Abstract
Cystic echinococcosis, caused by Echinococcus granulosus, is a widespread parasitic zoonosis causing economic loss and public health problems. Annexins are important proteins usually present in the plasma membrane, but previous studies have shown that an annexin B33 protein of E. granulosus (Eg-ANX) could be detected in the excretory/secretory products and cyst fluid. In this study, we cloned and characterized Eg-ANX. In silico analysis showed that the amino acid sequence of Eg-ANX was conserved and lacked any signal peptides. The phospholipid-binding activity of recombinant Eg-ANX (rEg-ANX) was tested; liposomes could bind to rEg-ANX only in the presence of Ca2+. In addition, we performed western blotting and immunohistochemical analyses to further validate the secretory properties of Eg-ANX. The protein could be detected in the cyst fluid of E. granulosus and was also present in the intermediate host tissues, which suggested that Eg-ANX might play an important role in parasite–host interaction.
Introduction
Echinococcus granulosus is a tapeworm with a two-host life cycle. Dogs and other canids are the definitive hosts and humans and domestic animals are the intermediate hosts for the metacestode (larval) stage. Echinococcus granulosus is the causative agent of cystic echinococcosis (CE), which is a substantial cause of morbidity and mortality in many parts of the world.1–3 This zoonosis is characterized by long-term growth of CE cysts in humans and domestic animals; more than 90% occur in the lung, liver, or both organs.4 The global burden of human CE exceeds 1 million disability-adjusted life years and losses are estimated at US$760 million a year. The livestock-associated losses range from US$1.2 billion to US$2.1 billion.5 The mortality rate of CE is about 2–4% in humans, and it may increase notably with poor treatment and care.6,7 Hence, CE remains an important zoonosis and public health threat.
Annexins belong to a multigene family, are widespread in all eukaryotes and are characterized by their ability to bind reversibly to phospholipids in a Ca2+-dependent manner. Structurally, annexins contain a variable N-terminal domain and a conserved core C-terminal domain.8 The C-terminal domain contains four repeat domains, and each repeat generally has type II and/or type III Ca2+-binding sites. Annexins are often associated with the plasma membrane, inner membrane, and cytoskeletal proteins, playing key roles in a broad range of important biological processes such as inflammatory response, apoptosis, signal transmission, membrane fusion and exocytosis, anticoagulation, ion channel regulation, and cell migration.8–11 In parasites, some annexins were considered to protect the parasite from structural breakdown during parasitism and to regulate the immune responses of the hosts, which is linked to parasite survival.12,13 For Taenia solium and Heterodera glycines, annexin was demonstrated to downregulate the immune response of the host.14,15 Annexin from Schistosoma mansoni was closely associated with tegument development, which is the most important host–parasite interface; therefore, annexin might be a potential vaccine candidate for S. mansoni.16 Annexin B30 of Clonorchis sinensis was shown to be involved in host–parasite interaction and affected the immune response of the host during C. sinensis infection.17
Interestingly, some annexins without any secretory signals have been found in the extracellular space (e.g., annexin A1, A2, and A5 of human and annexin B1 of T. solium).15,18–20 The mechanism by which this occurs remains unclear and is worthy of investigation.15,21 Recently, proteomic research on E. granulosus has shown that annexin B33 protein (Eg-ANX) could be detected in the excreted/secreted (ES) product and hydatid cyst fluid, which might potentially play important roles in parasite survival mechanisms during infection.22,23 However, the secretory properties of Eg-ANX in the proteomic research on E. granulosus was not very specific. In this study, we validated the existence of Eg-ANX in hydatid cyst fluid and found that Eg-ANX could be secreted in the host-derived layer (granulation tissue) of host liver. This could be an important finding of Eg-ANX in parasite–host interaction. In addition, to understand other characteristics of Eg-ANX gene, we identified and located Eg-ANX, and the classical Ca2+-dependent phospholipid-binding properties of Eg-ANX was also demonstrated.
Materials and Methods
Parasites and animals.
Cysts of E. granulosus were isolated from naturally infected sheep livers at a slaughterhouse in Qinghai Province, China. The liver of healthy sheep was obtained from Sichuan Province, China. The hydatid fluid and protoscoleces (PSCs) were separated aseptically by centrifugation at 600 × g for 5 minutes. Then the hydatid fluid was filtered through a 0.22-μm membrane and stored at −80°C for western blotting. Adult worms were collected from a 2-month-old dog 35 days postinfection with 20,000 PSCs. From the Laboratory Animal Center of Sichuan University (Chengdu, China), 6- to 8-week-old female-specific pathogen-free Institute for Cancer Research mice were purchased. Nine-week-old female New Zealand white rabbits and dogs were obtained from the Laboratory Animal Center of Sichuan Agricultural University. All animals were handled in strict accordance with the animal protection law of the People's Republic of China (a draft animal protection law was released on September 18, 2009). All procedures were approved by the Institute of Health Animal Care and Use Committee of Sichuan Agricultural University of China.
Bioinformatic analysis.
The complementary DNA (cDNA) sequence of Eg-ANX (EgrG_000041300) was obtained from GeneDB (http://www.genedb.org/Homepage). DNASTAR software (Madison, WI) was used to deduce the amino acid sequence of Eg-ANX. Multiple sequence alignment was performed using Clustal X software version 1.83 (EBI, Cambridge, UK). The phylogenetic tree was constructed using the neighbor-joining method with MEGA software (version 5.05, The Biodesign Institute, Tempe, AZ). Conserved domains were searched using SMART (http://smart.embl-heidelberg.de/). The physicochemical properties including molecular weight (MW) and isoelectric point (pI) were predicted by ProtParam (http://www.expasy.ch/tools/protparam.html). The signal sequence was predicted with the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP/).
Cloning, expression, and purification of recombinant Eg-ANX.
Total RNA from PSCs was isolated using the RNAprep Pure Tissue Kit (Tiangen, Beijing, China) and reverse transcribed into cDNA using the ThermoScript™ RT-PCR System for First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). The expression sequence of Eg-ANX was amplified by polymerase chain reaction (PCR) using primers 5′-CGCGGATCCATGAATTACGACTATAATAATGA-3′ and 5′-CCGGAATTCCTTCTCTCCCAAAAGGGT-3′ with BamHI and EcoRI restriction enzyme sites (underlined), respectively. The PCR products of Eg-ANX were ligated into the expression vector pET32a(+) (Novagen, Madison, WI). The recombinant plasmid was transformed into Escherichia coli BL21 (DE3) cells (Invitrogen, Carlsbad, CA). Subsequently, the transformants were induced with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG; Sigma, St Louis, MO) at 37°C for 6 hours. The bacterial cells were collected, resuspended in lysis buffer (50 mM NaH2PO4 [pH 8.0], 10 mM Tris-HCl [pH 8.0], and 100 mM NaCl), ultrasonicated on ice, and centrifuged at 4°C. The recombinant protein was purified using a Ni2+ affinity column (Bio-Rad, Hercules, CA) from the bacterial lysate following the manufacturer's instructions. The expression and purification of proteins were examined by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was determined with micro-bicinchoninic acid protein assay reagent (Pierce, Rockford, IL).
Sera.
Mouse anti-PSC sera was obtained as previously described.24 Every mouse was injected with 2,000 PSCs, and serum samples were collected after 9 months for subsequent western blot analysis. Polyclonal antibody against Eg-ANX was produced after a previous study.25 In brief, each rabbit was immunized with 200 μg recombinant Eg-ANX (rEg-ANX) emulsified in Freund's complete adjuvant (FCA; Sigma) subcutaneously, followed by two boosters (2 weeks apart) using the same route and dose in FCA. Two weeks after the final vaccination, rabbit anti-rEg-ANX serum was collected and the antibody titer was detected by enzyme-linked immunosorbent assay. Anti-rEg-ANX serum with high antibody titer was purified by HiTrap Protein A affinity chromatography (Bio-Rad) for subsequent western blotting and immunohistochemistry.
Quantitative real-time PCR.
To quantify the transcript level of Eg-ANX in PSCs, germinal layer, and laminated layer, real-time PCR was performed as described previously with primer pairs 5′-GGAGAACTGGCACGCAATC-3′/5′-TGGACTGAGGCAGCAAATAA-3′ (for Eg-ANX) and 5′-TTTGAGAAAGAGGCGGCTGAGATG-3′/5′-TAATAAAGTCACGATGACCGGGCG-3′ (for elongation factor 1 alpha).26 The data were calculated using the 2−ΔΔCT method.27
Western blot analysis.
The total proteins of PSCs and healthy sheep liver were obtained with a mammalian protein extraction kit (CWBIO, Beijing, China). The cyst fluid, total PSCs extracts, total liver extracts, and recombinant proteins were run on 12% SDS-PAGE and transferred onto nitrocellulose membranes, respectively. The membranes were washed with Tris-buffered saline Tween-20 buffer five times and subsequently blocked with 5% (w/v) skimmed milk at 37°C for 2 hours. After five washes, the membranes with cyst fluid, total PSCs extracts, and total liver extracts were incubated with rabbit anti-rEg-ANX serum (1:200 v/v dilution), while the membrane containing recombinant proteins was incubated with serum of anti-rEg-ANX rabbit or serum of infected mice (1:200 v/v dilutions) overnight at 4°C. After washing procedures, the membranes were incubated for 2 hours with 1:2,000 diluted horseradish peroxidase (HRP)–conjugated goat anti-rabbit IgG or anti-mouse IgG (Bio-Rad), respectively. The signals were detected by Enhanced HRP-DAB Chromogenic Substrate Kit (Tiangen).
Immunolocalization of Eg-ANX protein.
Immunolocalization of Eg-ANX in the different life cycle stages of E. granulosus and the host-derived layer was determined by immunofluorescence. Fresh adult worms, PSCs, the germinal layer, the healthy sheep liver, and the host-derived layer were washed in phosphate-buffered saline (PBS, pH 7.4) three times and then fixed in 4% (w/v) paraformaldehyde for 36 hours. These samples were paraffin embedded and sliced into 5 μm-thick sections. After dewaxing in xylene and dehydration in an ethanol series, the slices were treated with 0.01 M citrate buffer (pH 6.0) at 95°C for 15 minutes. Then 4% (w/v) bovine serum albumin in 0.01 M PBS was used to block the slices at 37°C for 2 hours, followed by washing three times with PBS for 5 minutes. The slices were incubated with anti-rEg-ANX antibodies or normal rabbit serum (1:200 v/v dilutions) overnight at 4°C. After three washes, some of the slices were incubated with fluorescein isothiocyanate–conjugated goat anti-rabbit IgG (H+L) (Bethyl Laboratories, Montgomery, TX), diluted 1:200 in 1% Evans Blue (Leagene Biotechnology, Beijing, China) at 37°C for 1 hour in darkness and imaged under a fluorescence microscope (Nikon, Tokyo, Japan). Others were incubated with HRP-conjugated goat anti-rabbit IgG (Boster, Wuhan, China) at 37°C for 1 hour. After washing with PBS, the slices were stained with DAB substrate (Boster) and the nuclei were counterstained with hematoxylin.
Phospholipid-binding bioactivity assay.
Liposomes were produced based on the previous description with some modifications.28 In brief, 8 mg phosphatidylcholine (Sigma) and 2 mg phosphatidylserine (Sigma) were mixed and dissolved into chloroform in a round bottomed flask, followed by rotary evaporation to remove the chloroform. Then the residue in the flask was dissolved in 50 mM Tris-HCl (pH 8.0) and ultrasonicated on ice for 5 minutes. The phospholipid-binding assay was performed as described by Choi and others.29 The experiment was divided into three sample groups (A, B, and C). The reaction mixture for each group was composed of 1 mM CaCl2 (except group C), 20 μg liposomes, and 10 μg Eg-ANX and supplemented with 50 mM Tris-HCl (pH 7.5) to a total volume of 200 μL. After incubation at 37°C for 30 minutes, all groups were centrifuged at 10,000 × g for 10 minutes to separate the bound (pellets) and unbound (supernatant) proteins. The supernatant samples were collected and the pellets were washed three times with 500 μL of 50 mM Tris-HCl. Subsequently, 1 mM EGTA (a kind of calcium chelating agent) and 50 mM Tris-HCl (total 200 μL) were added to the pellets of group B and incubated at 37°C for 30 minutes. The supernatant and pellets were separated by centrifuge at 12,000 × g for 10 minutes. All the supernatant and pellet samples were run on 12% SDS-PAGE and observed by Coomassie staining.
Statistical analysis.
Statistical analysis was performed with Student's t test for comparison between two experimental groups using the software package GraphPad Prism (San Diego, CA).
Results
Molecular characterization.
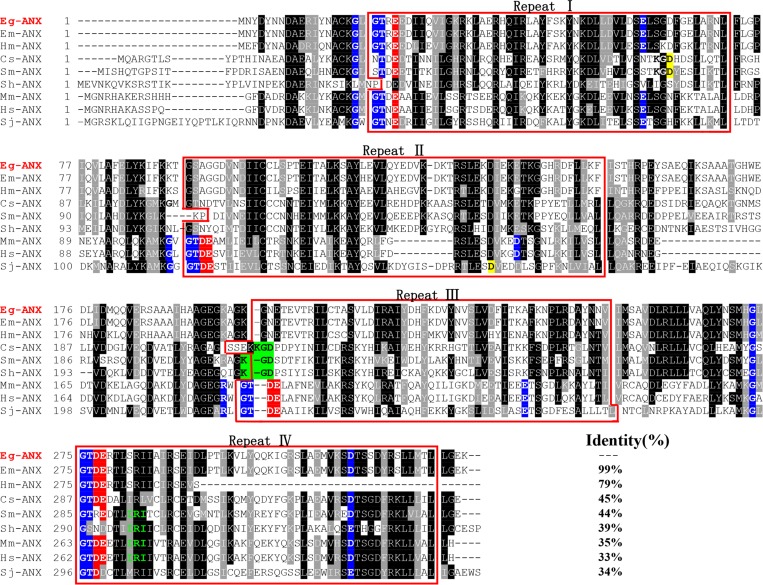
The Eg-ANX gene contained an open reading frame of 996 bp, encoding a putative protein of 331 amino acids with a predicted MW of 37.3 kDa and a pI of 5.9. Sequence analysis revealed that Eg-ANX did not have a signal peptide. Eg-ANX was aligned with other members of the annexin family (Figure 1 ), which showed that the core domain contained four annexin repeat domains, each repeat containing 52–62 amino acids. Alignment analysis revealed that Eg-ANX shared 95.8% identity with an annexin from Echinococcus multilocularis (Em-ANX) (GenBank: CDI96708.1), 79% identity with Hymenolepis microstoma annexin (Hm-ANX) (GenBank: CDS34213.1), and 34–45% identity with annexin from Schistosoma japonicum (Sj-ANX) (GenBank: CAX70812.1), S. mansoni (Sm-ANX) (GenBank: ACO90180.1), Schistosoma haematobium (Sh-ANX) (GenBank: KGB36040.1), and C. sinensis (Cs-ANX) (GenBank: GAA40121.2).
Figure 1.
Sequence alignment analysis of annexin B33 protein of Echinococcus granulosus (Eg-ANX) with homologous annexins. Alignment of the deduced amino acid sequence of E. granulosus annexin (GeneDB: EgrG_000041300) with homologs from other species. The following sequences were retrieved from the GenBank protein sequence database and aligned using the Clustal X program: Echinococcus multilocularis annexin (Em-ANX) (GenBank: CDI96708.1), Hymenolepis microstoma annexin (Hm-ANX) (GenBank: CDS34213.1), Clonorchis sinensis annexin (Cs-ANX) (GenBank: GAA40121.2), Schistosoma mansoni annexin (Sm-ANX) (GenBank: ACO90180.1), Schistosoma haematobium annexin (Sh-ANX) (GenBank: KGB36040.1), Mus musculus annexin (Mm-ANX) (GenBank: NP_081487.1), Homo sapiens annexin (Hs-ANX) (GenBank: CAG46637.1), and Schistosoma japonicum annexin (Sj-ANX) (GenBank: CAX70812.1). According to the Clustal X algorithm, regions of high identity and similarity between annexin sequences are shown as black and gray columns, respectively. The four repeat domains of annexin sequences are marked with red boxes. The actin-binding motifs (in repeat IV) are indicated by green letters. The type II Ca2+-binding sites are in white letters with blue backgrounds; the type III Ca2+-binding sites are in white letters with red and blue backgrounds. The KGD motifs are in black letters with green backgrounds. The percentage homology of Eg-ANX with other species is shown at the end of the alignments.
Annexins share a core domain made up of four similar repeats, and each repeat usually harbors type II and/or type III Ca2+-binding sites. Type II sites bind calcium ions using the sequence M-K/R-G/R-X-G-T-(38 residues)-D/E and type III using G-X-G-T-D/E.30,31 Some annexins contain both a type II and type III motif in all repeats (e.g., Homo sapiens (Hs)-ANX, Mus musculus (Mm)-ANX and Sj-ANX) (Figure 1). However, other annexins, such as Eg-ANX, Em-ANX, and Cs-ANX showed an absence of Ca2+-binding sites in some repeats. A KGD motif was found in repeat III of Cs-ANX, Sm-ANX, and Sh-ANX, where the Ca2+-binding site was lost. In repeat I or II of Sj-ANX, Sm-ANX, and Cs-ANX, the interval in the type II Ca2+-binding site was 42 amino acid residues. In addition, we identified an actin-binding (IRI) motif in repeat IV of Sm-ANX, Sh-ANX, Mm-ANX, and Hs-ANX but not in the Eg-ANX sequence.
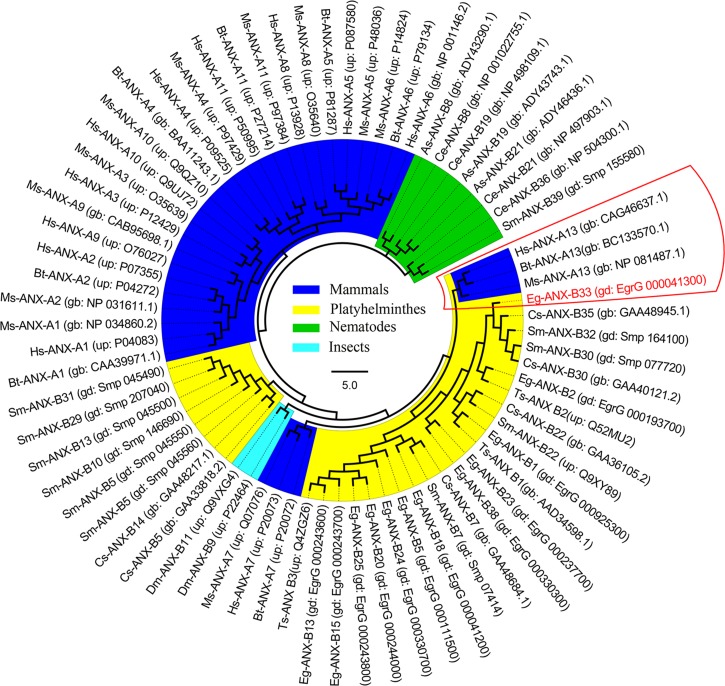
A phylogenetic tree was constructed based on multiple annexin sequences from various species (Figure 2 ), showing that Eg-ANX has near relationship with vertebrate annexin A13 family.
Figure 2.
Phylogenetic analysis of annexin B33 protein of Echinococcus granulosus (Eg-ANX) with homologous annexins. The phylogenetic tree was constructed based on the neighbor-joining method. gb = GenBank ID; gd = GeneDB ID; up = UniProt ID.
Cloning, expression, and purification of rEg-ANX.
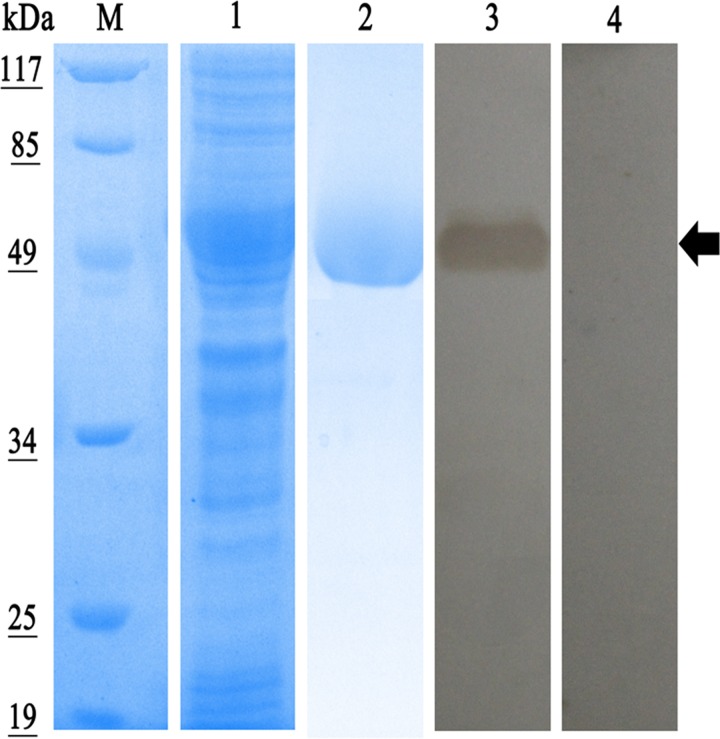
The Eg-ANX gene was amplified from PSCs and the fragment was expressed in E. coli with a ∼20-kDa epitope tag fusion peptide from pET-32a. The molecular mass of the recombinant protein was approximately 57 kDa (Figure 3 ). The rEg-ANX protein was partly expressed as soluble and was purified by Ni2+ affinity chromatography in non-denaturing conditions (Figure 3).
Figure 3.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot analysis of recombinant annexin B33 protein of Echinococcus granulosus (rEg-ANX). Expression, purification, and identification of rEg-ANX by serum of E. granulosus-infected mice. M = molecular mass marker in kDa; lane 1 = whole cell protein from isopropyl-β-d-1-thiogalactopyranoside–induced Escherichia coli cells containing pET-32a-Eg-ANX; lane 2 = sample after Ni column purification of recombinant protein; lane 3 = purified rEg-ANX was probed with the serum of E. granulosus infected mice; lane 4 = purified rEg-ANX was probed with native mouse serum.
The mRNA expressions at different parts of metacestode (larval) stage.
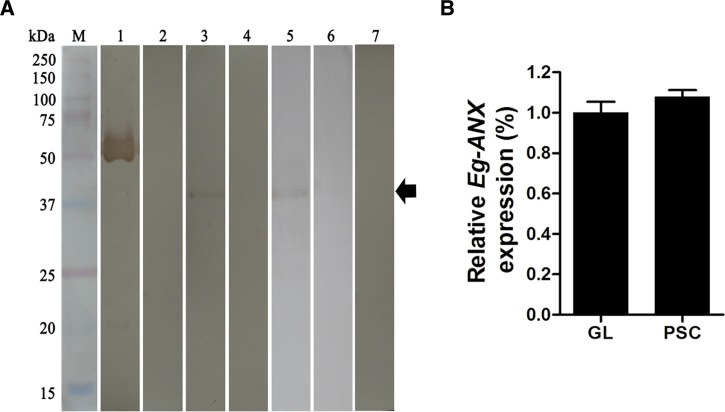
The transcripts of Eg-ANX were detected both at the PSCs and germinal layer, but no signal at laminated layer. And the transcriptional levels of PSCs and germinal layer showed no significant difference (Figure 4B ).
Figure 4.
Transcription and expression of endogenous annexin B33 protein of Echinococcus granulosus (Eg-ANX) in E. granulosus. (A) Identification of endogenous Eg-ANX from E. granulosus. M = molecular mass marker in kDa; lane 1 = purified rEg-ANX was probed with anti-rEg-ANX rabbit serum; lane 2 = purified rEg-ANX was probed with native rabbit serum; lane 3 = the anti-rEg-ANX rabbit serum detected a single band at a mass of 37 kDa in E. granulosus cystic fluid; lane 4 = the cystic fluid was probed with native rabbit serum; lane 5 = endogenous Eg-ANX from PSCs was probed with anti-rEg-ANX rabbit serum; lane 6 = the total protein of PSCs was probed with native rabbit serum; lane 7 = the total extracts of healthy sheep was probed with anti-rEg-ANX rabbit serum. (B) Endogenous Eg-ANX transcription of E. granulosus metacestode determined by quantitative real-time polymerase chain reaction. The transcripts of Eg-ANX in laminated layer were not detected. The elongation factor 1 alpha was used as the transcriptional control, and the data were presented as mean ± SD. GL = germinal layer; PSCs = protoscoleces.
Western blotting.
The rEg-ANX protein was recognized by antibodies from immunized rabbits as well as the sera from mice infected with E. granulosus, but was not recognized by negative controls (Figures 3 and 4A). These results showed strong immunogenicity and immunoreactivity of rEg-ANX. The native Eg-ANX protein in cyst fluid and PSCs was identified using rabbit anti-rEg-ANX antibody. A single band of ∼37.3 kDa was observed in each lane but not in the negative controls (Figure 4A). This result indicates that Eg-ANX is present in cystic fluid and PSCs.
Localization of Eg-ANX by immunohistochemistry.
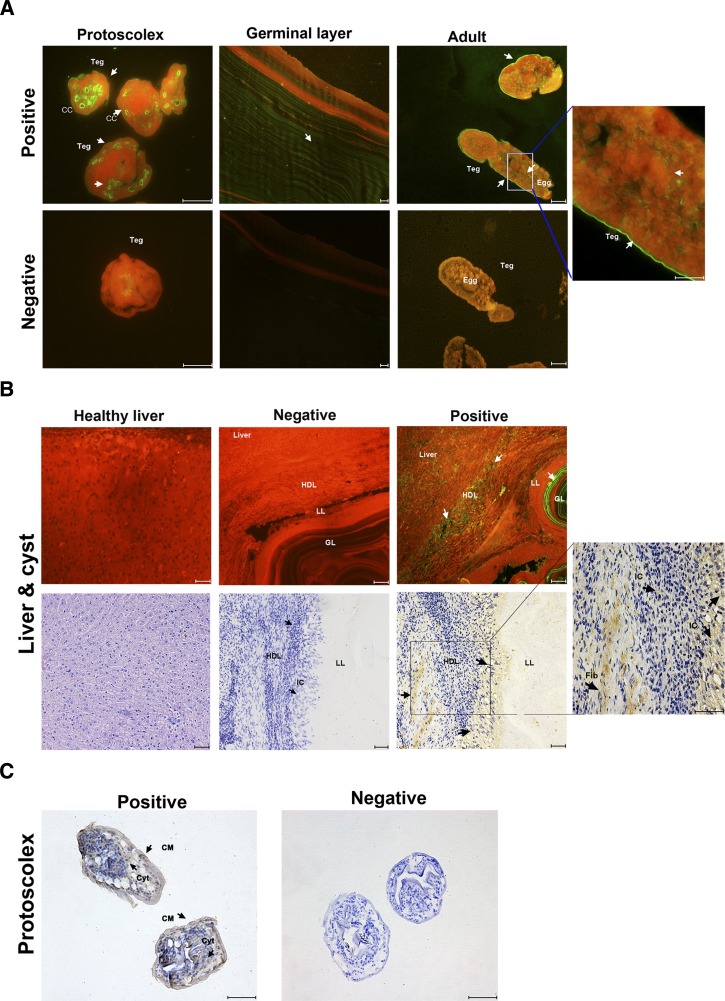
Localization of Eg-ANX in different life cycle stages of E. granulosus was performed by immunofluorescence using rabbit anti-rEg-ANX antibody. The results showed that the Eg-ANX was located on the tegument and eggs of adult worms. The weak fluorescence signals were detected in the whole germinal layer (Figure 5A ). For PSCs, the fluorescence and brown signals were both detected on the tegument and calcareous corpuscle (Figure 5A). At the cellular level, Eg-ANX was located in the cytosol and cellular membrane of protoscoleces (Figure 5C). In addition, weak fluorescent and brown signals were observed on the inflammatory cells and fibroblasts of host-derived layer. No fluorescence or brown signal was detected in controls (Figure 5B).
Figure 5.
Immunolocalization of annexin B33 protein of Echinococcus granulosus (Eg-ANX). (A) Immunofluorescence localization of Eg-ANX in different stages of E. granulosus. Eg-ANX in the protoscolex, germinal layer, and adult was immunofluorescently labeled using specific anti-rEg-ANX IgG (positive), or control pre-immune serum (negative), followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG. CC = calcareous corpuscle; Teg = tegument. (B) Immunohistochemical localization of Eg-ANX in the host-derived layer and host liver. Eg-ANX in the host liver and host-derived layer was labeled using specific anti-rEg-ANX IgG (positive) or control pre-immune serum (negative), followed by FITC-conjugated anti-rabbit IgG or horseradish peroxidase–conjugated goat anti-rabbit IgG. The healthy liver was incubated by specific anti-rEg-ANX IgG and stained with hematoxylin–eosin. Fib = fibroblasts; GL = germinal layer; HDL = host-derived layer; IC = inflammatory cells; LL = laminated layer. (C) Eg-ANX localization in cytosol, cellular membrane of protoscoleces. CM = cellular membrane; Cyt = cytosol. Fluorescence-labeled or brown-labeled regions are marked with arrows. Scale bars: 50 μm.
Phospholipid-binding bioactivity analysis.
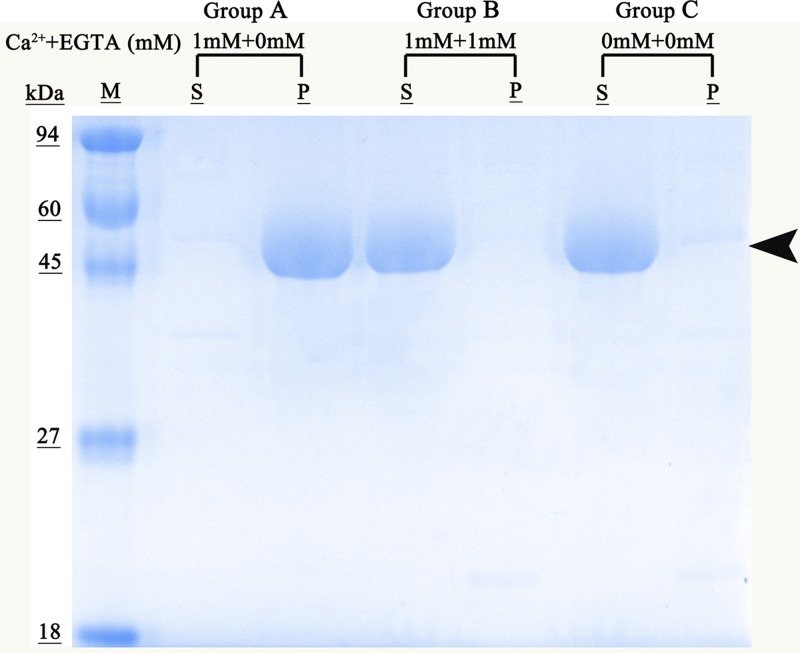
To examine the calcium-dependent phospholipid-binding properties of rEg-ANX, the recombinant protein was reacted with a lipidosome in the absence or presence of Ca2+. Mixtures were then centrifuged, bound protein located to the pellet and unbound protein to the supernatant. Recombinant Eg-ANX was observed only in the supernatant without Ca2+ (group C) (Figure 6 ), while rEg-ANX was observed in the pellets, but not in the supernatant, in the presence of 1 mM Ca2+ (group A) (Figure 6). When Ca2+ was removed from the recombinant protein by EGTA, rEg-ANX lost the ability to bind to lipidosomes and returned into the supernatant (group B).
Figure 6.
Phospholipid-binding properties of annexin B33 protein of Echinococcus granulosus (Eg-ANX). Lane M = molecular mass marker in kDa; Group A = rEg-ANX was incubated with liposomes in buffer containing 1 mM Ca2+; Group B = rEg-ANX was incubated with liposomes in buffer containing 1 mM Ca2+ and 1 mM EGTA was then added; Group C was the control group (no Ca2+ or EGTA). Ca2+ + EGTA (mM) = the concentrations of added Ca2+ and EGTA, respectively; P = pellet; S = supernatant.
Discussion
Annexins are a multigene family widely distributed in eukaryotes and are proposed to be involved in a wide range of important biological processes, such as membrane trafficking and fusion, anticoagulation, and anti-inflammatory action. Recent research has shown that annexins play important roles in host–parasite relationships. In T. solium metacestodes, annexin B1 was detected in cyst fluid and the sera of hosts as well as in the host-derived layer surrounding the cysts.32 Subsequently, other research demonstrated that T. solium annexin B1 was secreted by living cells and downregulated the host immune response, which suggested that secretion of annexin B1 might be a strategy for T. solium metacestodes to survive the host immune system.15 These results indicate the importance of parasite annexin and suggest a possible intervention target for the therapy and control of parasitic diseases. Two proteomic studies on the zoonotic pathogen E. granulosus found that an annexin was present in the ES product and hydatid cyst fluid.22,23 Therefore, we performed a primary study characterizing the Eg-ANX gene and validated the secretory and calcium ion binding properties of Eg-ANX protein.
As shown in many reports, annexins generally have Ca2+-binding sites in each annexin repeat domain, but some annexins have lost some of the Ca2+-binding sites.16,17,33,34 Our bioinformatic analysis showed that Eg-ANX has lost Ca2+-binding sites in repeats II and III, and this loss was not substituted by a “KGD” motif as in Em-ANX and Hm-ANX. However, we did identify a KGD motif in repeat III of Cs-ANX, Sm-ANX, and Sh-ANX, which may substitute for the loss of a Ca2+-binding site.33 Because of the Ca2+-binding sites in repeats I and IV, Eg-ANX had Ca2+-dependent phospholipid-binding properties, which might provide a link between Ca2+ signaling and membrane functions, such as the regulation of ion flux across membranes, organization of membranes, endocytosis, exocytosis, vesicle fusion, and so on.8 In the presence of EGTA, the Ca2+-dependent binding properties of Eg-ANX protein were lost. All this was consistent with previous reports, showing the reversible Ca2+-dependent phospholipid-binding properties of annexins, which are considered to be their biochemical hallmark.9,28,29 Further, the Eg-ANX sequence showed the absence of an IRI actin-binding motif, which is very common in other annexins (e.g., Sm-ANX, Sh-ANX, Mm-ANX, and Hs-ANX). This may suggest that Eg-ANX has lost the ability for membrane–actin interactions.34
Annexins are usually localized either in the cytosol or associated with cellular membranes and cytoskeletal proteins.8 Intriguingly, many previous reports have documented that some annexin could be secreted and released extracellularly despite the absence of any secretory signal peptide.15,32,35,36 In this study, based on an Eg-ANX-specific antibody, our western blot and immunolocalization analyses revealed that Eg-ANX could be secreted to extracellular space, even to the outside of E. granulosus. Given the fact that Eg-ANX has no signal peptide, we can hypothesize that Eg-ANX may have a special secretion pathway. Regrettably, the secretion mechanism of annexins is still at the stage of conjecture and hypothesis. Researchers hold diffident opinions on the secretion mechanism. Christmas and others37 reported that even though annexin A1 lacks hydrophobic signal sequences, the human prostate gland could selectively secrete high concentrations of the protein. Thus, the secretion of annexin A1 was considered to involve a highly selective mechanism and to be independent of a hydrophobic signal sequence and the endoplasmic reticulum. Faure and others38 believed that the secretion of annexin was caused by membrane disruption during exocytosis. Danielsen and others39 found a majority of enterocyte annexin A2 was located on the lumenal side of microvilli, implying an apical secretion by a “nonclassical” mechanism. More recent studies showed that annexin A2 was an important component of exosomes in dendritic cells40 and could be transferred from one cell to another in exosomes in a raft-associated manner.19,41 More research is required to better understand the secretory annexins.
In conclusion, we characterized a secretable annexin from E. granulosus, which had typical Ca2+-dependent phospholipid-binding properties. Our results revealed that the Eg-ANX was located on the inflammatory cells and fibroblasts of host-derived layer, which implied that this protein might be an important molecule in host–parasite interactions. The exact function of Eg-ANX, especially in the host–parasite interface, will be investigated further.
ACKNOWLEDGMENTS
We thank Xuan Zhou (Sichuan Agricultural University) for her crucial advice in our manuscript, Min Yan (Sichuan Agricultural University) for his assistance in animal experiment, and Jiahai Wang (Sichuan Agricultural University) for thoughtful comments.
Disclaimer: The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This study was supported by a grant from the Key Technology R&D Program of Sichuan Province, China (no. 2015NZ0041; http://www.scst.gov.cn/).
Authors' addresses: Xingju Song, Dandan Hu, Xiuqin Zhong, Ning Wang, Xiaobin Gu, Tao Wang, and Guangyou Yang, Department of Parasitology, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China, E-mails: 18728153735@163.com, 18783542005@163.com, xiuqinzhong@hotmail.com, wangningzhuhui@hotmail.com, guxiaobin198225@126.com, muhammad06@126.com, and guangyou1963@aliyun.com. Xuerong Peng, Department of Chemistry, College of Life and Basic Science, Sichuan Agricultural University, Ya'an, China, E-mail: 276361511@qq.com.
References
- 1.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 2.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, Gonzalez AE, Lorca M, Naquira C. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–394. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 3.Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris, France: World Organisation for Animal Health; 2001. [Google Scholar]
- 5.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti E, Junghanss T. Update on cystic hydatid disease. Curr Opin Infect Dis. 2009;22:497–502. doi: 10.1097/QCO.0b013e328330331c. [DOI] [PubMed] [Google Scholar]
- 8.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 9.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 10.Belluoccio D, Grskovic I, Niehoff A, Schlötzer Schrehardt U, Rosenbaum S, Etich J, Frie C, Pausch F, Moss SE, Pöschl E. Deficiency of annexins A5 and A6 induces complex changes in the transcriptome of growth plate cartilage but does not inhibit the induction of mineralization. J Bone Miner Res. 2010;25:141–153. doi: 10.1359/jbmr.090710. [DOI] [PubMed] [Google Scholar]
- 11.Fatimathas L, Moss SE. Annexins as disease modifiers. Histol Histopathol. 2010;25:527–532. doi: 10.14670/HH-25.527. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann A, Osman A, Leow CY, Driguez P, McManus DP, Jones MK. Parasite annexins—new molecules with potential for drug and vaccine development. BioEssays. 2010;32:967–976. doi: 10.1002/bies.200900195. [DOI] [PubMed] [Google Scholar]
- 13.Cantacessi C, Seddon JM, Miller TL, Leow CY, Thomas L, Mason L, Willis C, Walker G, Loukas A, Gasser RB. A genome-wide analysis of annexins from parasitic organisms and their vectors. Sci Rep. 2013;3:2898. doi: 10.1038/srep02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N, Hamamouch N, Li C, Hewezi T, Hussey RS, Baum TJ, Mitchum MG, Davis EL. A nematode effector protein similar to annexins in host plants. J Exp Bot. 2010;61:235–248. doi: 10.1093/jxb/erp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, Ren MY, Chen XQ, Wang XY, Li S, Lin JS, Liang C, Liang P, Hu Y, Lei HL. Calcium-dependent proapoptotic effect of Taenia solium metacestodes annexin B1 on human eosinophils: a novel strategy to prevent host immune response. Int J Biochem Cell Biol. 2008;40:2151–2163. doi: 10.1016/j.biocel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Tararam CA, Farias LP, Wilson RA, de Cerqueira Leite LC. Schistosoma mansoni annexin 2: molecular characterization and immunolocalization. Exp Parasitol. 2010;126:146–155. doi: 10.1016/j.exppara.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 17.He L, Ren MY, Chen XQ, Wang XY, Li S, Lin JS, Liang C, Liang P, Hu Y, Lei HL. Biochemical and immunological characterization of annexin B30 from Clonorchis sinensis excretory/secretory products. Parasitol Res. 2014;113:2743–2755. doi: 10.1007/s00436-014-3935-4. [DOI] [PubMed] [Google Scholar]
- 18.Yazid S, Sinniah A, Solito E, Calder V, Flower RJ. Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1. PLoS One. 2013;8:e58963. doi: 10.1371/journal.pone.0058963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259–6305. doi: 10.3390/ijms14036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Genderen HO, Kenis H, Hofstra L, Narula J, Reutelingsperger CP. Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta. 2008;1783:953–963. doi: 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 22.Virginio VG, Monteiro KM, Drumond F, de Carvalho MO, Vargas DM, Zaha A, Ferreira HB. Excretory/secretory products from in vitro-cultured Echinococcus granulosus protoscoleces. Mol Biochem Parasitol. 2012;183:15–22. doi: 10.1016/j.molbiopara.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Aziz A, Zhang W, Li J, Loukas A, McManus DP, Mulvenna J. Proteomic characterisation of Echinococcus granulosus hydatid cyst fluid from sheep, cattle and humans. J Proteomics. 2011;74:1560–1572. doi: 10.1016/j.jprot.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Dematteis S, Pirotto F, Marques J, Nieto A, Örn A, Baz A. Modulation of the cellular immune response by a carbohydrate rich fraction from Echinococcus granulosus protoscoleces in infected or immunized Balb/c mice. Parasite Immunol. 2001;23:1–9. doi: 10.1046/j.1365-3024.2001.00346.x. [DOI] [PubMed] [Google Scholar]
- 25.Nie HM, Xie Y, Fu Y, Yang Y, Gu XB, Wang S, Peng X, Lai W, Peng XR, Yang GY. Cloning and characterization of the fatty acid-binding protein gene from the protoscolex of Taenia multiceps. Parasitol Res. 2013;112:1833–1839. doi: 10.1007/s00436-013-3328-0. [DOI] [PubMed] [Google Scholar]
- 26.Hu DD, Song XJ, Xie Y, Zhong XQ, Wang N, Zheng Y, Gu XB, Wang T, Peng XR, Yang GY. Molecular insights into a tetraspanin in the hydatid tapeworm Echinococcus granulosus. Parasit Vectors. 2015;8:311. doi: 10.1186/s13071-015-0926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang KH, Guo YJ, Lu YM, Yan HL, Song YL, Wang F, Ding FX, Sun SH. Annexin B1 from Taenia solium metacestodes is a newly characterized member of the annexin family. Biol Chem. 2007;388:601–610. doi: 10.1515/BC.2007.071. [DOI] [PubMed] [Google Scholar]
- 29.Choi SH, Kwon SR, Lee EH, Kim KH. Molecular cloning, functional characterization and localization of an annexin from a fish gill fluke Microcotyle sebastis (Platyhelminthes: Monogenea) Mol Biochem Parasitol. 2009;163:48–53. doi: 10.1016/j.molbiopara.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 30.de la Torre Escudero E, Manzano Román R, Siles Lucas M, Pérez Sánchez R, Moyano JC, Barrera I, Oleaga A. Molecular and functional characterization of a Schistosoma bovis annexin: fibrinolytic and anticoagulant activity. Vet Parasitol. 2012;184:25–36. doi: 10.1016/j.vetpar.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao YJ, Yan HL, Ding FX, Lu YM, Sun SH. Annexin B1 at the host–parasite interface of the Taenia solium cysticercus: secreted and associated with inflammatory reaction. Acta Trop. 2007;101:192–199. doi: 10.1016/j.actatropica.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Morgan R, Martin Almedina S, Iglesias J, Gonzalez Florez M, Fernandez M. Evolutionary perspective on annexin calcium-binding domains. Biochim Biophys Acta. 2004;1742:133–140. doi: 10.1016/j.bbamcr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Konopka Postupolska D. Annexins: putative linkers in dynamic membrane-cytoskeleton interactions in plant cells. Protoplasma. 2007;230:203–215. doi: 10.1007/s00709-006-0234-7. [DOI] [PubMed] [Google Scholar]
- 35.Vergnolle N, Guimbaud R, Chaussade S, Buéno L, Escourrou J, Coméra C. Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflamm Bowel Dis. 2004;10:584–592. doi: 10.1097/00054725-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly S, Moss S. Annexins in the secretory pathway. Cell Mol Life Sci. 1997;53:533–538. doi: 10.1007/s000180050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christmas P, Callaway J, Fallon J, Jones J, Haigler H. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J Biol Chem. 1991;266:2499–2507. [PubMed] [Google Scholar]
- 38.Faure AV, Migné C, Devilliers G, Ayala Sanmartin J. Annexin 2 “secretion” accompanying exocytosis of chromaffin cells: possible mechanisms of annexin release. Exp Cell Res. 2002;276:79–89. doi: 10.1006/excr.2002.5512. [DOI] [PubMed] [Google Scholar]
- 39.Danielsen EM, van Deurs B, Hansen GH. “Nonclassical” secretion of annexin A2 to the lumenal side of the enterocyte brush border membrane. Biochemistry. 2003;42:14670–14676. doi: 10.1021/bi0355239. [DOI] [PubMed] [Google Scholar]
- 40.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 41.Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286:30911–30925. doi: 10.1074/jbc.M111.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]