At synaptic sites, the desensitization of presynaptic α7-nAChR decreases the release of GABA from GABAergic neurons or increases glutamate release from glutamatergic neurons after repetitive exposure to nicotine23,24. According to our report, cholinergic axons may regulate the excitatory rhythm of local neuronal circuits by desensitizing α7-nAChR. When exposed to fluctuant and low concentrations of choline, α7-nAChR transforms from a resting state to a desensitized state, from which it is able to subsequently recover. This transformation will regulate the activities of nearby GABAA or NMDA receptors, thereby altering local excitability. Neuronal nAChRs, especially the α7 subtype, have previously been related to multiple forms of time-dependent plasticity25. Acute or chronic nicotine treatment can facilitate long-term potentiation (LTP) by decreasing the threshold for LTP induction in the hippocampal CA1 region26. In hippocampal slices, exposure to nicotine promotes the induction of NMDA-mediated LTP27. According to our results, these events of synaptic plasticity in the hippocampus may be regulated by the effect of the desensitization of α7-nAChR on amino acid receptors, because the activation of the NMDA receptor directly induces LTP, whereas activation of the GABA receptor results in modulation of synaptic plasticity through a change in cellular excitability28,29.
In conclusion, our study provides insight into a mechanism by which the desensitization of α7-nAChR modulates cellular excitability and synaptic activity. The effect of this desensitization depends mostly upon the negative modulation of the GABAA receptor, as well as the positive modulation of the NMDA receptor.
Author contribution
Hai WANG and Ru-zhu CHEN designed the research and wrote the article; Lei SHEN conducted the experiments, analyzed the data, and wrote the article; and Wen-yu CUI conducted the experiments.
Acknowledgments
This study was supported by grants from the National New Drug Research and Development Key Project of China (No 2010zx09401–307) and the State Key Research Project of China (AWS11J003).
References
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012; 76: 116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL. Nicotinic ACh receptors in the hippocampal circuit: functional expression and role in synaptic plasticity. J Physiol 2014; 592: 4147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JS, Xie BX, Bian XL, Xue Y, Wei NN, Zhou JH, et al. Identification and in vitro pharmacological characterization of a novel and selective α7 nicotinic acetylcholine receptor agonist, Br-IQ17B. Acta Pharmacol Sin 2015; 36: 800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhang GF, Zhang X, Liu Q, Liu JG, Su DF, et al. Combined administration of anisodamine and neostigmine produces anti-shock effects: involvement of α7 nicotinic acetylcholine receptors. Acta Pharmacol Sin 2012; 33: 761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, et al. Impaired attention is central to the cognitive deficits observed in α7 deficient mice. Eur Neuropsychopharmacol 2007; 17: 145–55. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59: 1085–96. [DOI] [PubMed] [Google Scholar]
- Mazzuferi M, Palma E, Martinello K, Maiolino F, Roseti C, Fucile S, et al. Enhancement of GABAA-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A 2010; 107: 3180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 2010; 11: 682–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama Y, Hashimoto T, Kohayakawa H, Kiyoshi T, Nakamichi K, Kinoshita T, et al. In vivo pharmacological characterization of AC-3933, a benzodiazepine receptor partial inverse agonist for the treatment of Alzheimer's disease. Neuroscience 2014; 265: 217–25. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun XL. Desensitized nicotinic receptors in brain. Brain Res Rev 2005; 48: 420–37. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 2005; 28: 371–8. [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. Physiological concentrations of choline activate native α7-containing nicotinic acetylcholine receptors in the presence of PNU-120596 [1-(5-Chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea]. J Pharmacol Exp Ther 2010; 332: 588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin 2009; 30: 752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Cui W, Hu G, Wang H. Desensitization of α7 nicotinic receptors potentiated the inhibitory effect on M-current induced by stimulation of muscarinic receptors in rat superior cervical ganglion neurons. J Neural Transm 2005; 112: 1133–48. [DOI] [PubMed] [Google Scholar]
- Han FR, Wang H. Effects of desensitized nicotinic receptors on rotational behavior in a 6-hydroxydopamine model of Parkinson's disease. Neurosci Lett 2007; 415: 200–4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by α7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol 2007; 579: 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KD, Liu Q, Wu J, Lukas RJ. Kinetics of desensitization and recovery from desensitization for human α4β2-nicotinic acetylcholine receptors stably expressed in SH-EP1 cells. Acta Pharmacol Sin 2009; 30: 805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Sokolova E, Giniatullin R, Afzalov R, Nistri A. Recovery from desensitization of neuronal nicotinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. J Neurosci 1998; 18: 2458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaki T. N-glycosylation sites on the nicotinic ACh receptor subunits regulate receptor channel desensitization and conductance. Brain Res Mol Brain Res 2003; 114: 172–6. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signaling due to activation of α7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol (Lond) 2007; 582: 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Braga MFM, Pereira EF, Maelicke A, Albuquerque EX. α7 nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol 2000; 393: 59–67. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Edwin MM, Roger LP. Regulation of neuronal function by choline and 4OH-GTS-21 through α7 nicotinic receptors. J Neurophysiol 2003; 89: 1797–806. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci 2000; 20: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ. Diversity and patterns of regulation of nicotinic receptor subtypes. Ann N Y Acad Sci 1995; 757: 153–68. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 2011; 71: 155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Jia Y, Yang A, Sumikawa K. Nicotine reverses GABAergic inhibition of long-term potentiation induction in the hippocampal CA1 region. Brain Res 2000; 863: 259–65. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sumikawa K. Inactivation of α7 Ach receptors and activation of non-α7 Ach receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett 2000; 286: 134–8. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation — a decade of progress? Science 1999; 285: 1870–4. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol 1992; 68: 197–212. [DOI] [PubMed] [Google Scholar]