Abstract
In skeletal muscle excitation-contraction coupling, a voltage-gated calcium channel directly activates opening of the calcium release channel (RyR1) in the sarcoplasmic reticulum that supplies the calcium signal triggering contraction. In addition, a retrograde signal from the RyR1 facilitates gating of the voltage-gated calcium channel. Recent studies of RyR1 mutants, including the article by Bannister et al. in this issue of the Biophysical Journal, advance our understanding of the signaling mechanism, although the physiological significance of retrograde coupling remains elusive.
Main Text
Upon stimulation of skeletal muscle, the voltage-gated calcium channel CaV1.1 in the T-tubules senses the membrane depolarization and activates calcium release channels from the adjacent cisternae of the sarcoplasmic reticulum (SR) through physical interactions with the type 1 ryanodine receptor (RyR1). The calcium released from the SR is necessary and sufficient to activate contraction, whereas calcium influx is dispensable for skeletal muscle excitation-contraction (EC) coupling. In fact, L-type calcium currents (LTCC) through the adult CaV1.1a isoform are small, require high membrane potentials for activation, and activate slowly. Interestingly, these current properties depend on interaction of CaV1.1 with the RyR1. Twenty years ago, Nakai et al. (1) noticed that in cultured myotubes derived from dyspedic (RyR1-null) mice, the LTCC amplitude was dramatically reduced and its activation kinetics accelerated. Normal calcium current density could be restored by heterologous expression of RyR1. This observation gave rise to the bidirectional coupling hypothesis according to which CaV1.1 and RyR1 not only interact in EC coupling (termed “orthograde coupling”), but also in the retrograde direction in which the RyR1 supports normal channel function of CaV1.1.
In subsequent studies, the retrograde coupling model has been refined (Fig. 1). The >10-fold current reduction was in part explained by a reduced channel open probability and in part by a reduction in the number of the channels (3). The latter may be the consequence of decreased CaV1.1 expression in myotubes lacking SR calcium release and EC coupling. The effects on open probability were accompanied by an increased speed of current activation and an increased sensitivity to the LTCC agonist Bay K 8644 in dyspedic myotubes (3). Furthermore, pharmacological block of the RyR1 was shown to shift the voltage-dependence of activation and inactivation of the LTCC (4). Thus, retrograde coupling of the RyR1 and CaV1.1 is important to achieve normal current densities, gating, and the pharmacological properties of skeletal muscle LTCCs.
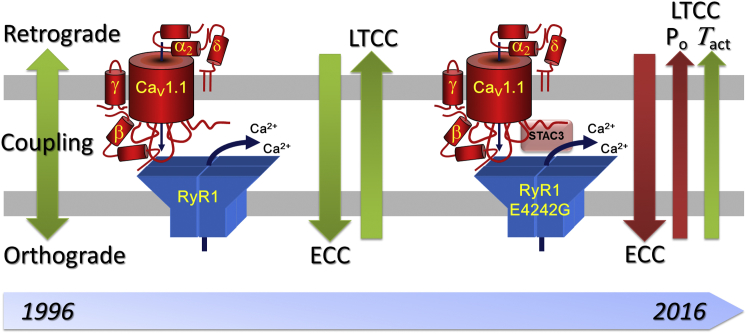
Figure 1.
Evolution of retrograde coupling in skeletal muscle. Initially orthograde excitation-contraction coupling (ECC) and retrograde coupling (RyR1-dependent augmentation of LTCC) were perceived as the two effects of a single coupling mechanism. Later it became clear that the molecular mechanisms are partially separate. Now Bannister et al. (2) demonstrate that, in the RyR1-E4242 mutation, the retrograde action on activation kinetics of LTCCs is unaffected, whereas the effects on CaV1.1 open probability and EC coupling are lost.
In parallel studies, important details about the molecular mechanism underlying retrograde coupling were elucidated. Using chimeras of CaV1.1 and other CaV isoforms, a sequence in the cytoplasmic loop connecting the homologous repeats II and III of CaV1.1 was identified, which is essential for skeletal muscle type EC coupling (5). Importantly, the same sequence is equally important for retrograde coupling; identifying the II-III loop as a critical component of bidirectional CaV1.1-RyR1 interactions and suggesting that activation of the RyR1 by CaV1.1 and the enhancement of CaV1.1 currents by RyR1 merely are two effects of the same coupling mechanism. However, applying a similar approach to the RyR1 indicated that the two coupling mechanisms can be separated. In chimeras of RyR1 with either RyR2 or RyR3, some RyR1 sequence domains were important for both EC coupling and retrograde coupling, while others supported only one or the other mechanism (6, 7). This demonstrated that the molecular mechanisms supporting EC coupling and retrograde coupling are in part overlapping and in part specific. However, due to its enormous size and complex structure, further attempts to dissect the specific structures underlying EC coupling and retrograde coupling were met with little success.
This is where studies of mouse mutants come into play. Malignant hyperthermia is a pharmacogenetic muscle disease characterized by an overly sensitive calcium release machinery that can be activated by excessive heat or exposure to volatile anesthetics. Many mutations in RyR1 and several in CaV1.1 have been linked to malignant hyperthermia and some of these have recently been introduced in mouse models. Interestingly, two such mutations in RyR1 affect the CaV1.1 EC-voltage sensing function as well as its current properties (8, 9, 10). In this issue of the Biophysical Journal, Bannister et al. (2) report on a new RyR1 mutation. The RyR1-E4242G mutation has previously been shown to ablate EC coupling (11). In the current study, the researchers from the University of Colorado demonstrate that the RyR1-E4242G mutation also reduces the LTCC amplitude by 80%. Biophysical analysis of the gating currents and tail currents indicated that about half of the reduced current amplitude in RyR1-E4242G myotubes was due to reduced expression of the channel and the other half due to reduction in the channel open probability. Interestingly, however, the activation kinetics of LTCC in the RyR-E4242G mutant was not affected. The perturbation of EC coupling and CaV1.1 open probability could indicate that the point mutation affects the intrinsic gating mechanisms of both the RyR1 and the CaV1.1. Alternatively, the mutation could affect only the gating of CaV1.1, resulting in reduced conduction and in a loss of voltage-sensing, and thus indirectly cause the loss of EC coupling. In any case, the observation that current kinetics is not affected by the RyR1-E4242G mutation indicates the existence of multiple retrograde coupling interactions.
If we picture EC coupling as a classical signal transduction cascade with sequential actions of the CaV1.1 and RyR1 in voltage-sensing and SR calcium release, respectively, the existence of such a complex retrograde signaling along the same pathway is puzzling. Even more so, when we consider that the physiological importance of LTCC is still highly disputed. Without a function to be regulated, the necessity of this signaling pathway is not obvious and retrograde coupling could be regarded as an orphan signaling pathway. On the other hand, if we picture the EC coupling system as a complex molecular machine, the fact that the normal function of each component depends on the functionality of any other component is not surprising at all. Or would a bicyclist be surprised if damage of his/her bicycle gears would not only result in failure to actuate the rear wheel but also cause a jammed or idle turning crank? Certainly not! Looking this way at the EC coupling machine accounts for multiple interactions in both directions and disconnects the existence of these interactions from the question as to its physiological function. The recent discoveries of hitherto unnoticed components of the EC coupling machine (12, 13) and the high-resolution structure information on the RyR1 promise that before long we may know how the multiple parts of the EC coupling machine fit together. In combination with further mutagenesis studies and studies of disease mutations like that by Bannister et al. (2), these advances will eventually contribute to a mechanistic model of the fascinating molecular machine that regulated the activation of skeletal muscle contraction.
Acknowledgments
Current research in the laboratory of B.E.F. is funded by the Austrian Science Fund (FWF) under grants P23479, P27031, and W1101.
Editor: Michael Pusch.
References
- 1.Nakai J., Dirksen R.T., Allen P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 2.Bannister R.A., Sheridan D.C., Beam K.G. Distinct components of retrograde Cav1.1-RyR1 coupling revealed by a lethal mutation in RyR1. Biophys. J. 2016;110:912–921. doi: 10.1016/j.bpj.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avila G., Dirksen R.T. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J. Gen. Physiol. 2000;115:467–480. doi: 10.1085/jgp.115.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannister R.A., Beam K.G. Ryanodine modification of RyR1 retrogradely affects L-type Ca2+ channel gating in skeletal muscle. J. Muscle Res. Cell Motil. 2009;30:217–223. doi: 10.1007/s10974-009-9190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabner M., Dirksen R.T., Beam K.G. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999;274:21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- 6.Nakai J., Sekiguchi N., Beam K.G. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J. Biol. Chem. 1998;273:13403–13406. doi: 10.1074/jbc.273.22.13403. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan D.C., Takekura H., Perez C.F. Bidirectional signaling between calcium channels of skeletal muscle requires multiple direct and indirect interactions. Proc. Natl. Acad. Sci. USA. 2006;103:19760–19765. doi: 10.1073/pnas.0609473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andronache Z., Hamilton S.L., Melzer W. A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice. Proc. Natl. Acad. Sci. USA. 2009;106:4531–4536. doi: 10.1073/pnas.0812661106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estève E., Eltit J.M., López J.R. A malignant hyperthermia-inducing mutation in RYR1 (R163C): alterations in Ca2+ entry, release, and retrograde signaling to the DHPR. J. Gen. Physiol. 2010;135:619–628. doi: 10.1085/jgp.200910328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannister R.A., Estève E., Beam K.G. A malignant hyperthermia-inducing mutation in RYR1 (R163C): consequent alterations in the functional properties of DHPR channels. J. Gen. Physiol. 2010;135:629–640. doi: 10.1085/jgp.200910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartz Hanson M., Niswander L.A. Rectification of muscle and nerve deficits in paralyzed ryanodine receptor type 1 mutant embryos. Dev. Biol. 2015;404:76–87. doi: 10.1016/j.ydbio.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson B.R., Wu F., Olson E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horstick E.J., Linsley J.W., Kuwada J.Y. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]