Abstract
Care practices and risk factors for diarrhea among impoverished communities across Mesoamerica are unknown. Using Salud Mesoamérica Initiative baseline data, collected 2011–2013, we assessed the prevalence of diarrhea, adherence to evidence-based treatment guidelines, and potential diarrhea correlates in poor and indigenous communities across Mesoamerica. This study surveyed 14,500 children under 5 years of age in poor areas of El Salvador, Guatemala, Mexico (Chiapas State), Nicaragua, and Panama. We compared diarrhea prevalence and treatment modalities using χ2 tests and used multivariable Poisson regression models to calculate adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) for potential correlates of diarrhea. The 2-week point prevalence of diarrhea was 13% overall, with significant differences between countries (P < 0.05). Approximately one-third of diarrheal children were given oral rehydration solution and less than 3% were given zinc. Approximately 18% were given much less to drink than usual or nothing to drink at all. Antimotility medication was given to 17% of diarrheal children, while antibiotics were inappropriately given to 36%. In a multivariable regression model, compared with children 0–5 months, those 6–23 months had a 49% increased risk for diarrhea (aRR = 1.49, 95% CI = 1.15, 1.95). Our results call for programs to examine and remedy low adherence to evidence-based treatment guidelines.
Introduction
In Central America, the burden of diarrheal disease among children under 5 years of age decreased by more than 80% between 1990 and 2010.1 Despite this success, the regional burden of diarrhea remains substantial. It is estimated that, in 2010, diarrhea caused 3,322 disability-adjusted life years and 29.7 deaths per 100,000 among children under 5 years of age in the region.1,2 Socioeconomic inequalities can lead to disparities in access to affordable health care, basic water and sanitation infrastructure, and maternal education, all of which may impact the health status of children.3 With a high level of socioeconomic inequality, indicated by a regional mean Gini coefficient of 49 in 2010,4 it should be no surprise that poor and indigenous communities in Mesoamerica bear a disproportionate health burden.5
Most diarrheal deaths are due to dehydration,6,7 and more than 90% of them can be prevented with use of oral rehydration solution (ORS).8 However, despite the overwhelming evidence of its life-saving ability,9 use of ORS has plateaued globally.6 Similarly, despite consistent evidence that it reduces stool frequency, diarrhea duration, and diarrhea recurrence,10–12 zinc supplementation during and after diarrhea episodes is thought to be rare.
The World Health Organization and the United Nations Children's Fund (UNICEF) created evidence-based recommendations regarding diarrhea treatment.7 Chief among them was the recommendation to give low-osmolarity ORS to prevent and treat dehydration in diarrheal patients and to give zinc supplements for 10–14 days to all children with diarrhea. The manual also recommended the continued feeding of children during and after illness. Equally important, the manual proscribed the use of antimotility medications in children and recommended against the routine use of antibiotics, except in cases of bloody diarrhea or severe dehydration caused by suspected cholera.
Our objective was to estimate the 2-week point prevalence of diarrhea among children under 5 years of age using population-based household survey data for the poorest quintile of families living in Guatemala, Mexico (Chiapas), Nicaragua, Panama, and El Salvador. We also sought to compare actual diarrhea treatment modalities with evidence-based guidelines and to identify diarrheal risk factors.
Materials and Methods
Data source.
We analyzed baseline measurement data from the Salud Mesoamérica Initiative (SMI), a result-based financing program that seeks to improve maternal and child health among the poorest quintile of persons living in Belize, Costa Rica, El Salvador, Guatemala, Honduras, Mexico (Chiapas State), Nicaragua, and Panama. The baseline survey methodology has previously been described in detail.13 In brief, within each country, the poorest fifth of municipalities were identified based on aggregate level measures. The exception was Mexico where the survey was limited to what is widely known to be the poorest state, Chiapas. Localities were selected from among all SMI municipalities, with probability proportional to size. Localities served as the primary sampling unit, with each consisting of approximately 150 households. On the basis of the results of our census, within each locality, SMI randomly selected 30 households with women 15–49 years of age or children under 5 years of age to complete detailed surveys. In addition, a sample of public health facilities in selected municipalities was surveyed. Field staff used DatStat Illume (DatStat, Seattle, WA) to conduct computer-assisted personal interviews. Surveys were conducted in Spanish or local indigenous languages, as appropriate.
The study received institutional review board approval from the University of Washington, partnering data collection agencies, and the Ministry of Health in each country. Written informed consent was obtained from all participants or their caretakers or guardians when applicable.
Study population.
Survey data regarding diarrhea were available for five countries: Guatemala (April 2013 to August 2013), Mexico (Chiapas State) (July 2012 to May 2013), Nicaragua (March 2013 to August 2013), Panama (April 2013 to August 2013), and El Salvador (March 2011 to July 2011). Analyses were limited to households with children under 5 years of age. Relevant data were not available for Costa Rica and Belize.
Diarrhea assessment.
As part of the household survey, mothers were asked, “In the last 2 weeks, has your child had diarrhea? If so, was there blood in the stool?” Following Demographic and Health Survey (DHS) and UNICEF Multiple Indicator Cluster Survey conventions, the local term for diarrhea was used and no clinical definition was provided to the caregiver.14 Children of caregivers who responded “yes, diarrhea with blood” or “yes, diarrhea without blood” were considered to have had diarrhea. Children of those responding “yes, diarrhea with blood” were considered to have had bloody diarrhea.
Data analysis.
Characteristics of children and their mothers were compared across the five countries using χ2 tests. Sociodemographic factors included the child's age in months, sex, and firstborn status. Maternal factors included indigenous ethnicity (Guatemala, Mexico, and Nicaragua only), age in years, highest level of education attained, literacy, marital status, and whether she was a housewife. Mothers in households where an indigenous language was spoken, whether it was their primary language, were considered indigenous. Literacy was defined as the ability to read the sentence, “La salud del niño es muy importante para su desarrollo en la vida,” or its equivalent in an indigenous language, in its entirety. Urban residence and a household asset index were also assessed. Water and sanitation-related variables included the principal source of household water (tap, borewell, other well or spring, and other), water treatment (boiling, chlorination, filtration, and other), the type of toilet used by the family (flush/pour flush, latrine/toilet with hole, dry toilet, no toilet, and other), and whether the toilet was shared with other homes. For children under 24 and 6 months of age, we also assessed current breast-feeding status and exclusive breast-feeding status, respectively.
Urban residence and indigeneity were not assessed in Panama because the sampled population was expected to be nearly entirely indigenous and rural. Language was not assessed in El Salvador because the entire population spoke Spanish. The household asset index was created by giving a point for owning specific household durable goods (e.g., a refrigerator or clothes washer), modes of transportation (scooter, car, or truck), land, and livestock. The sum was divided into quintiles, with the lower two quintiles categorized as “low,” the next two categorized as “medium,” and the remaining quintile categorized as “high.”
We used χ2 tests to compare diarrheal treatment and care-seeking behavior across countries. Factors of interest included whether the child was given ORS or zinc, how much the child was given to drink or eat, and whether the child received antimotility medication or antibiotics. Use of antibiotics was divided into appropriate use (for bloody diarrhea) and inappropriate use (for non-bloody diarrhea). Regarding fluid consumption, the mothers were asked, “When your child had diarrhea, did you give the child less to drink than usual, about the same as usual, more than usual, or nothing at all?” If the mothers responded that it was less than usual, the mothers were also asked, “Did you give them much less to drink than usual or a little less than usual?” Questions regarding food consumption followed the same pattern.
We also asked whether the mother sought medical advice or treatment and, if so, where. In Panama, we were able to link approximately 80% of women to their “usual” health facilities that we surveyed. Using these linked data, we assessed whether supply-side factors (availability of ORS, antibiotics, and educational materials in the health facilities) were associated with treatment modalities. Linkages were substantially less frequent in other countries, so they were not analyzed. In addition, we sought to determine whether the distribution of treatment modalities was affected by whether the mother sought medical advice or treatment.
Within each country, we used χ2 tests to compare differences in the prevalence of diarrhea by department (Guatemala, Nicaragua, Panama, and El Salvador) or health jurisdiction (Mexico). The proportion of children given ORS, zinc, less to eat or drink than usual, antimotility medication, and antibiotics were also compared across departments and jurisdictions.
Using χ2 tests, we compared the most commonly cited reasons why mothers did not seek care for their child with diarrhea between countries. These data were not available for El Salvador.
Statistical analysis.
We calculated combined and country-specific risk ratios (RRs) and 95% confidence intervals (95% CIs) for diarrhea using Poisson regression with robust variance estimates. We estimated RRs rather than odds ratios due to the greater ease of interpreting RRs. We used Poisson regression with robust variance estimators because resulting RRs were identical to those calculated by log-binomial regression, while reducing problems of model convergence.15 Univariable risk estimates with a P value < 0.10 were considered candidates for a multivariable regression model. Using variance inflation factors > 10 as a threshold, we found no evidence of collinearity among candidate variables. Candidates were ranked in order of the strength of association and added to the model one at a time. Retention of the newly added variable was based on having a Wald test P value of < 0.05. Once a candidate variable was included in the multivariable model, it was not removed if its P value became ≥ 0.05 after the addition of subsequent candidates. Our sole a priori confounder was country in the combined country analysis. All variables had less than 5% missing data.
Following the methods discussed in the previous paragraph, we also calculated RRs and 95% CIs for correlates of positive feeding practices. We defined positive feeding practice as giving an ill child approximately the same amount or more than the usual amount of food and drink.
We used Stata/IC 13.1 (StataCorp LP, College Station, TX) for the analyses and to account for the complex survey design. ArcGIS Desktop 10 (Environmental Systems Research Institute, Redlands, CA) was used to produce subnational maps. All P values are two-sided, and P = 0.05 was set as our a priori threshold for statistical significance.
Results
Study population.
We analyzed data from a total of 14,500 children (Table 1). The median child age range was 24–35 months overall and in every country, and the age distribution differed by country (P = 0.03). Indigenous children comprised 80.0% of the sample in Guatemala, 70.1% in Chiapas, and 11.0% in Nicaragua. The mean maternal age was 27.8 years overall, with a median maternal age range of 25–34 years in all countries. The overall proportion of literate women was 58.0% and ranged from 76.2% in El Salvador to 37.2% in Guatemala (P < 0.01). In the pooled data, 74.3% of households used tap water, ranging from 81.4% using tap water in Guatemala to 44.5% in Panama. Across countries, 63.6% of households treated their drinking water, with a high of 92.2% in Guatemala and a low of 20.9% in Nicaragua (P < 0.01). Overall, 51.7% of women boiled drinking water, 9.0% chlorinated drinking water, and less than 1% strained or filtered drinking water. Boiling water was the most common treatment method in Guatemala, Mexico, and Panama, whereas chlorination was the most common method in Nicaragua and El Salvador. There were significant differences in the type of household toilet facilities and the percentage of households who shared their toilet with other homes (P < 0.01).
Table 1.
Characteristics of children under 5 years of age and their mothers in the SMI, 2011–2013
| Guatemala (N = 4,100) | Mexico (N = 4,710) | Nicaragua (N = 1,312) | Panama (N = 913) | El Salvador (N = 3,465) | P* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Child's age (months) | 0.027 | ||||||||||
| 0–5 | 10.6 | 9.5–11.7 | 9.4 | 8.5–10.4 | 9.4 | 7.7–11.6 | 11.4 | 9.6–13.5 | 8.4 | 7.4–9.6 | |
| 6–11 | 11.1 | 10.3–12.0 | 10.0 | 9.2–10.8 | 12.2 | 10.6–14.1 | 10.5 | 8.6–12.9 | 9.4 | 8.3–10.7 | |
| 12–23 | 21.6 | 20.4–22.9 | 20.6 | 19.4–21.8 | 21.9 | 19.6–24.3 | 21.2 | 19.0–23.6 | 22.7 | 21.2–24.3 | |
| 24–35 | 21.4 | 20.0–22.9 | 19.9 | 18.8–21.1 | 19.3 | 17.4–21.5 | 21.7 | 19.8–23.8 | 21.2 | 19.8–22.8 | |
| 36–47 | 19.8 | 18.6–21.1 | 21.4 | 20.2–22.7 | 18.9 | 16.7–21.2 | 20.8 | 17.9–24.0 | 20.2 | 18.9–21.6 | |
| 48–59 | 15.4 | 14.3–16.6 | 18.8 | 17.7–19.9 | 18.2 | 16.3–20.4 | 14.4 | 12.1–16.9 | 18.1 | 16.8–19.4 | |
| Child's sex | 0.722 | ||||||||||
| Male | 50.0 | 48.3–51.6 | 49.8 | 48.1–51.4 | 50.0 | 47.1–52.9 | 48.0 | 44.8–51.2 | 48.0 | 46.3–49.7 | |
| Female | 50.0 | 48.4–51.7 | 50.2 | 48.6–51.9 | 50.0 | 47.1–52.9 | 52.0 | 48.8–55.2 | 52.0 | 50.3–53.7 | |
| Firstborn child | 20.5 | 18.8–22.3 | 16.6 | 14.9–18.4 | 28.5 | 25.3–32.1 | 12.9 | 10.5–15.7 | 29.1 | 26.9–31.4 | < 0.001 |
| Indigenous ethnicity | 80.0 | 74.2–84.8 | 70.1 | 63.4–76.1 | 11.0 | 4.6–24.1 | 0.0 | – | 0.0 | – | < 0.001 |
| Mother's age (years) | 0.013 | ||||||||||
| 15–24 | 34.2 | 32.2–36.3 | 35.9 | 33.7–38.1 | 40.8 | 36.9–44.7 | 38.2 | 34.9–41.6 | 38.6 | 36.0–41.4 | |
| 25–34 | 45.5 | 43.4–47.5 | 45.7 | 43.5–47.9 | 40.0 | 36.3–43.8 | 37.9 | 33.9–42.1 | 40.9 | 38.6–43.4 | |
| 35–49 | 20.3 | 18.6–22.1 | 18.4 | 16.6–20.3 | 19.2 | 16.6–22.1 | 23.9 | 20.9–27.2 | 20.4 | 18.4–22.6 | |
| Highest level of education attained | < 0.001 | ||||||||||
| None | 34.7 | 31.0–38.6 | 17.5 | 14.8–20.5 | 12.8 | 9.9–16.5 | 18.5 | 12.7–26.3 | 11.3 | 9.4–13.7 | |
| Primary | 50.5 | 47.9–53.1 | 52.3 | 48.8–55.9 | 53.9 | 49.2–58.5 | 55.9 | 50.0–61.6 | 58.1 | 54.9–61.3 | |
| Secondary | 7.8 | 6.3–9.6 | 19.4 | 17.4–21.6 | 24.1 | 21.0–27.6 | 19.2 | 15.3–23.9 | 26.4 | 23.4–29.6 | |
| High school or higher | 7.0 | 5.1–9.6 | 10.8 | 8.5–13.5 | 9.2 | 6.4–13.0 | 6.3 | 4.3–9.3 | 4.1 | 3.1–5.4 | |
| Mother is literate | 37.2 | 33.2–41.2 | 54.4 | 50.4–58.4 | 69.5 | 65.1–73.5 | 56.5 | 48.8–63.8 | 76.2 | 72.9–79.3 | < 0.001 |
| Marital status | < 0.001 | ||||||||||
| Single | 6.6 | 5.5–7.8 | 1.5 | 1.1–2.1 | 14.7 | 12.1–17.8 | 6.8 | 4.7–9.6 | 12.8 | 11.1–14.8 | |
| Married | 37.1 | 33.5–40.8 | 33.6 | 30.0–37.4 | 34.9 | 31.6–38.3 | 8.3 | 5.9–11.7 | 35.9 | 32.6–39.3 | |
| Domestic partnership | 52.0 | 48.2–55.8 | 60.3 | 56.5–63.9 | 44.2 | 40.6–47.8 | 79.1 | 74.2–83.3 | 40.4 | 37.5–43.4 | |
| Other | 4.4 | 3.7–5.1 | 4.6 | 3.9–5.5 | 6.2 | 4.5–8.6 | 5.8 | 4.0–8.2 | 10.9 | 9.3–12.8 | |
| Mother is a housewife | 93.8 | 91.5–95.5 | 92.8 | 90.8–94.5 | 86.2 | 82.8–89.1 | 94.5 | 91.6–96.4 | 89.9 | 88.0–91.6 | < 0.001 |
| Urban resident | 15.2 | 9.7–23.1 | 34.4 | 27.3–42.4 | 19.8 | 10.7–33.8 | 0.0 | – | 22.6 | 16.4–30.4 | 0.018 |
| Household asset index | < 0.001 | ||||||||||
| Low | 40.6 | 36.3–44.9 | 53.7 | 49.8–57.6 | 43.2 | 38.4–48.2 | 53.3 | 44.1–62.2 | 46.6 | 42.9–50.4 | |
| Medium | 42.7 | 39.7–45.7 | 31.9 | 29.4–34.6 | 45.6 | 41.5–49.8 | 27.9 | 22.7–33.8 | 37.2 | 34.5–40.0 | |
| High | 16.8 | 14.0–19.9 | 14.3 | 12.3–16.7 | 11.1 | 8.9–13.9 | 18.8 | 12.5–27.2 | 16.2 | 14.1–18.5 | |
| Principal source of household water | < 0.001 | ||||||||||
| Tap | 81.4 | 77.4–84.9 | 78.2 | 72.8–82.7 | 64.1 | 55.8–71.6 | 44.5 | 30.3–59.7 | 75.1 | 69.5–80.0 | |
| Borewell | 1.6 | 1.0–2.5 | 1.5 | 0.7–3.4 | 2.5 | 1.5–4.2 | 0.4 | 0.1–1.4 | 3.6 | 2.2–5.9 | |
| Other well or spring | 12.4 | 9.6–15.7 | 12.4 | 9.0–16.9 | 26.4 | 20.1–33.8 | 6.6 | 2.9–14.3 | 16.7 | 12.9–21.4 | |
| Other | 4.6 | 3.5–6.1 | 7.9 | 6.0–10.2 | 7.0 | 4.4–11.1 | 48.4 | 34.9–62.1 | 4.6 | 3.3–6.3 | |
| Treat drinking water† | 92.2 | 90.4–93.8 | 80.9 | 77.3–84.1 | 20.9 | 17.6–24.7 | 43.6 | 36.8–50.8 | 31.1 | 28.3–34.2 | < 0.001 |
| Boil‡ | 90.5 | 88.5–92.1 | 70.9 | 66.3–75.0 | 4.2 | 3.1–5.7 | 25.4 | 18.3–34.1 | 7.1 | 6.0–8.4 | < 0.001 |
| Chlorine‡ | 3.0 | 2.3–3.9 | 5.8 | 4.3–7.7 | 16.1 | 13.4–19.4 | 18.0 | 12.4–25.2 | 19.5 | 17.0–22.3 | < 0.001 |
| Water filter‡ | 0.6 | 0.3–1.0 | 0.3 | 0.2–0.7 | 0.4 | 0.2–1.0 | 1.7 | 0.7–3.9 | 1.1 | 0.6–2.1 | 0.036 |
| Other‡ | 0.7 | 0.4–1.2 | 9.1 | 7.2–11.5 | 1.0 | 0.6–1.8 | 3.0 | 1.8–5.0 | 2.4 | 1.7–3.5 | < 0.001 |
| Type of toilet used by family members | < 0.001 | ||||||||||
| Flush/pour flush toilet | 23.4 | 18.9–28.7 | 61.8 | 55.0–68.1 | 10.5 | 7.4–14.8 | 1.0 | 0.4–2.5 | 21.9 | 17.8–26.8 | |
| Latrine/toilet with hole | 65.4 | 60.5–70.0 | 34.8 | 28.9–41.3 | 74.0 | 69.6–77.9 | 27.7 | 17.7–40.5 | 52.8 | 48.1–57.4 | |
| Dry toilet | 4.8 | 3.5–6.6 | 0.9 | 0.4–1.8 | 0.1 | 0.0–0.6 | 0.2 | 0.1–1.0 | 13.9 | 10.9–17.7 | |
| No toilet | 6.2 | 4.4–8.8 | 2.5 | 1.3–4.6 | 15.1 | 11.9–19.0 | 18.7 | 11.5–28.9 | 11.3 | 8.6–14.8 | |
| Other | 0.1 | 0.1–0.4 | 0.1 | 0.0–0.3 | 0.2 | 0.1–0.6 | 52.3 | 37.2–67.0 | 0.0 | ||
| Toilet is shared with other homes§ | 5.2 | 4.1–6.6 | 12.3 | 10.5–14.4 | 17.0 | 13.5–21.2 | 9.3 | 6.0–14.1 | 18.5 | 16.4–20.8 | < 0.001 |
| Currently breast-feeding∥ | 77.2 | 74.7–79.6 | 77.8 | 75.2–80.1 | 71.8 | 67.7–75.5 | 81.4 | 75.0–86.5 | 80.8 | 77.8–83.4 | 0.003 |
| Exclusive breast-feeding¶ | 77.7 | 72.8–81.9 | 59.1 | 52.6–65.3 | 58.1 | 46.0–69.3 | 43.3 | 34.9–52.2 | 59.6 | 52.0–66.7 | 0.051 |
| Diarrhea in the past 2 weeks? | 0.004 | ||||||||||
| None | 84.8 | 82.6–86.8 | 88.4 | 87.0–89.6 | 84.6 | 81.4–87.3 | 89.4 | 85.5–92.3 | 85.5 | 84.0–86.9 | |
| Bloody diarrhea | 1.4 | 0.9–1.9 | 0.8 | 0.5–1.1 | 1.0 | 0.6–1.6 | 0.8 | 0.4–1.8 | 2.0 | 1.5–2.6 | |
| Non-bloody diarrhea | 13.8 | 12.1–15.8 | 10.9 | 9.7–12.2 | 14.5 | 11.9–17.4 | 9.8 | 7.0–13.5 | 12.5 | 11.2–14.0 | |
CI = confidence interval; SMI = Salud Mesoamérica Initiative.
Chi-square tests.
The proportion of respondents who used any form of drinking water treatment.
Respondents could choose more than one method of water treatment.
Only asked of those who had some type of toilet.
Current breast-feeding was only assessed for children less than 24 months of age.
Exclusive breast-feeding was only assessed for children less than 6 months of age.
Diarrhea prevalence.
The 2-week point prevalence of diarrhea was 13.0% overall, with 15.2% in Guatemala, 11.6% in Mexico, 15.4% in Nicaragua, 10.6% in Panama, and 14.5% in El Salvador (P < 0.01). Bloody diarrhea comprised between 6.5% (Nicaragua) and 13.8% (El Salvador) of diarrhea cases.
Diarrhea treatment.
Across countries, 36.0% of children with diarrhea were given ORS, ranging between 24.4% (Panama) and 46.5% (El Salvador) (P = 0.02) (Table 2). Zinc supplements were rare (< 3% overall) and did not vary appreciably between countries. There was significant variation in how much diarrheal children were given to drink (P < 0.01) or eat (P < 0.01) across countries. Overall, 18.0% of ill children were given much less to drink than usual or nothing at all, ranging from 13.8% in Panama to 18.4% in Mexico. The proportion of children fed nothing was 6.3% across countries, with this proportion rising to nearly 15% in Guatemala. Antimotility medication use was 17.1% overall, ranging from 2.0% to 20.7% in El Salvador and Mexico, respectively. Overall, antibiotics were given to 36.1% of children, with no appreciable variation between countries. Antibiotic use was also unrelated to whether the child had bloody or non-bloody diarrhea. Approximately half (51.7%) of mothers sought medical treatment or advice, and the majority (51.8%) of those did so in public clinics.
Table 2.
Treatment and parental care-seeking behavior for diarrheal children under 5 years of age in the SMI, 2011–2013
| Guatemala (N = 603) | Mexico (N = 545) | Nicaragua (N = 191) | Panama (N = 99) | El Salvador (N = 497) | P* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Given ORS | 34.6 | 29.9–39.6 | 32.2 | 27.5–37.4 | 41.6 | 34.5–49.1 | 24.4 | 15.8–35.8 | 46.5 | 41.5–51.6 | 0.017 |
| Given zinc | 1.2 | 0.4–3.6 | 2.1 | 0.9–4.7 | 3.9 | 2.0–7.3 | 0.0 | 5.1 | 3.4–7.6 | 0.213 | |
| How much was the child given to eat | 0.002 | ||||||||||
| Nothing | 14.6 | 11.4–18.5 | 5.6 | 3.2–9.6 | 5.0 | 2.6–9.3 | 3.4 | 1.2–8.9 | 5.1 | 3.1–8.4 | |
| Much less than usual | 19.3 | 15.9–23.4 | 16.6 | 13.1–20.7 | 14.6 | 9.1–22.7 | 12.2 | 7.7–18.8 | 22.9 | 18.2–28.4 | |
| A little less than usual | 43.6 | 39.5–47.8 | 46.1 | 41.1–51.2 | 47.5 | 40.3–54.8 | 34.4 | 26.4–43.4 | 37.3 | 32.1–42.8 | |
| About the same | 20.8 | 17.2–24.9 | 27.8 | 23.1–33.1 | 32.6 | 26.8–38.9 | 46.4 | 38.1–55.0 | 31.7 | 26.2–37.8 | |
| More than usual | 1.6 | 0.9–3.0 | 3.9 | 2.5–6.1 | 0.4 | 0.0–2.6 | 3.7 | 1.2–10.9 | 2.9 | 1.5–5.5 | |
| How much was the child given to drink | < 0.001 | ||||||||||
| Nothing | 3.9 | 2.4–6.1 | 0.8 | 0.2–2.4 | 1.7 | 0.5–5.5 | 1.0 | 0.1–6.8 | 1.4 | 0.6–3.3 | |
| Much less than usual | 13.2 | 10.1–17.1 | 17.6 | 13.7–22.3 | 16.5 | 11.4–23.2 | 12.8 | 8.1–19.7 | 13.7 | 10.1–18.4 | |
| A little less than usual | 39.0 | 34.0–44.2 | 34.9 | 30.1–40.1 | 46.6 | 39.8–53.5 | 29.6 | 21.0–39.9 | 16.7 | 12.6–21.7 | |
| About the same | 24.9 | 20.4–30.0 | 29.7 | 24.5–35.5 | 31.8 | 26.0–38.2 | 48.1 | 39.5–56.8 | 38.5 | 33.0–44.4 | |
| More than usual | 19.0 | 15.4–23.4 | 17.0 | 13.2–21.5 | 3.4 | 1.7–6.8 | 8.5 | 3.6–18.7 | 29.6 | 24.4–35.4 | |
| Given antimotility medication | 19.7 | 16.2–23.7 | 20.7 | 16.3–25.9 | 13.1 | 8.6–19.3 | 7.0 | 3.3–14.4 | 2.0 | 1.0–3.9 | 0.002 |
| Appropriate antibiotic use | 32.7 | 22.5–44.9 | 33.2 | 20.4–49.1 | 31.9 | 12.1–61.5 | 20.4 | 5.5–53.1 | 37.2 | 24.8–51.6 | 0.928 |
| Inappropriate antibiotic use | 32.7 | 28.2–37.6 | 40.4 | 34.1–46.9 | 31.1 | 23.8–39.4 | 22.9 | 14.9–33.4 | 34.8 | 29.2–41.0 | 0.056 |
| Sought advice or treatment | 61.4 | 56.8–65.9 | 52.2 | 46.6–57.8 | 48.0 | 39.3–56.8 | 49.1 | 36.0–62.3 | 50.4 | 44.8–55.9 | 0.165 |
| Where sought advice or treatment† | |||||||||||
| Public hospital | 1.0 | 0.4–2.8 | 5.7 | 2.8–11.1 | 24.3 | 13.2–40.5 | 14.2 | 3.3–44.5 | 5.2 | 2.8–9.7 | |
| Public clinic | 66.4 | 59.4–72.8 | 47.5 | 40.7–54.3 | 46.1 | 34.2–58.5 | 76.6 | 53.1–90.4 | 68.6 | 61.4–75.0 | |
| Private facility/provider | 2.9 | 1.4–6.1 | 13.8 | 9.2–20.0 | 8.4 | 5.1–13.6 | 0.0 | – | 13.3 | 9.3–18.8 | |
| Pharmacy | 17.3 | 12.5–23.4 | 27.3 | 21.8–33.7 | 17.8 | 10.5–28.6 | 0.0 | – | 4.1 | 2.1–7.9 | |
| Community health worker | 3.6 | 1.7–7.4 | 0.7 | 0.1–3.0 | 0.9 | 0.1–6.1 | 0.0 | – | 1.7 | 0.5–5.2 | |
| Other | 8.7 | 5.5–13.5 | 5.1 | 3.0–8.6 | 2.4 | 0.6–9.0 | 9.2 | 3.9–20.4 | 7.1 | 4.2–11.7 | |
CI = confidence interval; ORS = oral rehydration solution; SMI = Salud Mesoamérica Initiative.
Because of survey weighting, these sample sizes do not equal Table 1's sample size multiplied by the prevalence of diarrhea.
Chi-square tests.
Only asked of those who sought advice or treatment.
ORS (47.9% versus 23.3%, P < 0.01) and zinc (4.5% versus 0.8%, P < 0.01) usage were higher among children whose parents sought medical care, compared with children whose parents did not do so. They were also more likely to have been given antimotility medication (23.1% versus 10.7%, P < 0.01) and antibiotics (48.8% versus 22.7%, P < 0.01). The proportion who were given much less to drink or eat than usual or nothing at all was unrelated to care-seeking status (18.6% versus 17.3%, P = 0.60 and 25.0% versus 20.5%, P = 0.14, respectively).
Linked Panamanian household and facility data revealed no association between the availability of printed materials regarding signs and symptoms of children's risk in the facilities and use of ORS. There was also no association between the availability of ORS in the facility on the day of the survey and treatment with ORS. Mothers whose usual health facilities had antibiotics on the survey day were less likely (19.3%) to treat their children with antibiotics than were those whose health facility did not have antibiotics in stock (58.4%) (P = 0.01).
Subnational variation.
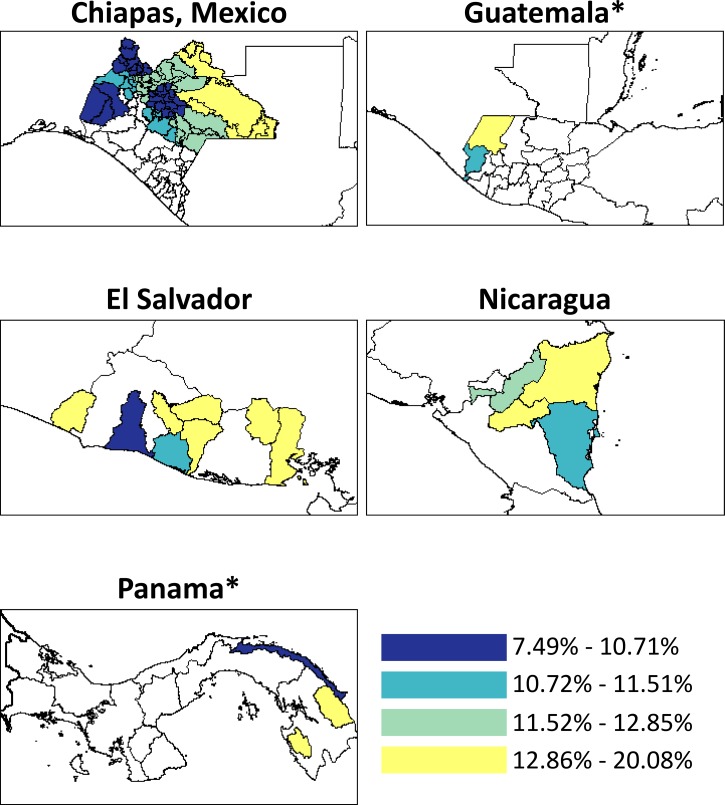
Diarrhea prevalence differed between the departments within Guatemala (P < 0.01) and Panama (P = 0.02) (Figure 1 ). ORS use varied by department in El Salvador (P < 0.01), and the proportion of diarrheal children receiving less to drink than usual varied between departments in Guatemala (P < 0.01) and Panama (P < 0.01) (Supplemental Figures 1 and 2, respectively). The proportion of diarrheal children receiving less food than usual varied between departments in El Salvador and Panama (P < 0.01) (Supplemental Figure 3). There was no significant variation within countries for levels of antimotility medication and antibiotic use among diarrheal children (Supplemental Figures 4 and 5, respectively).
Figure 1.
Two-week point prevalence of diarrhea among children under 5 years of age in Mesoamerica, 2011–2013. Colors represent quartiles of diarrhea prevalence across countries. Areas in white were not sampled. *Statistically significant differences in the prevalence between departments.
Reasons for not seeking medical care.
In Guatemala, Mexico, and Nicaragua, the most common reason for not seeking medical attention for pediatric diarrhea was that the child was treated at home (range = 47.6–65.3%), followed by the child not being sick enough to warrant medical care (range = 13.4–42.4%), and concerns that the health center did not have sufficient medications (range = 9.3–11.5%) (Table 3). In Panama, the most common reason was that the health center was too far (49.0%), followed by the child being treated at home (43.9%), and the child not being sick enough to warrant medical care (32.9%). The inability to find (24.7%) and pay for (29.6%) transportation were also notable obstacles in Panama.
Table 3.
Reasons why mothers did not seek care for their child with diarrhea in the SMI, 2011–2013
| Guatemala (N = 244) | Mexico (N = 252) | Nicaragua (N = 98) | Panama (N = 50) | P* | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Treated at home | 65.3 | 57.9–72.6 | 48.2 | 39.6–56.7 | 47.6 | 37.5–57.8 | 43.9 | 22.6–65.1 | 0.184 |
| Not sick enough to need care | 13.4 | 8.5–18.3 | 42.4 | 34.1–50.7 | 21.9 | 12.6–31.3 | 32.9 | 13.6–52.3 | 0.001 |
| Health center has insufficient medications | 11.4 | 6.4–16.5 | 11.5 | 6.4–16.5 | 9.3 | 3.2–15.5 | 5.0 | 0.0–11.1 | 0.675 |
| Health center is too far | 7.8 | 4.0–11.7 | 6.4 | 2.1–10.8 | 2.1 | 0.0–4.9 | 49.0 | 25.6–72.3 | < 0.001 |
| Could not pay for transportation | 0.6 | 0.0–1.5 | 1.5 | 0.0–4.0 | 10.9 | 4.9–16.9 | 29.6 | 12.0–47.2 | 0.001 |
| Too busy | 1.7 | 0.0–3.7 | 0.9 | 0.0–1.9 | 10.4 | 4.4–16.3 | 3.1 | 0.0–8.8 | < 0.001 |
| Care is too expensive | 7.4 | 4.0–10.8 | 3.6 | 0.8–6.4 | 1.0 | 0.0–3.0 | 14.2 | 3.6–24.8 | 0.051 |
| Went to the health center but there were no staff | 0.6 | 0.0–1.6 | 2.9 | 0.0–6.0 | 3.1 | 0.0–6.9 | 7.1 | 1.3–12.8 | 0.567 |
| Previous poor treatment by health center staff | 0.0 | – | 2.4 | 0.0–5.1 | 1.0 | 0.0–2.8 | 4.0 | 0.0–11.4 | 0.569 |
| Health center staff are difficult to deal with | 0.0 | – | 0.6 | 0.0–1.6 | 3.6 | 0.0–8.9 | 2.0 | 0.0–6.0 | 0.243 |
| Could not find transportation | 0.9 | 0.0–2.2 | 1.0 | 0.0–2.5 | 1.6 | 0.0–4.7 | 24.7 | 0.3–49.2 | 0.001 |
| Health center is poorly equipped | 1.2 | 0.0–2.5 | 0.7 | 0.0–1.8 | 2.5 | 0.0–5.9 | 0.0 | – | 0.374 |
| Health center staff are not trusted | 1.6 | 0.0–3.9 | 1.5 | 0.0–3.9 | 1.0 | 0.0–2.8 | 0.0 | – | 0.926 |
| Health center facilities are poor | 0.0 | – | 1.8 | 0.3–3.3 | 0.5 | 0.0–1.5 | 0.0 | – | 0.437 |
| Tried, but was denied attention | 0.0 | – | 0.7 | 0.0–1.7 | 1.0 | 0.0–3.0 | 2.0 | 0.0–6.0 | 0.766 |
| Religious or cultural beliefs | 0.4 | 0.0–1.0 | 1.1 | 0.0–3.4 | 0.0 | – | 0.0 | – | 0.525 |
| Health center staff are ill informed | 0.0 | – | 0.4 | 0.0–1.0 | 1.3 | 0.0–3.7 | 0.0 | – | 0.616 |
| Did not want to go alone | 0.6 | 0.0–1.7 | 0.4 | 0.0–1.1 | 0.0 | – | 0.0 | – | 0.714 |
| Could not get permission to go to the doctor | 0.0 | – | 0.3 | 0.0–0.9 | 0.0 | – | 0.0 | – | 0.836 |
| Did not know where to go | 0.0 | – | 0.2 | 0.0–0.7 | 0.0 | 0.0 | 0.846 | ||
| Other | 9.0 | 4.8–13.3 | 8.1 | 3.8–12.4 | 4.2 | 0.9–7.5 | 0.0 | – | 0.251 |
CI = confidence interval; SMI = Salud Mesoamérica Initiative.
Because of survey weighting, these sample sizes do not equal Table 2's sample size multiplied by the proportion of mothers who did not seek advice or treatment. Data not available for El Salvador and women could provide multiple reasons.
Chi-square tests.
Regression analyses.
Compared with children < 6 months old, those 6–23 months had a 49% (adjusted RR (aRR) = 1.49, 95% CI = 1.15, 1.95) increased risk for diarrhea, and those 36–59 months had a 31% (aRR = 0.69, 95% CI = 0.53, 0.91) decreased risk (Table 4). Water treatment with a method other than boiling, chlorination, or filtering was associated with 38% increased risk (aRR = 1.38, 95% CI = 1.07, 1.76), and male children experienced a 15% (aRR = 1.15, 95% CI = 1.02, 1.29) increased risk. Each additional year of maternal age decreased risk by 2% (aRR = 0.98, 95% CI = 0.97, 0.99) and use of “other” toilet facilities was associated with a 58% risk reduction (aRR = 0.42, 95% CI = 0.26, 0.69). Compared with Guatemalan children, Mexican children had a 23% (aRR = 0.77, 95% CI = 0.64, 0.93) reduced risk for diarrhea.
Table 4.
Predictors for diarrhea among children under 5 years of age in the SMI, 2011–2013
| Univariable (N = 14,500) | Multivariable (N = 14,406) | |||
|---|---|---|---|---|
| RR | 95% CI | aRR | 95% CI | |
| Child's age (months) | ||||
| 0–5 | 1.00 | Ref | 1.00 | Ref |
| 6–23 | 1.48 | 1.13–1.94** | 1.49 | 1.15–1.95** |
| 24–35 | 1.05 | 0.80–1.39 | 1.10 | 0.84–1.45 |
| 36–59 | 0.64 | 0.48–0.85** | 0.69 | 0.53–0.91** |
| Male child | 1.15 | 1.01–1.29* | 1.15 | 1.02–1.29* |
| Firstborn child | 1.49 | 1.25–1.77** | 1.11 | 0.93–1.32 |
| Mother's age (years) | 0.97 | 0.96–0.98** | 0.98 | 0.97–0.99** |
| Highest level of education attained | ||||
| None | 1.00 | Ref | ||
| Primary | 0.97 | 0.80–1.17 | ||
| Secondary | 1.10 | 0.89–1.35 | ||
| High school or higher | 1.05 | 0.84–1.32 | ||
| Mother is literate | 1.24 | 1.08–1.43** | ||
| Mother is a housewife | 1.04 | 0.81–1.34 | ||
| Urban resident | 1.03 | 0.87–1.22 | ||
| Household asset index | ||||
| Low | 1.00 | Ref | ||
| Medium | 1.09 | 0.93–1.27 | ||
| High | 1.05 | 0.85–1.30 | ||
| Source of water | ||||
| Tap | 1.00 | Ref | ||
| Borewell | 0.88 | 0.45–1.73 | ||
| Other well or spring | 1.11 | 0.92–1.34 | ||
| Other | 0.82 | 0.63–1.07 | ||
| Water treatment | ||||
| Drinking water is treated | 0.96 | 0.80–1.16 | ||
| Boil water† | 0.89 | 0.76–1.05 | ||
| Chlorinate water† | 1.13 | 0.84–1.53 | ||
| Filter water† | 0.97 | 0.40–2.36 | ||
| Other water treatment† | 1.24 | 0.98–1.57 | 1.38 | 1.07–1.76* |
| Toilet type | ||||
| Flush/pour flush toilet | 0.90 | 0.76–1.07 | 0.96 | 0.82–1.12 |
| Latrine/toilet with hole | 1.00 | Ref | 1.00 | Ref |
| Dry toilet | 1.16 | 0.79–1.70 | 1.22 | 0.80–1.87 |
| No toilet | 1.07 | 0.82–1.39 | 1.02 | 0.79–1.31 |
| Other | 0.47 | 0.31–0.73** | 0.42 | 0.26–0.69** |
| Toilet is shared with other homes‡ | 1.17 | 0.93–1.48 | ||
| Country | ||||
| Guatemala | 1.00 | Ref | 1.00 | Ref |
| Mexico | 0.77 | 0.65–0.91** | 0.77 | 0.64–0.93** |
| Nicaragua | 1.02 | 0.79–1.30 | 0.99 | 0.78–1.26 |
| Panama | 0.70 | 0.50–0.97* | 1.01 | 0.70–1.46 |
| El Salvador | 0.95 | 0.80–1.13 | 0.92 | 0.77–1.10 |
aRR = adjusted relative risk; CI = confidence interval; Ref = reference; RR = relative risk; SMI = Salud Mesoamérica Initiative.
P < 0.05; **P < 0.01.
Participants could select more than one type of water treatment.
Only asked of those who had some type of toilet.
In Guatemala, having a secondary-level education, compared with no education, was associated with a 43% increased risk for reported diarrhea (aRR = 1.43, 95% CI = 1.03, 1.99), and maternal literacy was associated with a 47% (aRR = 1.47, 95% CI = 1.18, 1.83) increased risk (Supplemental Table 1). Obtaining water from a spring or well other than a borewell was also associated with increased risk in Guatemala (aRR = 1.39, 95% CI = 1.11, 1.75). In Mexico, use of a dry toilet increased risk for diarrhea nearly three-fold (aRR = 2.71, 95% CI = 1.57, 4.70) (Supplemental Table 2). In Panama, using a water source other than a tap, borewell, or other well or spring was associated with an 80% increased risk (aRR = 1.80, 95% CI = 1.10, 2.93), and filtering water was associated with more than a doubling of risk (aRR = 2.52, 95% CI = 1.61, 3.93) (Supplemental Table 3). There were no unique findings in the Nicaragua- or El Salvador-specific analyses (Supplemental Tables 4 and 5, respectively).
In the sub-analysis of children under 24 months of age, current breast-feeding was not significantly associated with diarrheal status and risk increased with age (Supplemental Table 6). For children under 6 months of age, exclusive breast-feeding was not a statistically significant predictor (RR = 0.73, 95% CI = 0.47, 1.14) (Supplemental Table 7).
Our region-wide assessment of the correlates of positive feeding practices among children with diarrhea found that children whose households used a borewell were three times more likely to provide adequate food and drink to ill children, as compared with those who used tap water (aRR = 3.06, 95% CI = 1.75, 5.36) (Supplemental Table 8). Children whose households treated their drinking water were less likely to receive adequate food and drink (aRR = 0.69, 95% CI = 0.48, 0.99) and children in Panama were more likely to receive adequate food and drink (aRR = 2.18, 95% CI = 1.38, 3.44) compared with children in Guatemala. Country-specific analyses of correlates of positive feeding practices are provided in Supplemental Tables 9– 13.
Discussion
To our knowledge, this is the first analysis to examine diarrhea burden, care practices, and risk factors across multiple countries in Mesoamerica. Approximately one in eight poor Mesoamerican children had an episode of diarrhea in the 2 weeks before being surveyed, with significant variation in prevalence between countries and across departments in Guatemala and Panama. Moreover, evidence-based guidelines for diarrhea treatment were often not followed. Our findings call for continued efforts to reduce diarrhea incidence and to better align treatment practices with evidence-based recommendations.
We were unable to find recent peer-reviewed estimates of the 2-week point prevalence of diarrhea in the countries included in our analysis. However, the 2012 Mexican Health and Nutrition Survey estimated the prevalence to be 13.4% in Chiapas,16 which is similar to our finding. Nevertheless, as noted above, the burden of diarrhea among children under 5 years of age in these countries has decreased over the last 20 years.1 In addition to the benefit of ORS usage, improvement may be due to regional improvements in education and sanitation,17 diarrheal disease or cholera control programs,18 and the introduction of rotavirus vaccine, which has effectively reduced rotavirus-associated diarrhea incidence and pediatric-associated hospitalization.19,20 Although these successes are to be applauded, our prevalence maps highlight areas where prevention efforts should be redoubled or new interventions undertaken.
Overall, diarrhea treatment was poorly aligned with evidence-based recommendations. Though ORS usage has improved over the last two decades,21 qualitative studies are needed to understand why nearly two-thirds of children with diarrhea were not given ORS and why many were given much less to drink or eat than usual or nothing at all. For example, interventions could address the common belief in parts of Guatemala and Nicaragua that ORS is useful only for certain types of diarrhea.22,23 Generally, interventions should seek to increase ORS desirability as well as accessibility. Low zinc utilization was expected since, at the time of the surveys, zinc for management of diarrhea was not a part of the national norms in these countries. Despite being contraindicated for non-bloody/non-cholera diarrhea in this age group, use of antimotility medications and antibiotics was not uncommon. Our finding that use of these medications was significantly and substantially higher among children whose parents sought medical care is particularly worrisome. This suggests that health-care providers may have been unaware of the guidelines or perhaps succumbed to parental pressure for medication.24 However, this association can also be explained by the fact that prescriptions are legally necessary for obtaining antibiotics in all of the surveyed countries. Though lower than the proportion among those who sought medical care, children treated at home were also given antimotility medications and antibiotics. Follow-up studies should examine the reasons for these practices, and beliefs surrounding the use of these medications to develop interventions to reduce their contraindicated use.
The assessment of diarrheal risk factors revealed two unexpected findings. In Guatemala, we found that children of literate and educated women were at greater risk for diarrhea. On the basis of a previous analysis of DHSs using maternal recall for diarrhea,25 we believe this is most likely due to underreporting of diarrhea by less educated and illiterate mothers. We also found no significant association between current or exclusive breast-feeding and risk for diarrhea. However, both were in the direction of reduced diarrheal risk, and it is possible that, given a larger sample size, both would be significant.
The prevalence of diarrhea found in these poor areas under study indicates how underserved they are. SMI is implementing evidence-based interventions to improve maternal and child health in the region, including interventions related to diarrhea. The initiative encourages the inclusion of zinc for management of diarrhea in national norms, and this was incorporated in El Salvador, Guatemala, Nicaragua, and Panama between 2013 and 2014. In Guatemala, the initiative is currently strengthening child enrollment in health services to promote use of ORS and zinc for dehydration and diarrhea. In Mexico, SMI is working to ensure that children have access to ORS for management of diarrhea. In Nicaragua, the initiative is helping update standards for the use of zinc in diarrhea treatment and has invested in community promotion and distribution of ORS and zinc for treatment of diarrhea. In Panama, SMI is developing a strategy for community distribution of zinc and ORS and has been involved in improving access to, treatment of, and consumption of safe water in 24 indigenous communities. Finally, in El Salvador, SMI is strengthening service provision by community health teams, including the use of ORS and zinc for treatment of diarrhea. Follow-up household and facility-based surveys will assess the effectiveness of these and other SMI interventions.
Our analysis must be interpreted in light of some limitations and its strengths. We used maternal recall to assess diarrhea occurrence and treatment, both of which are subject to recall bias. However, our prevalence estimates are likely to be conservative since previous studies show that recall beyond 2–3 days results in underestimation of diarrhea burden.25,26 We also lacked data regarding diarrhea severity and duration, both of which could be associated with care seeking and treatment behavior. However, additional research and validation are needed to identify supplementary questions that could assess these factors using large-scale household surveys like SMI.14 In addition, except for in Mexico, data were collected between March and August, which prevents us from being able to adjust our prevalence estimates for seasonality.27 However, our survey window bridged the expected viral and bacterial diarrhea seasonal peaks,27–29 with a trough in between, so it provides a good cross-section of prevalence. Furthermore, our ability to match households with their usual health facilities was weak in certain countries. Follow-up SMI studies will improve household and facility linkages. Our large sample size is a strength of this study. Moreover, we used a standard methodology to allow comparability within and between countries and to ensure high quality data.
Our study revealed that, despite decades of progress, the burden of diarrhea among poor and disenfranchised children under 5 of years in Mesoamerica remains sizeable. In addition, treatment practices often do not follow national guidelines. Our findings call for accelerated efforts to reduce the burden of diarrhea in poor areas in Mesoamerica. These efforts should focus on community programs to increase awareness and to ensure that health professionals are following national guidelines in treating diarrhea.
Supplementary Material
ACKNOWLEDGMENTS
We extend our gratitude to Adrienne Chew for copyediting the draft manuscript.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the Inter-American Development Bank, its Board of Directors, or the countries they represent. This paper was presented in part at the 9th European Congress on Tropical Medicine and International Health, Basel, Switzerland, September 8, 2015.
Footnotes
Financial support: All phases of this study were supported by the Bill & Melinda Gates Foundation, the Spanish Agency for International Development Cooperation, and the Carlos Slim Health Institute through the Inter-American Development Bank.
Authors' addresses: Danny V. Colombara, Bernardo Hernández, Claire R. McNellan, Sima S. Desai, Marielle C. Gagnier, Annie Haakenstad, Casey Johanns, Erin B. Palmisano, Alexandra Schaefer, Paola Zúñiga-Brenes, Nicholas Zyznieuski, and Ali H. Mokdad, Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, E-mails: dvc2@uw.edu, bhp3@uw.edu, crmcn@uw.edu, sdesai89@uw.edu, gagnierm@uw.edu, ahaak@uw.edu, cjohanns@uw.edu, palmie@uw.edu, amschae@uw.edu, nzyznie@uw.edu, and mokdaa@uw.edu. Diego Ríos-Zertuche, Paola Zúñiga-Brenes, and Emma Iriarte, Salud Mesoamérica Initiative, Inter-American Development Bank, Panamá, Panamá, E-mails: diegori@iadb.org, mpzuniga@iadb.org, and emmai@iadb.org.
References
- 1.Institute for Health Metrics and Evaluation GBD Compare: Central Latin America, Diarrheal Diseases, Both Sexes, Under 5 Years. 2015. http://vizhub.healthdata.org/gbd-compare/ GBD 2010, Released 3/2013. Available at.
- 2.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Wagstaff A, Bustreo F, Bryce J, Claeson M, WHO-World Bank Child Health and Poverty Working Group Child health: reaching the poor. Am J Public Health. 2004;94:726–736. doi: 10.2105/ajph.94.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The World Bank, Development Research Group GINI Index (World Bank Estimate) 2015. http://data.worldbank.org/indicator/SI.POV.GINI Available at.
- 5.Locklear TD, Perez A, Caceres A, Mahady GB. Women's health in Central America: the complexity of issues and the need to focus on indigenous healthcare. Curr Womens Health Rev. 2013;9:30–40. [Google Scholar]
- 6.Santosham M, Chandran A, Fitzwater S, Fischer-Walker C, Baqui AH, Black R. Progress and barriers for the control of diarrhoeal disease. Lancet. 2010;376:63–67. doi: 10.1016/S0140-6736(10)60356-X. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization, Department of Child and Adolescent Health and Development . The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: Department of Child and Adolescent Health and Development, World Health Organization; 2005. [Google Scholar]
- 8.Munos MK, Walker CLF, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39:i75–i87. doi: 10.1093/ije/dyq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santosham M, Greenough WB. Oral rehydration therapy: a global perspective. J Pediatr. 1991;118:S44–S51. doi: 10.1016/s0022-3476(05)81425-8. discussion S52. [DOI] [PubMed] [Google Scholar]
- 10.Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics. 2008;121:326–336. doi: 10.1542/peds.2007-0921. [DOI] [PubMed] [Google Scholar]
- 11.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, Rosado JL, Roy SK, Ruel M, Sazawal S, Shankar A. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;135:689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119:1120–1130. doi: 10.1542/peds.2006-3481. [DOI] [PubMed] [Google Scholar]
- 13.Mokdad AH, Colson KE, Zúñiga-Brenes P, Ríos-Zertuche D, Palmisano EB, Alfaro-Porras E, Anderson BW, Borgo M, Desai S, Gagnier MC, Gillespie CW, Giron SL, Haakenstad A, Romero SL, Mateus J, McKay A, Mokdad AA, Murphy T, Naghavi P, Nelson J, Orozco M, Ranganathan D, Salvatierra B, Schaefer A, Usmanova G, Varela A, Wilson S, Wulf S, Hernandez B, Lozano R, Iriarte E, Regalia F. Salud Mesoamérica 2015 Initiative: design, implementation, and baseline findings. Popul Health Metr. 2015;13:3. doi: 10.1186/s12963-015-0034-4. http://www.pophealthmetrics.com/content/13/1/3 Available at. Accessed February 20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer Walker CL, Fontaine O, Black RE. Measuring coverage in MNCH: current indicators for measuring coverage of diarrhea treatment interventions and opportunities for improvement. PLoS Med. 2013;10:e1001385. doi: 10.1371/journal.pmed.1001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Oropeza Abúndez C. Instituto Nacional de Salud Pública (Mexico) Encuesta Nacional de Salud Y Nutrición 2012: Resultados Nacionales. Primera edición. Cuernavaca, México: Instituto Nacional de Salud Pública and Secretaría de Salud; 2012. [Google Scholar]
- 17.Gutiérrez G, Tapia-Conyer R, Guiscafreì H, Reyes H, Martinez H, Kumate J. Impact of oral rehydration and selected public health interventions on reduction of mortality from childhood diarrhoeal diseases in Mexico. Bull World Health Organ. 1996;74:189. [PMC free article] [PubMed] [Google Scholar]
- 18.Velázquez FR, Garcia-Lozano H, Rodriguez E, Cervantes Y, Gómez A, Melo M, Anaya L, Ovalle JC, Torres J, De Jesus BD, Alvarez-Lucas C, Breuer T, Muñoz O, Kuri P. Diarrhea morbidity and mortality in Mexican children: impact of rotavirus disease. Pediatr Infect Dis J. 2004;23:S149–S155. doi: 10.1097/01.inf.0000142463.72442.91. [DOI] [PubMed] [Google Scholar]
- 19.Becker-Dreps S, Paniagua M, Dominik R, Cao H, Shah NK, Morgan DR, Moreno G, Espinoza F. Changes in childhood diarrhea incidence in Nicaragua following 3 years of universal infant rotavirus immunization. Pediatr Infect Dis J. 2011;30:243–247. doi: 10.1097/INF.0b013e3181f87ffe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, Coronado L, Patel MM, Parashar UD, Cortese MM. Reduction of diarrhea-associated hospitalizations among children aged < 5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30:S16–S20. doi: 10.1097/INF.0b013e3181fefc68. [DOI] [PubMed] [Google Scholar]
- 21.Gorter AC, Sánchez G, Pauw J, Pérez RM, Sandiford P, Smith GD. Childhood diarrhea in rural Nicaragua: beliefs and traditional health practices [in Spanish] Boletin Oficina Sanit Panam Pan Am Sanit Bur. 1995;119:377–390. [PubMed] [Google Scholar]
- 22.Arvelo W, Degollado J, Reyes L, Álvarez A. Perceptions regarding oral rehydration solutions for the management of diarrhea in Guatemalan children: implications for diarrheal management in the Americas. Rev Panam Salud Publica. 2013;34:121–126. [PubMed] [Google Scholar]
- 23.Smith GD, Gorter A, Hoppenbrouwer J, Sweep A, Perez RM, Gonzalez C, Morales P, Pauw J, Sandiford P. The cultural construction of childhood diarrhoea in rural Nicaragua: relevance for epidemiology and health promotion. Soc Sci Med. 1993;36:1613–1624. doi: 10.1016/0277-9536(93)90350-d. [DOI] [PubMed] [Google Scholar]
- 24.Homedes N, Ugalde A. Patients' compliance with medical treatments in the third world. What do we know? Health Policy Plan. 1993;8:291–314. [Google Scholar]
- 25.Boerma JT, Black RE, Sommerfelt AE, Rutstein SO, Bicego GT. Accuracy and completeness of mothers' recall of diarrhoea occurrence in pre-school children in demographic and health surveys. Int J Epidemiol. 1991;20:1073–1080. doi: 10.1093/ije/20.4.1073. [DOI] [PubMed] [Google Scholar]
- 26.Alam N, Henry FJ, Rahaman MM. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18:697–700. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 27.Villa S, Guiscafreì H, Martinez H, Munoz O, Gutierrez G. Seasonal diarrhoeal mortality among Mexican children. Bull World Health Organ. 1999;77:375. [PMC free article] [PubMed] [Google Scholar]
- 28.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Oliveira LH, Danovaro-Holliday MC, Andrus JK, de Fillipis AMB, Gentsch J, Matus CR, Widdowson M. Sentinel hospital surveillance for rotavirus in Latin American and Caribbean countries. J Infect Dis. 2009;200:S131–S139. doi: 10.1086/605060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.