Abstract
Aim:
CYP2J3 in myocardium metabolizes arachidonic acid to 4 regioisomeric epoxyeicosatrienoic acids (EETs), which have diverse biological activities in rat heart. In this study we examined whether CYP2J3 was involved in cardioprotective effects of ophiopogonin D (OPD), a steroidal glycoside isolated from Chinese herb Radix ophiopogonis.
Methods:
Rat cardiomyoblast cell line (H9c2 cells) was tested. Intracellular Ca2+ concentrations ([Ca2+]i) were measured using Fluo-4/AM. The expression of calcium-regulating molecules and ER stress signaling molecules was measured with qRT-PCR and Western blot analyses. Cell apoptosis was quantified with Hoechst 33258 staining and TUNEL assay. The level of 14,15-DHET, a stable metabolite of 14,15-EET, was assessed with ELISA.
Results:
Angiotensin II (10−6 mol/L) significantly decreased the expression of calcium-regulating molecules (SERCA2a, PLB, RyR2 and FKBP12.6), and elevated [Ca2+]i in H9c2 cells. Furthermore, angiotensin II markedly increased the expression of ER stress signaling molecules (GRP78, CHOP, p-JNK and cleaved caspase-12) and ER stress-mediated apoptosis. OPD (100, 250 and 500 nmol/L) dose-dependently increased CYP2J3 expression and 14,15-DHET levels in normal H9c2 cells. Pretreatment of H9c2 cells with OPD suppressed angiotensin II-induced abnormalities in Ca2+ homeostasis, ER stress responses and apoptosis. Overexpression of CYP2J3 or addition of exogenous 14,15-EET also prevented angiotensin II-induced abnormalities in Ca2+ homeostasis, whereas transfection with CYP2J3 siRNA diminished the effects of OPD on Ca2+ homeostasis. Furthermore, the intracellular Ca2+ chelator BAPTA suppressed angiotensin II-induced ER stress responses and apoptosis in H9c2 cells.
Conclusion:
OPD is a novel CYP2J3 inducer that may offer a therapeutic benefit in treatment of cardiovascular diseases related to disturbance of Ca2+ homeostasis and ER stress.
Keywords: ophiopogonin D, cardiomyocytes, angiotensin II, CYP2J3, epoxyeicosatrienoic acid, ER stress, Ca2+ homeostasis, BAPTA
Introduction
Ca2+ is a ubiquitous cellular second messenger that participates in various intracellular processes, ranging from fertilization to gene expression and cell survival1. In cardiomyocytes, Ca2+ cycling is essential for effective myocyte contraction and relaxation2, and the precise control of intracellular Ca2+ homeostasis relies on a series of specialized regulatory proteins, including transcription factors, ion channels, and Ca2+ binding proteins. During a normal cardiac cycle, Ca2+ enters the cell via L-type channels through a membrane potential-dependent mechanism. This leads to Ca2+-dependent Ca2+ release from the sarcoplasmic reticulum (SR) into the cytosol through ryanodine receptors (RyR2), which is modulated by calstabin 2 (FKBP12.6) and, ultimately, the initiation of the contractile cycle3. The cardiac diastole is primarily mediated by the activation of the SR Ca2+ ATPase (SERCA2a), which pumps Ca2+ back into the SR. SERCA2a activity is negatively regulated by phospholamban (PLB), another SR membrane protein4. Dephosphorylated PLB inhibits SERCA2a activity by inhibiting its apparent affinity for Ca2+ and reduces Ca2+ re-uptake into the SR, whereas PLB phosphorylation reverses the inhibition of SERCA2a activity and increases SR Ca2+ uptake5. Ca2+ homeostasis is critical for maintaining normal cardiac performance, and evidence suggests that altered Ca2+ homeostasis and expression of Ca2+ cycling proteins are responsible for heart disease6,7.
The endoplasmic reticulum (ER) is a central subcellular organelle that performs multiple specialized functions in eukaryotic cells, including Ca2+ storage, phospholipid and sterol synthesis, and protein folding and maturation8. Various cellular stresses, such as the excess accumulation of unfolded proteins, oxidative stress, viral infection, glucose deprivation, and Ca2+ depletion from the ER stores, can contribute to ER stress9. To alleviate this stress, the ER triggers an evolutionarily-conserved signaling cascade: the unfolded protein response (UPR). Ca2+ overload and depletion or alterations in the ER Ca2+ pool can directly induce the UPR10,11, which is mediated by ER transmembrane receptor proteins, including inositol-requiring kinase 1 (IRE1), RNA-activated protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF6). The activation of these receptor proteins upregulates ER chaperones, including glucose-regulated protein 78 (GRP78, BiP). However, if ER function is severely impaired due to excessive or prolonged exposure to stress, the apoptotic pathway is triggered via the activation of C/EBP homologous protein (CHOP), c-Jun NH2-terminal kinase (JNK), or caspase-1212. A previous study suggests that ER stress-mediated apoptosis occurs in a number of diseases, including heart failure13.
Human cytochrome P450 2J2 (CYP2J2) and its rat homolog, CYP2J3, are highly expressed in the myocardium, and this isozyme metabolizes arachidonic acid (AA) into four different epoxyeicosatrienoic acid (EET) regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EET)14. Reports indicate that the function of CYP2J3-derived EETs is to act an endothelium-derived hyperpolarizing factor (EDHF) that exerts vasodilating effects and hyperpolarizes vascular smooth muscle cells (VSMCs)15. Accumulating evidence suggests that EETs have crucial and diverse protective roles within the cardiovascular system. Specifically, EETs have been reported to possess anti-arrhythmic16, anti-inflammatory17, anti-apoptotic18, and anti-oxidant19 functions. Additionally, CYP2J3 overexpression and increased levels of EETs have been reported to decrease ER stress signaling and ER stress-mediated apoptosis in rats with heart failure by maintaining SERCA2a activity and intracellular Ca2+ homeostasis20. Additionally, research indicates that decreased SERCA2a expression and activity are hallmarks of heart failure in both experimental animal models and patients21. Restoration of SERCA2a activity and expression by gene transfer or the overexpression of its upstream regulators improves cardiac function in animal models and patients with heart failure22,23. Dysregulation of RyR2 and its regulatory subunit, FKBP12.6, which have been observed in failing hearts, facilitates Ca2+ leakage during the diastole, thus depleting SR Ca2+ stores, and may also be involved in defective Ca2+ cycling in failing hearts24.
Ophiopogonin D (OPD), a steroidal glycoside isolated from Radix ophiopogonis (a tuber of Ophiopogon japonicus KerGawl, Liliaceae), is a traditional Chinese herbal medicine that has been used to treat inflammatory and cardiovascular diseases25. OPD has also been suggested to be the chief active component of the traditional Chinese herbal prescription drug, Shendong yin, which has been used to prevent and treat cardiovascular diseases26,27. OPD is protective, serving as an antioxidant against H2O2-induced endothelial injury, and it displays mild anti-apoptotic properties28. OPD is also protective against doxycycline (DOX)-induced autophagy in cardiomyocytes by inhibiting DOX-induced activation of JNK and ERK1/2, resulting in ROS scavenging and reduced oxidative stress29. Although the CYP2J3/EETs system and ER stress-associated pathways are both vital to cardiovascular diseases, the underlying mechanism of the beneficial effects of OPD on cardiac myocytes via CYP2J3 is unknown. Thus, we investigated whether OPD upregulates CYP2J3/EETs, maintains Ca2+ homeostasis and reduces ER stress in cardiomyocytes.
Materials and methods
Drugs and reagents
OPD (purity: 98% by HPLC) was obtained from the National Institutes for Control of Pharmaceutical and Biological Products (Beijing, China). Angiotensin II (Ang II), thapsigargin (TG) and tunicamycin (TM) were purchased from Sigma-Aldrich (St Louis, MO, USA). 2-(2-Propynyloxy)-benzenehexanoic acid (PPOH), 1, 2-bis (o-aminophenoxy) ethane-N, N, N', N'-tetra-acetic acid (BAPTA), 11,12-EET, 14,15-EET and 14,15-EEZE were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Trizol reagent was obtained from Invitrogen (Carlsbad, CA, USA). The TransScript™ First-Step RT-PCR SuperMixFast and SYBR® Green Master Mix were purchased from TransGen Biotech Co, Ltd (Beijing, China). The antibodies against GAPDH, SERCA2a, p-PLB, GRP78, CHOP, p-JNK and caspase-12 were obtained from Cell Signaling Technology Inc (Danvers, MA, USA). The antibody against CYP2J3 was obtained from Bioss (Boston, MA, USA). The antibody against RyR2 was obtained from Alomone Labs (Jerusalem, Israel). The antibody against FKBP12.6 was obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). The Fluo-4/AM Ca2+ sensor was obtained from Dojindo Laboratories (Tokyo, Japan). A TACS®2 TdT-Fluor In Situ Apoptosis Detection Kit and a 14,15-DHET ELISA kit were purchased from Detroit R & D Inc (Detroit, MI, USA).
Cell cultures and treatment
A rat cardiomyoblast cell line (H9c2 cells) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and an antibiotic mixture of penicillin (100 IU/mL) and streptomycin (100 μg/mL). The cells were seeded on 6-, 12-, or 24-well culture plates for each experiment and grown in a 37 °C humidified incubator containing 5% CO2. OPD, TG, TM, BAPTA, 14,15-EET and 14,15-EEZE were dissolved in dimethyl sulfoxide (DMSO) and diluted with DMEM. DMSO was the vehicle, and the final concentration never exceeded 5‰ (v/v).
RNA isolation and quantitative reverse-transcriptase PCR
The total RNA was extracted, and the expression of specific mRNAs was quantified with quantitative real-time PCR (qRT-PCR), as previously described30. Briefly, the total RNA was prepared from the H9c2 cells using Trizol reagent. The RNA integrity and concentration were measured using a DU-600 ultraviolet spectrophotometer (Beckman Coulter, Ca, USA). All RNA samples with an OD260/OD280 between 1.8 and 2.0 were used for quantitative real-time PCR. The total RNA was reverse-transcribed with the Transcriptor First-strand cDNA Synthesis Kit to obtain the cDNAs. Quantitative RT-PCR was performed in an ABI Prism 7500 real-time PCR instrument (Applied Biosystems, MA, USA) using the TransScript™ SYBR® Green Master Mix kit, according to the instructions provided. Each target gene was quantified, with GAPDH serving as an internal control. The primer sequences are listed in supplementary Table 1.
Protein preparation and Western blot
The protein was extracted from the treated H9c2 cells as previously described31. Briefly, after the protein concentration was quantified with a BCA assay (Beyotime, Haimen, China), the supernatants were stored at -80 °C until they were separated via SDS-PAGE and electro-transferred onto polyvinylidene difluoride membranes (PVDF; 0.45 μm HATF, Millipore, USA). Next, the membranes were blocked with 5% nonfat dry milk in TBST (20 mmol/L Tris, 140 mmol/L NaCl, and 0.1% Tween-20) for 1 h at room temperature before being incubated overnight at 4 °C with an anti-SERCA2a rabbit polyclonal antibody (1:750), anti-p-PLB rabbit polyclonal antibody (1:750), anti-RyR2 mouse polyclonal antibody (1:500), anti-FKBP12.6 mouse polyclonal antibody (1:750), anti-CYP2J3 mouse polyclonal antibody (1:500), anti-GRP78 rabbit polyclonal antibody (1:500), anti-CHOP mouse polyclonal antibody (1:500), anti-p-JNK rabbit polyclonal antibody (1: 500), or anti-GAPDH mouse monoclonal antibody (1:1000 in 0.5% milk in TBST). After five washes with TBST, the membranes were incubated with HRP-conjugated goat-anti-mouse or -anti-rabbit IgG secondary antibodies (1:2000) for 1 h at room temperature. After five washes with TBST, the membranes were treated with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Waltham, MA, USA) and visualized using an ImageQuant LAS 500 (Healthcare Bio-Sciences AB, Sweden).
siRNA transfection
The siRNA for rat CYP2J3 and the negative control non-silencing RNA were designed and synthesized by Invitrogen (Carlsbad, CA, USA). The siRNA sequences are as follows: forward, 5′-GUAACUGGCAUGCCCUUAATT-3′, and reverse, 5′-UUAAGGGCAUGCCAGUUACTT-3′. The transfection was performed according to the manufacturer's instructions. Briefly, the H9c2 cells were seeded in 12-well plates, incubated overnight in complete medium, and then transfected with the indicated siRNA or control siRNA (final concentration 100 nmol/L) for 48 h using Lipofectamine 2000 (Carlsbad, CA, USA).
Intracelluar Ca2+ measurements
The intracellular Ca2+ concentrations ([Ca2+]i) were measured using an intracellular Ca2+ indicator Fluo-4/AM (Dojindo Laboratories, Japan) according to the supplier's instructions, as previously described32, with some modifications. Briefly, the H9c2 cells were loaded with 5 μmol/L Fluo-4/AM for 60 min at 37 °C in the dark. Then, they were washed with Hanks' balanced salt solution (HBSS; with magnesium and Ca2+). The cells were viewed under a confocal microscope (100×oil immersion objective, Olympus, Tokyo, Japan) and fluorescence was induced (λex 494 nm; λem 516 nm). Then, [Ca2+]i was calculated as follows: [Ca2+]i=Kd [F–Fmin]/[Fmax–F], where the Kd for Fluo-4/AM is 400 nmol/L, F is the mean fluorescent intensity of the cells, Fmin is the fluorescent intensity when the cells were treated with 5 mmol/L EGTA (Sigma, USA), and Fmax is the fluorescent intensity when the cells were treated with 0.1% Triton X-100 (Sigma, USA). The fluorescent intensities were quantified using Zeiss LSM Image Software (Carl Zeiss, Germany).
Hoechst 33258 staining
The H9c2 cells (5×105/well) were cultured in 6-well plates and incubated with 20 mmol/L Hoechst 33258 dye for 20 min at 37 °C, according to the manufacturer's instructions (Dojindo Laboratories, Tokyo, Japan). The cells were then visualized under a fluorescent microscope (Olympus, Tokyo, Japan) (λex 350 nm; λem 460 nm).
TUNEL assay
Apoptosis was quantified using Trevigen's TACS®2 TdT-Fluoro Apoptosis Detection Kit, according to the manufacturer's instructions. Briefly, the medium was removed, and the cells were washed once with room temperature PBS and then dehydrated using an alcohol gradient (70%, 95%, 100%) for 5 min at each concentration, followed by air drying for 10 min and cell immobilization in PBS for 10 min. Subsequently, the cells were covered with CytoninTM for 30 min and then washed two times in deionized water. After immersion in TdT Labeling Buffer for 5 min, the cells were covered with Labeling Reaction Mix, incubated for 60 min at 37 °C in a humidified chamber, blocked with Stop Buffer for 5 min, and incubated with Strep-Fluor Solution for 20 min in the dark, after which the labeled DNA fragments were detected under a fluorescent microscope (Olympus, Tokyo, Japan).
Evaluation of the 14,15-DHET levels by ELISA
A stable metabolite of 14,15-EET, 14,15-dihydroxyeicosatrienoic acid (14,15-DHET), was measured with ELISA according to the manufacturer's instructions, as previously described33, with some modifications. Briefly, the culture medium was collected and centrifuged, and the amount of 14,15-DHET released in the culture medium was measured (yellow product) on a Victor 3 luminometer (PerkinElmer, Fremont, CA, USA) at 450 nm. The 14,15-DHET concentration was calculated from a standard curve.
Lentivirus construction and transfection
The coding sequence of CYP2J3 was amplified by qRT-PCR using the following primer sequences: forward, 5′-GTGTGCTTCTGCTCCCTGAG-3′, and reverse, 5′- TTGTGCTCCATTTGAGTAAAG-3′. The segment and the pGC-FU plasmid (Shanghai GenePharma Co, China) were digested with Age I and ligated with T4 DNA ligase to produce pGC-FU-CYP2J3. The plasmids were subsequently purified and sequenced. To generate recombinant lentiviral LV-CYP2J3, 293T cells were co-transfected with the pGC-FU plasmid (5 μg) containing the CYP2J3 cDNA, and the pHelper1.0 (2.5 μg) and pHelper 2.0 (2.5 μg) plasmids using Lipofectamine 2000 (50 μL). The supernatant was harvested 48 h later and mixed with the lentiviral solution (volumetric ratio of 5:1), followed by an overnight incubation at 4 °C. Then, the supernatant was centrifuged at 4000×g for 20 min, and the lentivirus was resuspended in cell culture medium and incubated with the H9c2 cells. We generated a lentiviral vector that expressed GFP alone as a control. The H9c2 cells were transfected with lentiviral vectors for 72 h and then used in the experiments.
Statistical analysis
All data are expressed as the mean±SEM. The differences were compared using Student's t-tests or one-way ANOVA followed by a Newman-Keuls' multiple comparisons test, as appropriate. P<0.05 was considered to be statistically significant. GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA) was used to analyze the data.
Results
Effects of OPD on the CYP2J3/EETs system
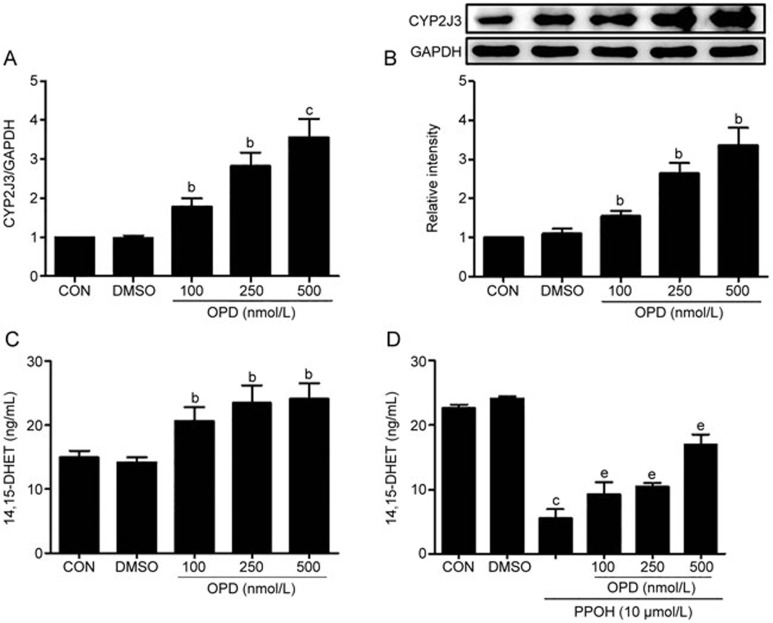
As shown in Figure 1A and B, OPD significantly increased the expression of CYP2J3 mRNA in a dose-dependent manner; the expression of the CYP2J3 protein increased, as well. One major role of CYP2J3 is to metabolize AA into four biologically active EET regioisomers, which are subsequently metabolized into less biologically active forms, DHETs, by sEH6. Thus, we measured the levels of 14,15-DHET in cultured cells, and Figure 1C shows that the levels of 14,15-DHET were increased in the OPD-treated cells compared with the controls. The selective CYP2J3 signaling antagonist PPOH was used to confirm the importance of OPD24, and the 14,15-DHET levels were decreased in the PPOH-treated cells compared with the controls. The H9c2 cells that were pretreated with OPD exhibited reduced PPOH-mediated effects in cardiomyocytes (Figure 1D), suggesting that OPD increased CYP2J3 activity. Thus, OPD can significantly increase both CYP2J3 expression and activity in cardiomyocytes.
Figure 1.
OPD increases CYP2J3 expression and 14,15-DHET levels in cardiomyocytes. H9c2 cells were treated as described in the Methods. CYP2J3 expression was quantified by qRT-PCR (A) and Western blotting (B). (C) 14,15-DHET levels were measured with an ELISA. (D) H9c2 cells were pretreated with OPD as indicated in the Methods, and 14,15-DHET levels were increased compared with the PPOH-treated group. Data are presented as the mean±SEM. n=3–4. bP<0.05, cP<0.01 vs the controls; eP<0.05 vs the PPOH-treated group.
Effects of CYP2J3/EETs on intracellular Ca2+ homeostasis
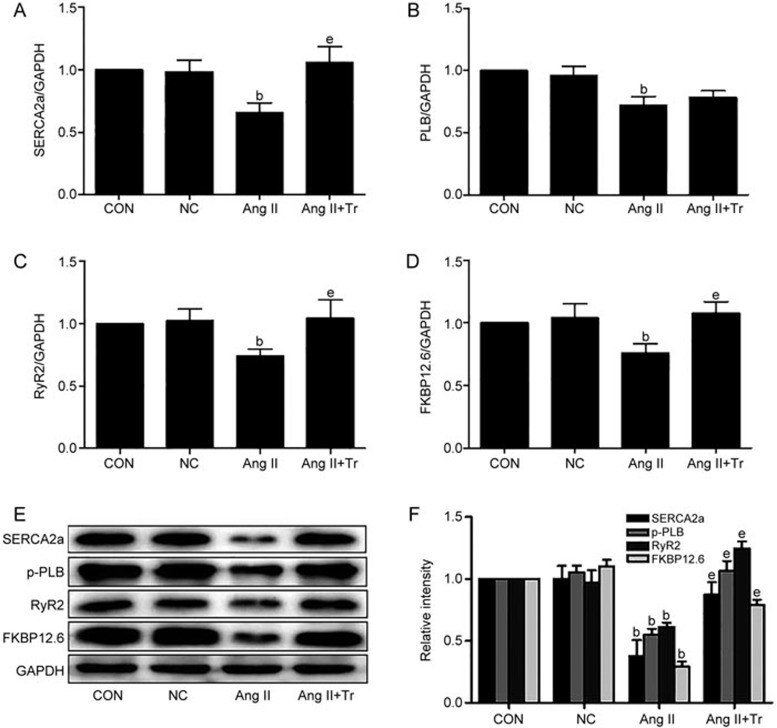
We transfected the H9c2 cells with a LV-CYP2J3 lentivirus to overexpress CYP2J3 and showed that the levels of the CYP2J3 mRNA and protein were significantly increased after transfection compared with the controls (supplementary Figure 1A and 1B). We evaluated the effects of CYP2J3 overexpression on the expression of Ca2+-regulating molecules (SERCA2a, PLB, RyR2 and FKBP12.6), which are required to maintain intracellular Ca2+ homeostasis. All four Ca2+-regulating proteins were down-regulated in the H9c2 cells, similar to the cells treated with Ang II 10−6 mol/L, and this agreed with the data showing decreased protein levels for these molecules. The CYP2J3 intervention significantly reversed the down-regulated expression of the Ca2+-regulating molecules compared with the Ang II-treated group (Figure 2A–2F). Thus, CYP2J3 overexpression maintains Ca2+ homeostasis in cardiomyocytes treated with Ang II.
Figure 2.
CYP2J3 overexpression restores the expression of Ca2+-regulating molecules in Ang II (10−6 mol/L)-treated cardiomyocytes. qRT-PCR was used to quantify the levels of the SERCA2a, PLB, RyR2, and FKBP12.6 mRNAs (A–D), and Western blotting was used to measure the expression of the corresponding proteins (E and F). The housekeeping gene GAPDH was used as a loading control. Data are presented as the mean±SEM. n=3–4. bP<0.05 vs the controls; eP<0.05 vs the Ang II-treated group. Tr, transfected with CYP2J3 lentiviral vectors for 72 h.
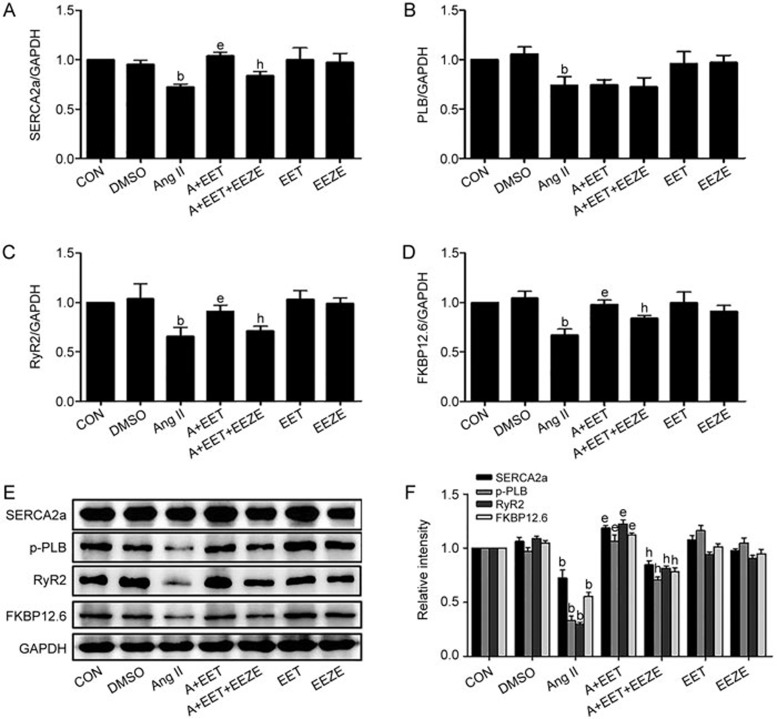
EETs are major metabolites of CYP2J3; therefore, to investigate the function of EETs in intracellular Ca2+ homeostasis, H9c2 cells were cultured with 11,12- or 14,15-EET (2 mmol/L each) for 2 h before treatment with Ang II for 24 h. Each EET attenuated the Ang II-induced down-regulation of SERCA2a expression, and 14,15-EET had a greater effect than 11,12-EET (supplementary Figure 2A and 2B). We then treated the H9c2 cells with different concentrations of 14,15-EET (0.5–2.5 μmol/L). The addition of exogenous 14,15-EET to cardiomyocytes significantly reversed the Ang II-induced down-regulation of the expression of the Ca2+-regulating molecules (SERCA2a, PLB, RyR2 and FKBP12.6) in a dose-dependent manner (supplementary Figure 3A–3F). However, there was no significant difference in the expression of the PLB mRNA between the Ang II group and the 14,15-EET co-treated group. To confirm these data, we applied 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 10 μmol/L), a selective inhibitor of EET34, and observed that 14,15-EEZE abolished the beneficial effects of 14,15-EET on the expression of the Ca2+-modulating proteins (Figure 3A–3F). Therefore, 14,15-EET significantly protects Ca2+ homeostasis in cardiomyocytes.
Figure 3.
14,15-EET restores the expression of the Ca2+-regulating molecules in the Ang II 10−6 mol/L-treated cardiomyocytes. The effects of 14,15-EET (2.5 μmol/L) and/or 14,15-EEZE (10 μmol/L) on the expression of the Ca2+ handling molecules are shown. qRT-PCR was used to quantify the expression of the SERCA2a, PLB, RyR2, and FKBP12.6 mRNAs (A–D), and Western blotting was used to measure the expression of the corresponding proteins (E and F). Data are presented as the mean±SEM. n=3–5. bP<0.05 vs the controls; eP<0.05 vs the Ang II-treated group; hP<0.05 vs the Ang II+14,15-EET-treated group.
Effects of OPD on intracellular Ca2+ homeostasis
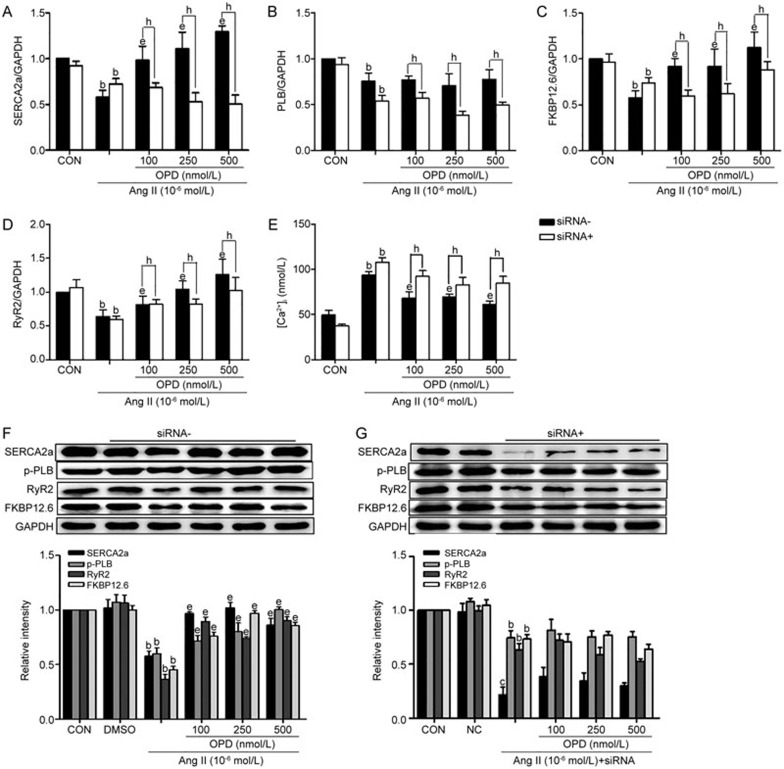
To examine whether OPD offered protection against Ang II-induced Ca2+ homeostasis in cardiomyocytes, H9c2 cells were pretreated with different concentrations of OPD for 2 h and then treated with 10−6 mol/L Ang II, or Ang II alone for an additional 24 h. Ang II alone reduced the expression of the SERCA2a, PLB, RyR2 and FKBP12.6 mRNAs, whereas pretreatment with OPD reversed the Ang II-mediated effects, with the exception of PLB expression, in the Ang II-treated and OPD-treated groups (Figure 4A–4D). Additionally, incubation with Ang II for 24 h significantly reduced the levels of the SERCA2a, p-PLB, RyR2 and FKBP12.6 proteins compared with the controls, whereas OPD prevented the Ang II-mediated effects on these proteins (Figure 4F).
Figure 4.
OPD maintains Ca2+ homeostasis in the Ang II-treated cardiomyocytes and CYP2J3 knockdown abolishes the protective effects of OPD. H9c2 cells were pretreated as indicated in the Methods. The expression of the SERCA2a, PLB, RyR2, and FKBP12.6 mRNAs was measured by qRT-PCR (A–D), and the expression of the corresponding proteins was measured by Western blotting (F and G). (E) Intracellular Ca2+ concentrations were measured with a Fluo-4/AM kit. Relative intracellular Ca2+ concentrations were expressed as a percentage of the control. Data are presented as the mean±SEM. n=3–6. bP<0.05, cP<0.01 vs the controls; eP<0.05 vs the Ang II-treated group; hP<0.05 vs the OPD+Ang II-treated group.
The data showed that OPD and the CYP2J3/EETs system individually modulated the expression of the Ca2+-handling molecules that were decreased by Ang II and may protect Ca2+ homeostasis in cardiomyocytes. OPD significantly increased CYP2J3 expression and activity in cardiomyocytes, and its protective effects toward Ca2+-handling molecules and Ca2+ homeostasis were associated with the up-regulation of the CYP2J3/EETs levels. To confirm our hypothesis, we knocked down CYP2J3 expression with an siRNA (supplementary Figure 4A and 4B) to verify the involvement of CYP2J3 in OPD-mediated protection against distorted Ca2+ homeostasis. Figure 4A–4G shows that the OPD-induced inhibition of the Ang II-mediated down-regulation of Ca2+ handling proteins (SERCA2a, PLB, RyR2 and FKBP12.6) was significantly abolished by silencing CYP2J3 expression. Thus, the OPD-mediated protection of Ca2+ homeostasis in cardiomyocytes may depend on up-regulated CYP2J3 expression.
The Ca2+ concentrations were measured using Fluo-4/AM fluorescent labeling, and Figure 4E shows that after treatment with 10−6 mol/L Ang II for 24 h, the ratio of the fluorescent intensity of the intracellular Ca2+ in the H9c2 cells was significantly increased compared with the controls. However, pretreatment with OPD (100, 250 and 500 nmol/L) decreased the fluorescent intensities of Ca2+ compared with the Ang II-treated group. Furthermore, after CYP2J3-knockdown, the OPD-induced attenuation of the Ang II-induced increase in intracellular Ca2+ concentrations was diminished.
Thus, OPD suppresses pharmacological stress-induced increases in intracellular Ca2+ concentrations at least in part by upregulating CYP2J3 expression.
Effects of OPD on ER stress signaling and apoptosis in cultured cardiomyocytes
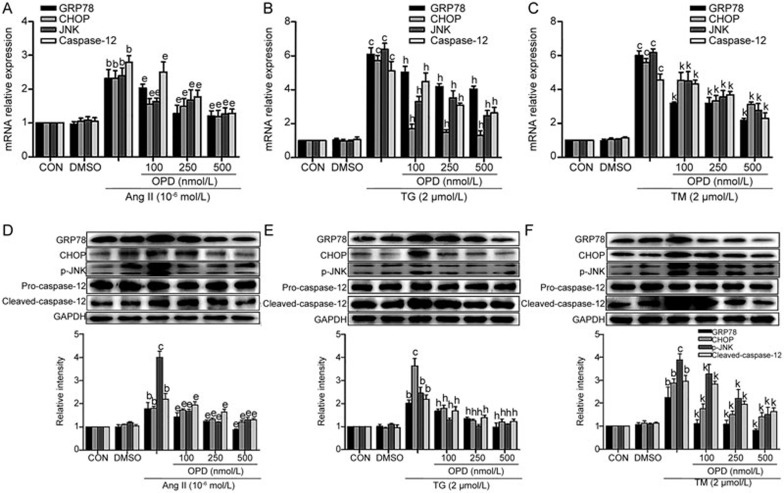
The elevation of cytosolic Ca2+ concentrations is a common mechanism in abnormal ER stress and stress-mediated apoptosis35. Thus, we assessed whether OPD conferred protection against Ang II-induced ER stress and apoptosis. H9c2 cells were pretreated with 100, 250, and 500 nmol/L OPD for 2 h and then treated with 10−6 mol/L Ang II, or Ang II alone for an additional 24 h. We measured ER stress signaling, and qRT-PCR and Western blot confirmed that the expression of the ER stress molecules GRP78, CHOP, p-JNK, and cleaved caspase-12 was markedly elevated after Ang II treatment (Figure 5A and 5D). Interestingly, the induction of the ER stress signaling markers was suppressed by treatment with OPD (Figure 5A and 5D). To understand how OPD inhibits ER stress signaling in cultured cells, we treated H9c2 cells with TG or TM (positive controls for ER stress) and OPD. Similar to the data for the Ang II-treated cells, OPD significantly decreased TG- (Figure 5B and 5E) and TM-induced (Figure 5C and 5F) ER stress signaling. Thus, the induction of ER stress-mediated apoptotic signaling pathways in cardiomyocytes is attenuated by OPD.
Figure 5.
OPD reduces ER stress signaling and stress-mediated apoptosis in cultured cardiomyocytes. H9c2 cells were pretreated with OPD as indicated in the Methods. GRP78, CHOP, JNK and caspase-12 mRNA levels were measured by qRT-PCR (A–C), and the expression of the corresponding proteins was measured by Western blotting (D–F). Data are presented as the mean±SEM. n=3–6. bP<0.05, cP<0.01 vs the controls; eP<0.05 vs the Ang II; hP<0.05 vs TG; kP<0.05 vs TM. DMSO, dimethyl sulfoxide; TG, thapsigargin; TM, tunicamycin.
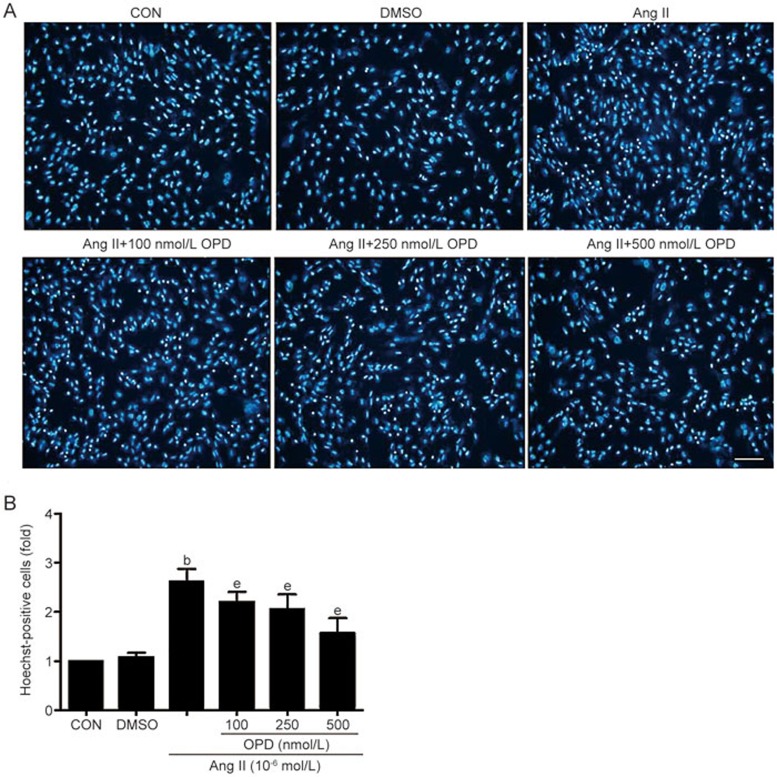
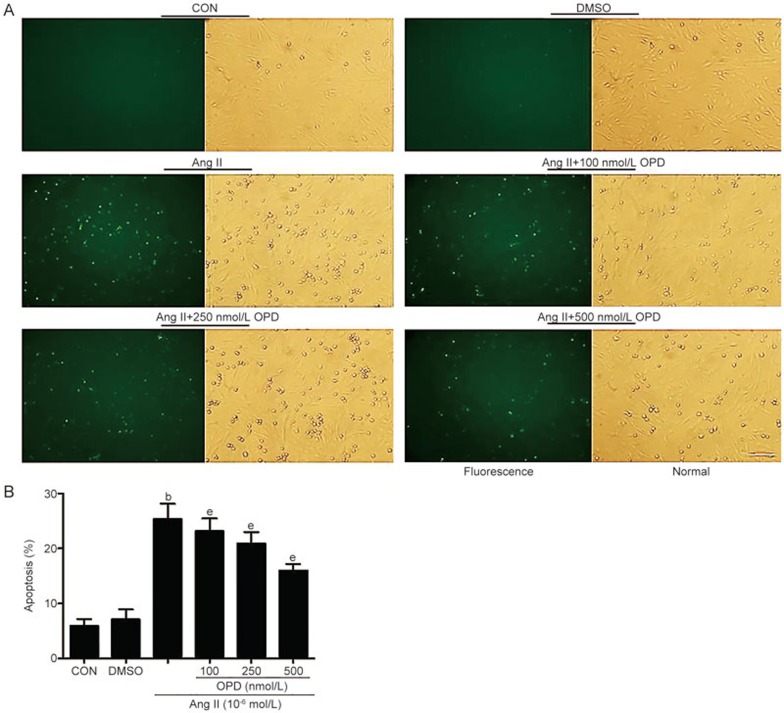
We used Hoechst 33258 staining to quantify the number of cardiomyocytes that underwent apoptosis. Compared with the controls, there were more Hoechst-positive cardiomyocytes in the cultures treated with Ang II. The number of Hoechst-positive cells was reduced when the cells were pretreated with OPD (Figure 6A and B), and TUNEL staining revealed similar results (Figure 7A and B). Thus, OPD inhibits ER stress-mediated apoptosis.
Figure 6.
OPD reduces ER stress-mediated apoptosis in cultured cardiomyocytes. (A) Hoechst 33258 staining was used to quantify the number of cardiomyocytes that underwent apoptosis. (B) The number of Hoechst-positive cells (fold) was counted in six random fields per group. Scale bar=20 μm. Data are presented as the mean±SEM. n=3–6. bP<0.05 vs the controls; eP<0.05 vs the Ang II-treated group.
Figure 7.
OPD reduces ER stress-mediated apoptosis in cultured cardiomyocytes. (A) TUNEL staining was used to quantify the number of cardiomyocytes that underwent apoptosis. (B) The percentage of apoptotic H9c2 cells were calculated from the ratio of TUNEL-labeled cells to total cells. Scale bar=20 μm. Data are presented as the mean±SEM. n=3–6. bP<0.05 vs the controls; eP<0.05 vs the Ang II-treated group.
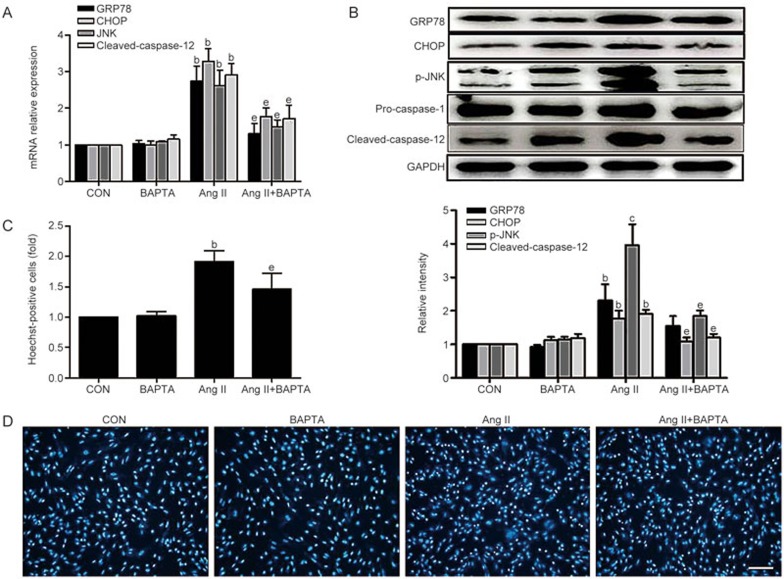
We assessed whether increased intracellular Ca2+ concentrations induced an aberrant ER stress response in H9c2 cells using BAPTA (10 μmol/L), an intracellular Ca2+ chelator, and showed that BAPTA reduced the ER stress response. The data show that the addition of BAPTA inhibited the ER stress response and stress-mediated apoptosis in Ang II-treated cardiomyocytes (Figure 8A–D).
Figure 8.
BAPTA attenuates ER stress signaling in cardiomyocytes exposed to Ang II. H9c2 cells were pretreated with BAPTA as indicated in the Methods. GRP78, CHOP, JNK and caspase-12 mRNA levels were assessed by qRT-PCR (A), and the expression of the corresponding proteins was measured by Western blotting (B). (C and D) Hoechst 33258 staining was used to count the number of cardiomyocytes that underwent apoptosis. The number of Hoechst-positive cells (fold) was counted in six random fields from each group. Data are presented as the mean±SEM. n=3–6. bP<0.05, cP<0.01 vs the control group; eP<0.05 vs the Ang II-treated group.
In summary, cytosolic Ca2+ overload triggers the ER stress response, and OPD treatment reduced the intracellular Ca2+ overload by up-regulating CYP2J3/EETs expression and suppressing ER stress and stress-mediated apoptosis in cardiomyocytes.
Discussion
To our knowledge, this is the report of OPD-induced CYP2J3 expression. Specifically, the upregulation of a CYP2J3/EETs-mediated myocardial protection program may be important for the cardioprotective effects of OPD against intracellular Ca2+ overload, ER stress, and ER stress-mediated apoptosis.
CYP2J3 is the predominant AA epoxygenase that metabolizes AA to EETs, which are highly expressed in cardiomyocytes and vascular endothelial cells14. EETs have diverse biological effects within the cardiovascular system, and CYP2J3 overexpression has been used to study EET biosynthesis and heart disease development. CYP2J3 overexpression prevented the formation of arrhythmogenic substrates by maintaining gap junction integrity and reducing the development of atrial fibrosis in cardiac hypertrophy16. The upregulation of CYP2J3/11,12-EETs during ischemic post-conditioning (HPost) induced cardioprotection by inhibiting apoptosis via the mitochondrial pathway18. In the CYP2J3-expressing SHR rats, systolic blood pressure was significantly decreased, cardiac output was improved, cardiac collagen content was reduced, and ANP was increased in the myocardium and plasma36. Here, we report that CYP2J3 overexpression significantly prevented the Ang II-induced down-regulation of Ca2+-regulating molecules (Figure 2). Additionally, the 14,15-EET intervention reversed the down-regulation of Ca2+-regulating molecule expression, and 14,15-EEZE, a selective EET antagonist, abolished these effects (Figure 3). Thus, CYP2J3-derived EETs may exert their cardioprotective functions by maintaining intracellular Ca2+ homeostasis. This idea is consistent with previous studies in which a CYP2J3 transgene maintained SERCA2a activity and intracellular Ca2+ homeostasis in rats with heart failure20. We found that OPD increased the expression of the CYP2J3 mRNA and protein (Figure 1) the up-regulated activity of the CYP2J3 gene may increase EET synthesis. Indeed, we demonstrated that OPD increased the levels of 14,15-DHET, a stable metabolite of 14,15-EET, in cardiomyocytes. Ours is the report to show that OPD can upregulate the CYP2J3/EETs system. Additionally, we found that OPD protected cardiomyocytes from cellular Ca2+ overload and reversed the down-regulation of Ca2+ modulating proteins; these effects were abolished by silencing CYP2J3 expression. Thus, OPD protected Ca2+ homeostasis in cardiomyocytes, which depends on its ability to upregulate the CYP2J3/EETs system. Additionally, siRNA-mediated pregnane X receptor (PXR) knockdown diminished the effects of OPD on CYP2J3 expression (data not shown). OPD had no significant effect on the expression of the PXR mRNA and protein but promoted PXR translocation from the cytoplasm to the nucleus (data not shown). Although the transcription of many P450 enzymes is induced by nuclear receptor activation, until now, there have been no reports showing that PXR can directly regulate CYP2J3 transcription. However, further investigation is warranted to study the mechanisms by which OPD influences CYP2J3 expression and activity.
In our study, the expression of SERCA2a, PLB, RyR2 and FKBP12.6 was reduced in the Ang II-treated cardiomyocytes, which agrees with published findings37,38. Both OPD and CYP2J3/EETs reversed the down-regulation of these Ca2+ handling proteins. However, the expression of PLB mRNA was not different in the Ang II, OPD, or CYP2J3/EETs intervention groups, even though mRNA stability may not be exclusively responsible for the changes in protein function. The post-transcriptional modifications that alter protein translation or stabilization are critical for regulating protein expression. PLB can be phosphorylated at serine-16 by protein kinase A (PKA), and at theronine-17 by Ca2+/calmodulin-dependent protein kinase II (CaMKII)39. This phosphorylation prohibits PLB from inhibiting SERCA2a, and Ca2+ uptake is accelerated. We also observed that the phosphorylation of the PLB protein can be reversed by OPD or CYP2J3/EETs, but not Ang II, and this may be an underlying mechanism by which OPD and CYP2J3/EETs maintain SERCA2a expression in cardiomyocytes. RyR2 can also be phosphorylated by both PKA and CaMKII5, which affect RyR2 activity. However, all of the potential functional consequences of RyR2 phosphorylation (decrease, increase, or no effect on activity) have been reported40,41,42. In cardiomyocytes, Ca2+ homeostasis depends on a tight balance between SR Ca2+ reuptake and leakage. We observed that OPD also attenuated Ang II-induced intracellular Ca2+ overload. Therefore, we concluded that OPD promoted Ca2+ homeostasis by regulating the Ang II-induced dysfunction in Ca2+ handing proteins.
Impaired intracellular Ca2+ homeostasis is a documented and common feature of heart disease, which is a consequence of the dysregulation of Ca2+-cycling proteins43,2,3. Modifications in Ca2+ homeostasis are also a major mechanism that produces abnormal ER stress and apoptosis44; uncontrolled increases in the Ca2+ concentrations and Ca2+ depletion are known to trigger apoptosis by directly activating the ER-resident proteins CHOP, p-JNK and caspase-1212,44,45,46. Additionally, Ang II can directly cause contractile dysfunction and heart failure, and the underlying mechanism may be partially associated with disturbances in Ca2+ homeostasis and down-regulation of Ca2+-modulating proteins47,48. Our data support a model of Ang II-induced apoptosis in which increased cytoplasmic Ca2+ concentrations lead to ER Ca2+ leakage. The CHOP, JNK and caspase-12 ER stress pathways were significantly induced in the Ang II-treated cardiomyocytes. Although the ER stress-induced apoptotic pathway has not been fully characterized, three hallmarks of the process have been identified, including the upregulation of pro-apoptotic transcription factor CHOP, JNK12,44,45 and the activation of the ER-localized caspase-1245,46. After triggering ER stress, GRP78 expression is also dramatically elevated, which may be a biomarker of ER stress. Our data suggest that BAPTA, an intracellular Ca2+ chelator, inhibited aberrant ER stress and apoptosis (Figure 8), a finding that is consistent with other reports49. We also treated the H9c2 cells with TG or TM as positive controls for ER stress, and OPD reduced the Ang II-, TG-, and TM-induced ER stress and stress-mediated apoptosis (Figure 5). Importantly, OPD reversed the down-regulation of the Ca2+-regulating protein and attenuated Ang II-induced intracellular Ca2+ overload (Figure 4). Therefore, OPD may confer protective effects against ER stress-mediated cardiomyocyte apoptosis by maintaining intracellular Ca2+ homeostasis.
Studies that screen CYP2J3 inducers that regulate the CYP2J3/EETs system may offer new leads for treating cardiovascular disease. Xenobiotic modulation of CYP subtype genes is mediated by activated nuclear receptors, and CYP2J3 appears to be unresponsive to typical xenobiotic inducers. A previous study indicated that butylated hydroxyanisole (BHA) is a novel inducer of CYP2J2 expression in HepG2 cells, and BHA (10–100 μmol/L) increased CYP2J2 mRNA and protein expression50. Here, the induction of CYP2J3 (∼2.8-fold) by OPD (250 nmol/L) was comparable with its induction with 100 μmol/L BHA. Thus, OPD is a novel and potent natural product that can induce CYP2J3 expression and activity in vivo.
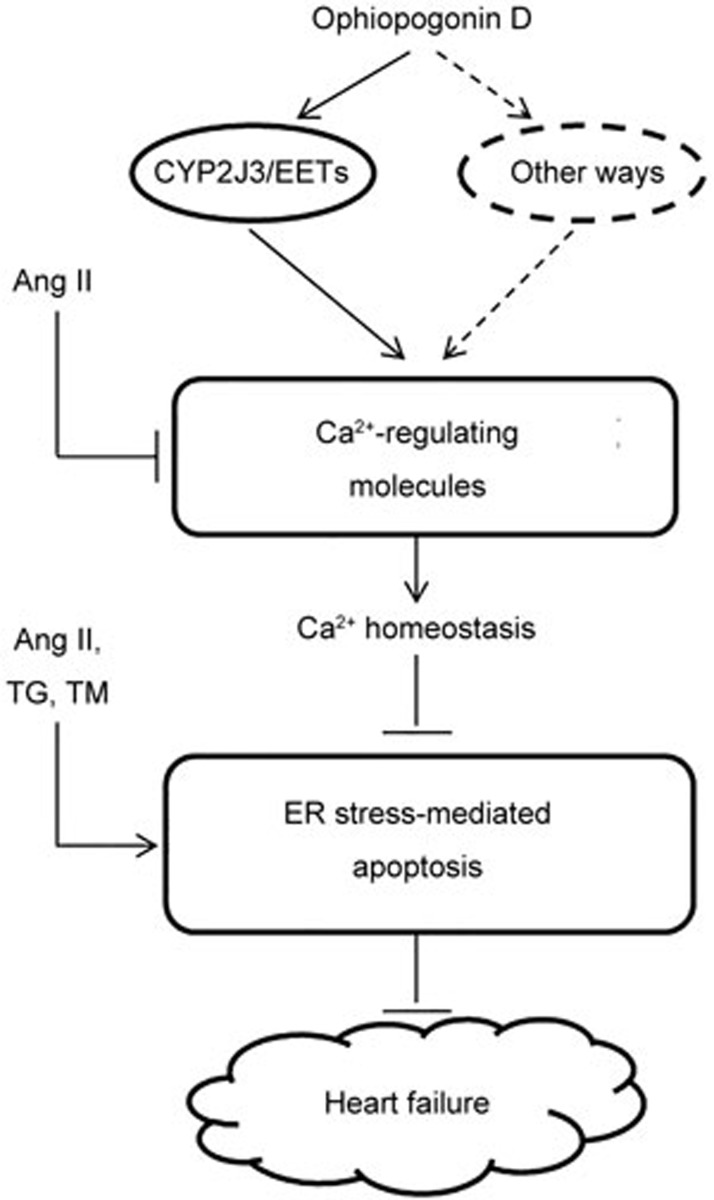
In summary (Figure 9), we offer insights into the role of CYP2J3/EETs in protecting cardiomyocytes. OPD significantly reduced intracellular Ca2+ overload by upregulating the levels of CYP2J3/EETs. OPD also suppressed drug-induced ER stress and stress-mediated apoptosis in cardiomyocytes. We suggest that the CYP2J3/EETs-mediated myocardial protection program may be essential for the cardioprotective effects of OPD. More studies are needed to assess the physiological significance of ophiopogonin D-activated CYP2J3 expression in animal models using CYP2J3 overexpression or knockout approaches.
Figure 9.
A simplified scheme indicates the potential pathways induced by OPD in cardiomyocytes. As depicted, OPD reverses the Ang II-induced decrease in the expression of the calcium handling proteins and protects calcium homeostasis in cardiomyocytes at least partly by upregulating CYP2J3 expression. Elevation of the cytosolic calcium concentrations is a common mechanism of abnormal ER stress and stress-mediated apoptosis. OPD suppresses Ang II-, TG-, and TM-induced ER stress-mediated apoptosis in cardiomyocytes. CYP2J3/EETs-mediated myocardial protection program may be essential for the cardioprotective effects of OPD. ↓ indicates activation or induction, and ┴ indicates inhibition or blockade.
Author contribution
Yu-guang WANG designed this study; Tao ZHOU, Wen-ting YOU and Yu-guang WANG performed most of the experiments, analyzed the data and drafted the manuscript; Zeng-chun MA, Qian-de LIANG, Xiang-lin TANG, Hong-ling TAN, and Cheng-rong XIAO assisted with in the experiments; Bo-li ZHANG and Yue GAO reviewed the manuscript.
Acknowledgments
This work was supported by the National Basic Research Program of China (“973 Program”) (No 2011CB505304 and 2012CB518402), and the Scientific and Technological Major Special Project Major Creation of New Drugs (No 2009ZX09501-304).
Footnotes
Supplementary information is available at the Acta Pharmacologica Sinica's website.
Supplementary Information
Primer sequences used for qRT-PCR
Stable H9c2 cell lines with CYP2J3 overexpression.
11,12-EET and 14,15-EET restored SERCA2a expression in cardiomyocytes treated with AngII
14,15-EET restored calcium-regulating molecules expression in cardiomyocytes treated with AngII
Knocking down CYP2J3 expression using its specific siRNA
References
- Clapham DE. Calcium signaling. Cell 2007; 131: 1047–58. [DOI] [PubMed] [Google Scholar]
- Wang W, Barnabei MS, Asp ML, Heinis FI, Arden E, Davis J, et al. Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance. Nat Med 2013; 19: 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med 2000; 6: 1221–7. [DOI] [PubMed] [Google Scholar]
- Gianni D, Chan J, Gwathmey JK, del Monte F, Hajjar RJ. SERCA2a in heart failure: role and therapeutic prospects. J Bioenerget Biomembr 2005; 37: 375–80. [DOI] [PubMed] [Google Scholar]
- Camors E, Valdivia HH. CaMKII regulation of cardiac ryanodine receptors and inositol triphosphate receptors. Frontiers Pharmacol 2014; 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang H, Xia W, Tang Y, Li H, Huang C. NADPH oxidase inhibition ameliorates cardiac dysfunction in rabbits with heart failure. Mol Cell Biochem 2010; 343: 143–53. [DOI] [PubMed] [Google Scholar]
- Kawase Y, Ladage D, Hajjar RJ. Rescuing the failing heart by targeted gene transfer. J Am Coll Cardiol 2011; 57: 1169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obesity Metab 2010; 12 Suppl 2: 108–15. [DOI] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest 2002; 110: 1383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 2002; 32: 269–78. [DOI] [PubMed] [Google Scholar]
- Verkhartsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 2005; 85: 201–79. [DOI] [PubMed] [Google Scholar]
- Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radical Biol Med 2015; 78: 30–41. [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res 2010; 107: 1185–97. [DOI] [PubMed] [Google Scholar]
- Askari A, Thomson SJ, Edin ML, Zeldin DC, Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat 2013; 107: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Narayanan J, Harder D, Maier KG. Identifying endothelium-derived hyperpolarizing factor: recent approaches to assay the role of epoxyeicosatrienoic acids. Jap J Pharmacol 2001; 86: 369–75. [DOI] [PubMed] [Google Scholar]
- Westphal C, Spallek B, Konkel A, Marko L, Qadri F, DeGraff LM, et al. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PloS One 2013; 8: e73490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000; 21: 125–7. [DOI] [PubMed] [Google Scholar]
- Wang HX, Zhang DM, Zeng XJ, Mu J, Yang H, Lu LQ, et al. Upregulation of cytochrome P450 2J3/11,12-epoxyeicosatrienoic acid inhibits apoptosis in neonatal rat cardiomyocytes by a caspase-dependent pathway. Cytokine 2012; 60: 360–8. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, et al. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther 2011; 339: 451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ni L, Yang L, Duan Q, Chen C, Edin ML, et al. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol Pharmacol 2014; 85: 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, et al. Chronic phospholamban–sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 1999; 99: 313–22. [DOI] [PubMed] [Google Scholar]
- White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A 2000; 97: 5428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR. SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. Br J Pharmacol 2014; 171: 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A 2006; 103: 511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou J, Tian Y, Tang Y, Yan J, Yu B. Antithrombotic activities of aqueous extract from Radix Ophiopogon japonicus and its two constituents. Biol Pharm Bull 2006; 29: 1267–70. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhu D, Qi J, Qin M, Yu B. Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J Pharm Biomed Anal 2010; 52: 757–62. [DOI] [PubMed] [Google Scholar]
- Lu LY, Zheng GQ, Wang Y. An overview of systematic reviews of shenmai injection for health care. Evidence-based Complement Alter Med 2014; 2014: 840650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Jiang F, Wang B, Yu Y, Zhang X, Yin Z, et al. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J Ethnopharmacol 2010; 128: 438–45. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Meng C, Zhang XM, Yuan CH, Wen MD, Chen Z, et al. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J Pharmacol Exp Ther 2015; 352: 166–74. [DOI] [PubMed] [Google Scholar]
- Wang YG, Zhou JM, Ma ZC, Li H, Liang QD, Tan HL, et al. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: a possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem Biol Interact 2012; 200: 11–20. [DOI] [PubMed] [Google Scholar]
- Zhou T, Wang YG, Ma ZC, Xiao Y, Tang XL, Tan HL, et al. Ginkgolide B induces CYP3A4 expression through activation of human pregnane X receptor. Chin Pharmacol Bull 2014; 30: 931–6. [Google Scholar]
- Zhao L, Wu J, Zhang X, Kuang H, Guo Y, Ma L. The effect of Shenmai injection on the proliferation of rat airway smooth muscle cells in asthma and underlying mechanism. BMC Complement Altern Med 2013; 13: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 2005; 65: 4707–15. [DOI] [PubMed] [Google Scholar]
- Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG, et al. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Disease 2013; 4: e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 2008; 27: 285–99. [DOI] [PubMed] [Google Scholar]
- Xiao B, Li X, Yan J, Yu X, Yang G, Xiao X, et al. Overexpression of cytochrome P450 epoxygenases prevents development of hypertension in spontaneously hypertensive rats by enhancing atrial natriuretic peptide. J Pharmacol Exp Ther 2010; 334: 784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Tang XY, Dai DZ, Dai Y. Reversal of isoproterenol-induced downregulation of phospholamban and FKBP12.6 by CPU0213-mediated antagonism of endothelin receptors. Acta Pharmacol Sin 2007; 28: 1746–54. [DOI] [PubMed] [Google Scholar]
- Saliaris AP, Amado LC, Minhas KM, Schuleri KH, Lehrke S, St John M, et al. Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and beta-adrenergic hyporesponsiveness in heart failure. Am J Physiol Heart Circ Physiol 2007; 292: 1328–35. [DOI] [PubMed] [Google Scholar]
- Mattiazzi A, Kranias EG. The role of CaMKII regulation of phospho-lamban activity in heart disease. Frontiers Pharmacol 2014; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000; 101: 365–76. [DOI] [PubMed] [Google Scholar]
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 2002; 91: 1015–22. [DOI] [PubMed] [Google Scholar]
- Li J, Imtiaz MS, Beard NA, Dulhunty AF, Thorne R, vanHelden DF, et al. ss-Adrenergic stimulation increases RyR2 activity via intracellular Ca2+ and Mg2+ regulation. PloS One 2013; 8: e58334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell 2007; 131: 1047–58. [DOI] [PubMed] [Google Scholar]
- Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012; 10: 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Wang L, Wang B, Wang T, Yang G, Shen L, et al. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt. Cell Death Disease 2014; 5: e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YR, Xu Y, Fang JF, Zhou F, Deng XW, Zhang YQ. Bufotalin-induced apoptosis in osteoblastoma cells is associated with endoplasmic reticulum stress activation. Biochem Biophys Res Commun 2014; 451: 112–8. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension 2005; 46: 426–32. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, et al. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 1997; 29: 859–70. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Cardiac Failure 2008; 14: 355–67. [DOI] [PubMed] [Google Scholar]
- Lee AC, Murray M. Up-regulation of human CYP2J2 in HepG2 cells by butylated hydroxyanisole is mediated by c-Jun and Nrf2. Mol Pharmacol 2010; 77: 987–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used for qRT-PCR
Stable H9c2 cell lines with CYP2J3 overexpression.
11,12-EET and 14,15-EET restored SERCA2a expression in cardiomyocytes treated with AngII
14,15-EET restored calcium-regulating molecules expression in cardiomyocytes treated with AngII
Knocking down CYP2J3 expression using its specific siRNA