Abstract
Although artemisinin resistance has yet to be reported in Africa, surveillance of the efficacy of artemisinin-based combination therapies (ACTs) is warranted. Here, the efficacy of artesunate + sulfadoxine–pyrimethamine (AS+SP) and artemether–lumefantrine (AL) was evaluated in Mali. Randomized open-label comparative in vivo assay of AS+SP versus AL were carried out using the 28-day follow-up World Health Organization protocol. Patients with uncomplicated falciparum malaria and at least 6 months of age were recruited between October 2010 and January 2014. A subset of these patients was selected to measure Plasmodium falciparum clearance time. Polymerase chain reaction-corrected adequate clinical and parasitological responses were 100% for AS+SP and 98.2% for AL with no significant difference (P = 0.06). The reinfection rates were comparable (P = 0.63) with 8.0% for AS+SP and 12.6% for AL. Individuals under 8 years were more susceptible to treatment failure (relative risk = 1.9; 95% confidence interval = 1.2, 3.3). Median parasite clearance half-life was 1.7 hours (interquartile range [IQR] = 1.3–2.2) for AS+SP and 1.9 hours (IQR = 1.5–2.5) for AL with no statistically significant difference (P = 0.24). Efficacy of AS+SP and AL was high. This study provides baseline information on parasite clearance half-lives after ACT treatment, particularly AS+SP, in Mali.
Introduction
Malaria remains a major public health problem with estimates of 198 million (124–283 million) clinical cases and 367,000–755,000 deaths reported worldwide in 2013, 90% of which occurred in sub-Saharan Africa.1 In Mali, of the 39,283 deaths due to malaria, 28,859 were children under 5 years of age in 2010.2 Artemisinin-based combination therapies (ACTs) are the first-line treatments of uncomplicated malaria in endemic countries. Artemisinins are a powerful class of antimalarial drugs that are able to rapidly reduce malaria parasite load and morbidity.3–5 These drugs along with quinine are the most efficacious antimalarial drugs currently used to control malaria. Unfortunately, artemisinin resistance, defined as delayed parasite clearance, has emerged in southeastern Asia.6–8 Mutations in the kelch 13 propeller domain have been associated with artemisinin resistance in vitro and in vivo in southeast Asia.9,10 To date, artemisinin resistance has not been reported in Africa and ACTs remained highly efficacious.10–14 However, sub-Saharan Africa remains at risk because of drug pressure from wide use of ACTs and intercontinental movement of people. Furthermore, circulation of substandard or counterfeit drugs and nonadherence to treatment course are factors that contribute to rapidly selecting resistant parasites.14–16 Since 2006, Mali adopted artemether–lumefantrine (AL) and artesunate–amodiaquine as first-line treatments for uncomplicated malaria management. Large quantities of artesunate + sulfadoxine–pyrimethamine (AS+SP) were donated to Mali by the Chinese cooperation in 2010. China is a major partner of Mali, supporting its heath system since the 1960s. This cooperation allowed the training of many Malian health professionals and the building of three hospitals. When thousands of doses of AS+SP were donated, it was critical to assess the efficacy of this combination and compare it to one of the standard first-line ACT, that is, AL.
Materials and Methods
Study sites.
The study was conducted in three clinical trial sites of the Malaria Research and Training Center, namely Banambani, Sotuba, and Kolle.
Banambani village is located 30 km north of Bamako, the capital city of Mali, in the Savanna area. The population is estimated to be 1,400 inhabitants. Malaria transmission is seasonal (June–November). Malaria incidence in children under 5 years of age is 2.1 episodes per child per year. The entomological inoculation rate is 137–167 infective bites per person per year.17 Sotuba is a peri-urban area of Bamako with 6,472 inhabitants. The entomological inoculation rate is 4–12 infective bites per person per year.17 Kolle is a village of 2,500 inhabitants located 57 km southwest of Bamako in the Sudan savanna area and 9 km away from the Niger River. The entomological inoculation rate is nearly identical to the level of Banambani.17
Study volunteers were recruited between October 2010 and January 2011 in Sotuba and Kolle and between January 2013 and January 2014 in Banambani.
Anopheles gambiae s.l. and Anopheles funestus s.l. are the two Anopheles complex species responsible for malaria transmission in all three study sites.
Sample size calculation and sampling.
The sample size was calculated using a non-inferiority hypothesis of AS+SP versus AL, which had an efficacy rate of 95%.18 A non-inferiority range of 5%, an alpha risk of 5%, a power of 80%, and lost to follow-up of 10% were assumed to yield a total sample size of 503 subjects. Volunteers were recruited by applying a simple random sampling among patients visiting our medical teams during the study periods. Volunteers were then assigned to either AS+SP or AL through a computer-generated random list (Excel; Microsoft Corporation, Redmond, WA).
A nested parasite clearance evaluation sub-study was conducted on the cohort of volunteers recruited between January 2013 and January 2014. Among the volunteers recruited during that period, a systematic sampling was used to draw the volunteers included into the nested sub-study. The first volunteer was randomly determined and then every 6th volunteer was selected until reaching the 45 patients of the sub-study.
Study design.
We used the standard World Health Organization (WHO) 28-day in vivo antimalarial efficacy protocol.19 Patients aged 6 months and older suffering from uncomplicated falciparum malaria were recruited. Cases of coinfection with other malaria parasite species were excluded. Inclusion parasitemia ranges were 1,000–200,000 trophozoites/μL. Pregnant women, breast-feeding mothers, and patients allergic to any of the study drugs were systematically excluded. Exclusion criteria also included general signs of danger in children under 5 years of age, severe falciparum malaria and severe illness according to WHO 2003 protocol.19 Patients previously treated by any antimalarial drug in the preceding 14 days before inclusion were excluded. During scheduled visits at days 0, 1, 2, 3, 7, 14, 21, and 28, each enrolled patient was subjected to full clinical examination, collection of dry blood spots on 3 MM filter papers (Whatman®, Maidstone, United Kingdom) hemoglobin measurements and parasitemia assessments. Drugs were administered to volunteers over the first 3 days by the research teams. Doses were repeated whenever there was immediate vomiting. In case of repeated vomiting, patients were excluded and treated by parenteral route. A presence of other Plasmodium species or a parasitemia outside the range of 1,000–200,000/μL, as confirmed by the third reader, was excluded.
Follow-up ended when the following criteria were met: 1) lost to follow-up, 2) refusal, 3) occurrence of any sign of severe malaria, and 4) any treatment failure. Volunteers selected for parasite clearance evaluation were checked for parasitemia at hours 0 (before first drug administration), 8, 16, 24, 36, 48, and 60. After hour 60, those individuals continued to be followed as part of the main study until day 28 or when one of the above study termination criteria was met.
Study drugs.
The drugs tested included fixed dose tablets of AL (20/120 mg, Coartem® Novartis, Basel, Switzerland) and co-blistered tablets of AS+SP (Artecospe®, Guilin Pharmaceutical Co. Ltd., Guilin, China) in three different packages (3 × 50 mg + 1 × 500/25 mg, 6 × 50 mg + 2 × 500/25 mg, and 6 × 100 mg + 3 × 500/25 mg). For AL, the standard dosing was used, that is, one tablet for patients weighing 5–14 kg, two tablets for 15–24 kg, three tablets for 25–34 kg, and four tablets for ≥ 35 kg taken at hours 0, 8, 24, 36, 48, and 60. For AS+SP, tablets of SP were given at a unique dose of 25 mg/kg of sulfadoxine and 1.25 mg/kg of pyrimethamine on day 0 along with artesunate at 4 mg/kg on days 0, followed with AS at 4 mg/kg on days 1 and 2.
Quality control of thick and thin blood smears.
Previously described methods were used to perform quality control of all slides.20 Parasitemia was estimated with Giemsa-stained thick smears by counting the number of asexual stages per 300 white blood cells (WBCs) or more. Indeed, the quotient from the division of the asexual stage counts by ≥ 300 WBCs was multiplied by 7,500 to estimate parasitemia per microliter of blood. Thin smears were used to search the presence of other Plasmodium species before inclusion on day 0 or to check the appearance of other malaria parasite species during follow-up. All thick/thin smears were read a second time by an independent qualified microscopist. A third read was done if the first two readers had significant qualitative or quantitative discrepancies. Qualitative discrepancy was deemed met when the second reader observed another malaria parasite species. Quantitative discrepancy was estimated by dividing the difference of the two counts by their mean. Third read was warranted for discrepancy > 25% (significant) when parasitemia was comprised in 1–999/μL and > 50% (significant) for parasitemia ≥ 1,000/μL. The mean value of the two counts was used in the absence of significant discrepancy between the two first readers. However, in case of significant discrepancy the mean value of the third read and the closest read was retained.
Molecular correction.
Msp2 gene and two microsatellites (Ca1 and Ta99) were genotyped by nested polymerase chain reaction (PCR) to discriminate recrudescent parasites from new infections as previously described.21,22 Three qualified investigators assessed gel pictures. Outcomes were classified: 1) recrudescence, if the alleles on day 0 and days of treatment failure were the same for msp2, Ca1, and Ta99 or if similar alleles were found on day 0 and days of treatment failure for all the three markers in the presence of additional bands; 2) reinfection, if the alleles on day 0 and days of treatment failure were distinct for any of the three markers; and 3) indeterminate, if either or both the day 0 and days of treatment samples could not be amplified.
Data analysis.
Data collected in case report forms were entered in Microsoft Excel (Microsoft® Corporation). Efficacy data were analyzed using GraphPad Prism 6 (http://www.graphpad.com/scientific-software/prism/). Parasite clearance was analyzed with the Worldwide Antimalarial Resistance Network parasite clearance estimator (www.wwarn.org) and the WHO online parasite clearance estimator (http://www.who.int/malaria/areas/treatment/drug_efficacy/en/). This software provided individual estimation of parasite clearance half-life, which were used to perform different comparisons using GraphPad Prism 6. Pearson's χ2 test and Fisher's exact test were used for comparisons of efficacy data whereas Mann–Whitney test was used for parasite clearance data. A P value ≤ 0.05 was considered statistically significant.
Ethical clearance.
The study protocol obtained ethical clearance from WHO and the Ethics Committee of Faculty of Pharmacy and Faculty of Medicine and Odonto-stomatology, University of Science, Techniques and Technologies of Bamako, Mali. Community permission and individual informed consent and/or informed assent were obtained after explanation of benefits and probable risks related to the study. Data were anonymized to guarantee confidentiality of volunteers' identities. All patients seen by our medical team received free medical care including treatments for concomitant diseases.
Results
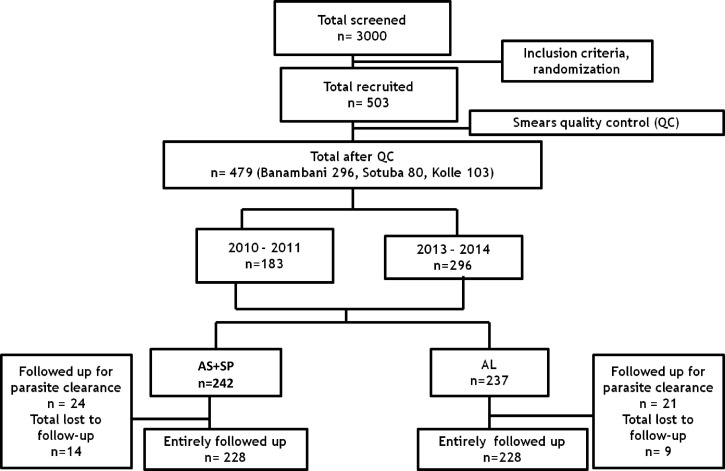
Of 3,000 patients screened during the study period, 503 volunteers were enrolled (Figure 1 ). After quality control of all thick/thin smears, 479 volunteers were included in the final analyses. Between October 2010 and January 2011, 103 patients were enrolled in Kolle, 80 in Sotuba and between January 2013 and January 2014, 296 were enrolled in Banambani. Patients were randomized by treatment arm with 242 in AS+SP and 237 in AL arms. Because of refusal and travel 14 volunteers were lost to follow-up in AS+SP arm and nine in AL arm. Consequently, 228 volunteers were successfully followed up in each treatment arm.
Figure 1.
Study profile. AL = artemether + lumefantrine; AS+SP = artesunate + sulfadoxine–pyrimethamine; QC = quality control.
Baseline characteristics of the patients are presented in Table 1. The two treatment arms were comparable for all assessed parameters. Patients under 5 years represented less than 28% in each group.
Table 1.
Baseline characteristics of subjects by treatment arm
| Characteristics | AS+SP | AL |
|---|---|---|
| Size (n) | 242 | 237 |
| Age (year) | ||
| Mean ± standard deviation | 8.83 ± 7.18 | 8.98 ± 7.06 |
| Minimum–maximum | 1.08–43.00 | 0.92–48.00 |
| Age range (%) | ||
| 5 years | 27.3 | 27.5 |
| ≥ 5 years | 72.7 | 72.5 |
| Sex (%) | ||
| Female | 50.0 | 41.3 |
| Male | 50.0 | 58.7 |
| Weight (kg) | ||
| Mean ± standard deviation | 22.32 ± 12.55 | 22.73 ± 11.65 |
| Minimum–maximum | 7.00–67.00 | 7.00–62.00 |
| Hemoglobin concentration (g/dL) | ||
| Mean ± standard deviation | 10.94 ± 2.00 | 10.89 ± 2.01 |
| Minimum–maximum | 5.20–17.90 | 5.50–17.70 |
AL = artemether + lumefantrine; AS+SP = artesunate + sulfadoxine–pyrimethamine.
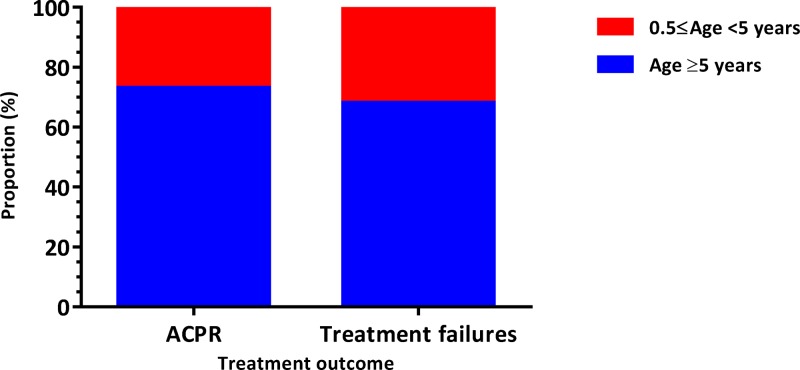
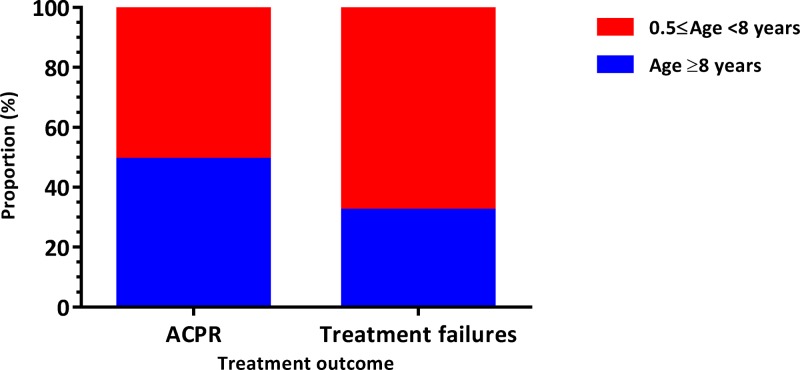
Before PCR correction, the rate of adequate clinical and parasitological response (ACPR) was significantly higher in the AS+SP group than in the AL group (P = 0.016; Pearson's χ2 test) with 91.2% (N = 228) and 83.8% (N = 228), respectively (Table 2). The risk of occurrence of treatment failures in AL group was 9% higher than AS+SP group (relative risk [RR] = 1.09; 95% confidence interval [CI95] = 1.0, 1.2). When we compared the occurrence of treatment failures between groups of individuals under 5 years versus those ≥ 5 years (Figure 2 ), we found no difference between the two groups (RR = 1.23; CI95 = 0.7, 2.1). However, when we performed this comparison between patients under 8 years versus those ≥ 8 years (Figure 3 ), the risk of failure was significantly higher in AL group (RR = 1.94; CI95 = 1.2, 3.3).
Table 2.
Treatment responses of study drugs before PCR correction
| Characteristics | AS+SP | AL |
|---|---|---|
| n (%) | n (%) | |
| Treatment arm outcomes | ||
| ACPR | 208 (91.2) | 191 (83.8) |
| LPF | 16 (7.0) | 28 (12.3) |
| LCF | 4 (1.8) | 9 (4.0) |
| Size | 228 | 228 |
ACPR = adequate clinical and parasitological response; AL = artemether + lumefantrine; AS+SP = artesunate + sulfadoxine–pyrimethamine; LCF = late clinical failure; LPF = late parasitological failure; PCR = polymerase chain reaction. AS+SP vs. AL regarding treatment efficacy (i.e., ACPR), P = 0.016 (Pearson's χ2 test).
Figure 2.
Distribution of treatment responses by age ranges (0.5 ≤ age < 5 years vs. age ≥ 5 years). ACPR = adequate clinical and parasitological response. Relative risk = 1.2; 95% confidence interval = 0.7, 2.1.
Figure 3.
Distribution of treatment responses by age ranges (0.5 ≤ age < 8 years vs. age ≥ 8 years). ACPR = adequate clinical and parasitological response. Relative risk = 1.9; 95% confidence interval = 1.2, 3.3.
Among the parasitological failures, five samples from AL arm and two from AS+SP arm failed to yield PCR results. In a per protocol analysis excluding these seven samples, the rates of ACPR for the two treatment arms were comparable (P = 0.06; Fisher's exact test) with 100% for AS+SP and 98.2% for AL. The parasite reinfection rate for AS+SP was 8.0%. There was no significant difference with the reinfection rate of AL, which was 12.6% (P = 0.11; Pearson's χ2 test).
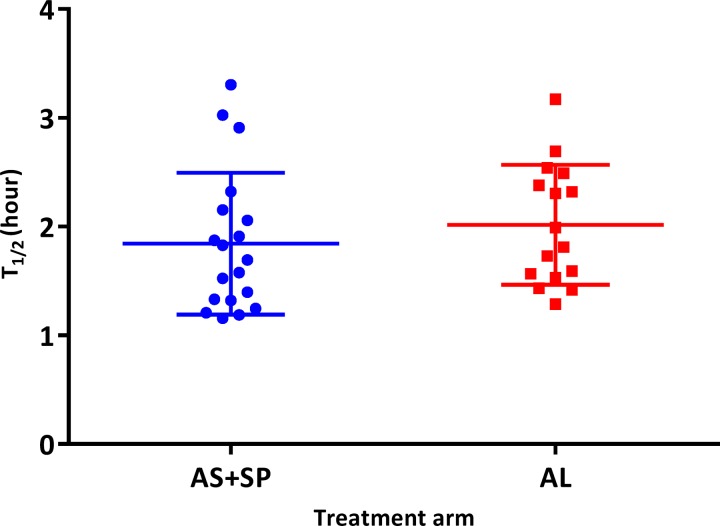
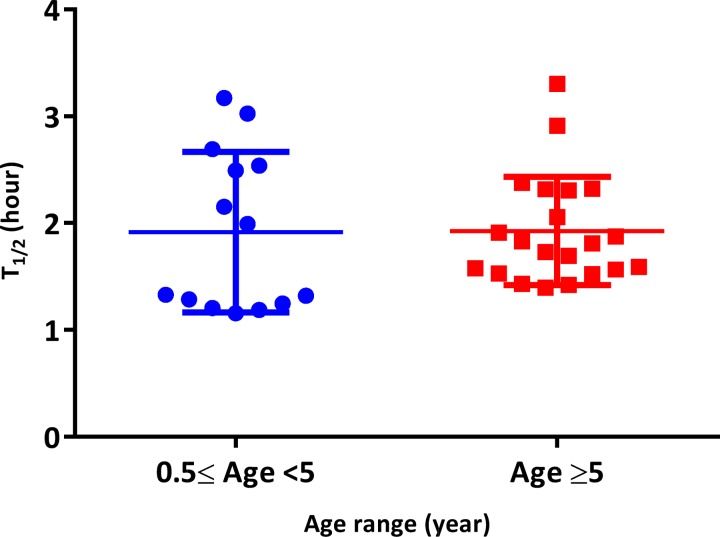
From the 45 subjects followed for parasite clearance assessment, 10 did not meet the software requirements (too few data points; too low parasitemia; if the first zero parasitemia is replaced by software detection limit, which was 26 parasites/μL with the last positive parasitemia exceeding 1,000; and if the last positive parasitemia was > 1,000 with no zero parasitemia) and were therefore removed. After analysis, the medians of parasite clearance half-life (T1/2) were 1.7 hours (interquartile range [IQR] = 1.3–2.2) for AS+SP and 1.9 hours (IQR 1.5–2.5) for AL (Table 3). The distribution of T1/2 by treatment arm and by age range (Figures 4 and 5 ) showed no significant difference between AS+SP and AL (P = 0.24, Mann–Whitney test) and in subjects < 5 and ≥ 5 years (P = 0.70, Mann–Whitney test). Both parasite clearance estimation methods gave the same outcome and were highly correlated (r = 0.73; P < 0.001). The proportions of negative parasitemia 24 hours after the treatment (or day 1) were 73.9% (N = 23) for AS+SP and 65.0% (N = 20) for AL (P = 0.53). Proportions of patients with positive smears at days 2 and 3 (48 hours) were 0% in both. All volunteers included in the parasite clearance assessment presented adequate clinical and parasitological response by day 28 of follow-up regardless of treatment arm (Table 3).
Table 3.
Parasite clearance parameters by treatment arm
| Characteristics | AS+SP | AL |
|---|---|---|
| Total size (analyzed size for T1/2) | 24 (19) | 21 (16) |
| Parasite clearance half-life, T1/2 (hour)* | ||
| Median | 1.7 | 1.9 |
| IQR | 1.3–2.2 | 1.5–2.5 |
| Negative parasitemia at hour 24, % (n/N) | 73.9 (17/23) | 65.0 (13/20) |
| Day 2 positive parasitemia, % (n/N) | 0.0 (0/23) | 0.0 (0/20) |
| Day 3 positive parasitemia, % (n/N) | 0.0 (0/23) | 0.0 (0/20) |
| ACPR, % (n/N) | 100 (23/23) | 100 (20/20) |
ACPR = adequate clinical and parasitological response; AL = artemether + lumefantrine; AS+SP = artesunate + sulfadoxine–pyrimethamine; IQR = inter-quartile range. AS+SP vs. AL regarding negative parasitemia at hour 24, P = 0.53 (Pearson's χ2 test).
Those results are given by Worldwide Antimalarial Resistance Network's online parasite clearance estimator.
Figure 4.
Distribution of parasite clearance half-life by treatment arms. AL = artemether + lumefantrine; AS+SP = artesunate + sulfadoxine–pyrimethamine; T1/2 = parasite clearance half-life. AS+SP vs. AL, P = 0.24 (Mann–Whitney test). Results of both parasite clearance estimation methods were similar, r = 0.73 and P = 0.001.
Figure 5.
Distribution of parasite clearance half-live by age ranges < 5 years vs. ≥ 5 years, P = 0.70 (Mann–Whitney test). T1/2 = parasite clearance half-life. Results of both parasite clearance estimation methods were similar, r = 0.73 and P = 0.001.
Discussion
AL and AS+AQ have been used as first-line treatments for malaria in Mali since 2006, whereas AS+SP has been widely used in the main hospitals and many secondary health-care centers of the country as part of Chinese and Malian governments' efforts to control malaria. This study sought to provide evidence of the efficacy of the later regimen in the treatment of uncomplicated malaria as the partner drug is used in intermittent preventive treatment in pregnant women (IPTp) and seasonal malaria chemoprevention (SMC) in children in Africa.23–26 We evaluated efficacy of the combinations AL and AS+SP in children aged 6 months and older and adults living in a malaria-endemic area in Mali. We showed that AS+SP is as efficacious as the first-line treatment ACT with a high cure rate. In addition to high ACPR, other parameters such as parasite clearance time were comparable between the two regimens.
Our findings are in line with results from previous studies that showed that ACTs had a high cure rate and short parasite clearance time in malaria-endemic areas in Mali.27–32 Although we did not observe a statistically significant difference in terms of reinfection rate between the two arms of treatment when the entire population was used, children under 8 years of age in the AL group were more likely to experience reinfection than children of the same age in the AS+SP group. This observation could be explained by the fact that volunteers were followed during 28 days instead of 42 days or it could be due to differences in the intensity of transmission between the study sites.
Parasitemia at day 3 was shown to be a good indicator of delayed parasite clearance.33 Our results show that all patients cleared their parasite by day 2 in both treatment groups, indicative of a fast parasite clearance. These results are comparable to data reported from previous studies.27–32
SP is recommended in IPTp and has been used in other interventions such as SMC in Africa.23–26 There is a concern about the rapid emergence of SP resistance. One limitation of this study is the lack of genotyping data on molecular markers of SP resistance before and after treatment. However, our treatment failure rates for AS+SP arm were too low to provide adequate power to compare the rates of resistance-mediating polymorphisms in dihydropteroate synthase and dihydrofolate reductase before the treatment and after the follow-up. Although the addition of artesunate to SP may delay the emergence of SP resistance, the implementation of AS+SP at large scale in areas with high rate of SP resistance could be a threat to artemisinin derivatives. More importantly, the unavailability of this ACT as a fixed-dose combination could jeopardize patient adherence and increase the likelihood of using the artesunate tablets for monotherapy. This could add concerns to potential emergence of artemisinin resistance.
Conclusion
AS+SP and AL were highly efficacious in Mali 4–7 years after the implementation of the ACTs. This study provides baseline information on parasite clearance half-lives after ACT treatment, particularly for AS+SP, in Mali.
ACKNOWLEDGMENTS
We are grateful to all study subjects for their participation; the populations of Sotuba, Kolle, and Banambani; and health authorities of Kati for their kind help during this study.
Footnotes
Financial support: This study was primarily funded by Bill & Melinda Foundation through WHO to MRTC for in vivo assessment of two ACT efficacies in 2010 in Mali. French Mérieux Foundation provided additional fund through the grant 01/2007 Christophe Mérieux Award.
Authors' addresses: Karamoko Niaré, Issaka Sagara, Mahamadou S. Sissoko, Cheick Oumar Guindo, Nana H. Cissé, Abdoulaye A. Djimdé, and Ogobara K. Doumbo, Département d'Epidémiologie des Affections Parasitaire(DEAP), Malaria Research and Training Center (MRTC), Université des Sciences, des Techniques et des Technologies de Bamako (USTTB), Bamako, Mali, E-mails: karaniare@icermali.org, isagara@icermali.org, mssissoko@icermali.org, guindo.cheickoumar@yahoo.fr, nanahouma@yahoo.fr, adjimde@icermali.org, and okd@icermali.org. Antoine Dara, Division of Malaria Research, Institute for Global Health, University of Maryland School of Medicine, Baltimore, MD, E-mail: adara@medicine.umaryland.edu. Cheick Oumar Coulibaly, World Health Organization Office, Bamako, Mali, E-mail: coulibalyc@who.int. Pascal Ringwald, Drug Resistance and Containment, Global Malaria Programme, World Health Organization, Geneva, Switzerland, E-mail: ringwaldp@who.int. Françoise Benoit-Vical, Laboratoire de Chimie de Coordination (LCC), Centre National de la Recherche Scientifique (CNRS), Toulouse, France, E-mail: francoise.vical@inserm.fr. Antoine Berry, Service de Parasitologie-Mycologie, Centre Hospitalier Universitaire (CHU) Toulouse, Toulouse, France, and Centre de Physiopathologie de Toulouse Purpan (CPTP), Université de Toulouse, Toulouse, France, E-mail: antoine.berry@yahoo.fr.
References
- 1.WHO . World Malaria Report 2014. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Cumming JN, Ploypradith P, Posner GH. Antimalarial activity of artemisinin (Qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–297. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 4.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meshnick SR. Artemisinin antimalarials: mechanisms of action and resistance. Med Trop. 1998;58:13–17. [PubMed] [Google Scholar]
- 6.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Tracking Resistance to Artemisinin Collaboration (TRAC) Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski B, Lelièvre J, Barragán MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, Tagbor H, Williams J, Bojang K, Njie F, Desai M, Kariuki S, Gutman J, Mathanga DP, Mårtensson A, Ngasala B, Conrad MD, Rosenthal PJ, Tshefu AK, Moormann AM, Vulule JM, Doumbo OK, Ter Kuile FO, Meshnick SR, Bailey JA, Juliano JJ. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2014;211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, Dorsey G, Rosenthal PJ. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One. 2014;9:e105690. doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, Mumba D, Kekre M, Yavo W, Mead D, Bouyou-Akotet M, Apinjoh T, Golassa L, Randrianarivelojosia M, Andagalu B, Maiga-Ascofare O, Amambua-Ngwa A, Tindana P, Ghansah A, MacInnis B, Kwiatkowski D, Djimde AA. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2015;211:1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 15.White NJ. Antimalarial drug resistance and combination therapy. Philos Trans R Soc Lond B Biol Sci. 1999;354:739–749. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White NJ, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc Biol Sci. 2003;270:545–554. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dicko A, Sagara I, Diemert D, Sogoba M, Niambele MB, Dao A, Dolo G, Yalcouye D, Diallo DA, Saul A, Miller LH, Toure YT, Klion AD, Doumbo OK. Year-to-year variation in the age-specific incidence of clinical malaria in two potential vaccine testing sites in Mali with different levels of malaria transmission intensity. Am J Trop Med Hyg. 2007;77:1028–1033. [PubMed] [Google Scholar]
- 18.Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Dicko Y, Dara N, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. Randomized, multicentre assessment of the efficacy and safety of ASAQ—a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:125. doi: 10.1186/1475-2875-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . Assessment of Therapeutic Efficacy of Antimalarial Drugs for Uncomplicated Falciparum Malaria. Geneva, Switzerland: World Health Organization; 2003. WHO/HTM/RBM/2003.50. [Google Scholar]
- 20.Guindo MA, Shott JP, Saye R, Diakité ML, Sanogo S, Dembele MB, Keita S, Nagel MC, Ellis RD, Aebig JA, Diallo DA, Doumbo OK. Promoting good clinical laboratory practices and laboratory accreditation to support clinical trials in sub-Saharan Africa. Am J Trop Med Hyg. 2012;86:573–579. doi: 10.4269/ajtmh.2012.11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranford-Cartwright LC, Taylor J, Umasunthar T, Taylor LH, Babiker HA, Lell B, Schmidt-Ott JR, Lehman LG, Walliker D, Kremsner PG. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans R Soc Trop Med Hyg. 1997;91:719–724. doi: 10.1016/s0035-9203(97)90539-3. [DOI] [PubMed] [Google Scholar]
- 22.Su X, Ferdig M, Huang Y, Huynh CQ, Liu A, You J, Wootton JC, Wellems TE. A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science. 1999;286:1351–1353. doi: 10.1126/science.286.5443.1351. [DOI] [PubMed] [Google Scholar]
- 23.Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 24.WHO . WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 25.Dicko A, Sagara I, Sissoko MS, Guindo O, Diallo AI, Kone M, Toure OB, Sacko M, Doumbo OK. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children in Mali. Malar J. 2008;7:123. doi: 10.1186/1475-2875-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . Seasonal Malaria Chemoprevention with Sulfadoxine-Pyrimethamine Plus Amodiaquine in Children: A Field Guide. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 27.Djimdé A, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, Dama S, Ouologuem D, Dicko A, Doumbo OK. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–461. [PubMed] [Google Scholar]
- 28.Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Niambele MB, Sissoko M, Dicko A, Djimde A, Doumbo OK. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 29.Kayentao K, Maiga H, Newman RD, McMorrow ML, Hoppe A, Yattara O, Traore H, Kone Y, Guirou EA, Saye R, Traore B, Djimde A, Doumbo OK. Artemisinin-based combinations versus amodiaquine plus sulphadoxine-pyrimethamine for the treatment of uncomplicated malaria in Faladje, Mali. Malar J. 2009;8:5. doi: 10.1186/1475-2875-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagara I, Rulisa S, Mbacham W, Adam I, Sissoko K, Maiga H, Traore OB, Dara N, Dicko YT, Dicko A, Djimdé A, Jansen FH, Doumbo OK. Efficacy and safety of a fixed dose artesunate-sulphamethoxypyrazine-pyrimethamine compared to artemether-lumefantrine for the treatment of uncomplicated falciparum malaria across Africa: a randomized multi-centre trial. Malar J. 2009;8:63. doi: 10.1186/1475-2875-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagara I, Fofana B, Gaudart J, Sidibe B, Togo A, Toure S, Sanogo K, Dembele D, Dicko A, Giorgi R, Doumbo OK, Djimde AA. Repeat artemisinin-based combination therapies in a malaria hyperendemic area of Mali: efficacy, safety, and public health impact. Am J Trop Med Hyg. 2012;87:50–56. doi: 10.4269/ajtmh.2012.11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiga AW, Fofana B, Sagara I, Dembele D, Dara A, Traore OB, Toure S, Sanogo K, Dama S, Sidibe B, Kone A, Thera MA, Plowe CV, Doumbo OK, Djimde AA. No evidence of delayed parasite clearance after oral artesunate treatment of uncomplicated falciparum malaria in Mali. Am J Med Trop Hyg. 2012;87:23–28. doi: 10.4269/ajtmh.2012.12-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepniewska K, Ashley E, Lee SJ, Anstey N, Barnes KI, Binh TQ, D'Alessandro U, Day NP, De Vries PJ, Dorsey G, Guthmann JP, Mayxay M, Newton PN, Olliaro P, Osorio L, Price RN, Rowland M, Smithuis F, Taylor WR, Nosten F, White NJ. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]