Abstract
Intermittent preventive treatment (IPT) and insecticide-treated bed nets are the standard of care for preventing malaria in pregnant women. Since these preventive measures reduce exposure to malaria, their influence on the antibody (Ab) response to the parasite antigen VAR2CSA was evaluated in pregnant Cameroonian women exposed to holoendemic malaria. Ab levels to full-length VAR2CSA (FV2), variants of the six Duffy binding like (DBL) domains, and proportion of high avidity Ab to FV2 were measured longitudinally in 92 women before and 147 women after IPT. As predicted, reduced exposure interfered with acquisition of Ab in primigravidae, with 71% primigravidae being seronegative to FV2 at delivery. Use of IPT for > 13 weeks by multigravidae resulted in 26% of women being seronegative at delivery and a significant reduction in Ab levels to FV2, DBL5, DBL6, proportion of high avidity Ab to FV2, and number of variants recognized. Thus, in women using IPT important immune responses were not acquired by primigravidae and reduced in a portion of multigravidae, especially women with one to two previous pregnancies. Longitudinal data from individual multigravidae on IPT suggest that lower Ab levels most likely resulted from lack of boosting of the VAR2CSA response and not from a short-lived Ab response.
Introduction
In sub-Saharan Africa, an estimated 32 million women are at risk of becoming infected with Plasmodium falciparum during pregnancy, thereby, increasing their risk of severe anemia, spontaneous abortions, stillbirths, and low birth weight babies.1–3 These poor pregnancy outcomes occur when P. falciparum-infected erythrocytes (IE) sequester in the placenta and induce an inflammatory response.2,4–6 Sequestration is mediated by the binding of the parasite antigen VAR2CSA to host chondroitin sulfate A (CSA) localized on syncytial trophoblasts.7–10 Fortunately, antibodies (Abs) to placental-binding IE reduce sequestration and improve pregnancy outcomes.11–17 Abs to VAR2CSA are acquired over successive pregnancies that reduce the risk of placental malaria (PM).7,12,15
Intermittent preventive treatment (IPT) with sulfadoxine–pyrimethamine (SP) and insecticide-treated bed nets (ITN) are the recommended standard of care for preventing malaria during pregnancy in areas with moderate to high transmission.18 The original guidelines recommended that women take three doses of SP, with the first dose in the second trimester after quickening and the next two doses at least 1 month apart. Subsequently, use of SP at each prenatal visit after the first trimester has become the policy.19 Unfortunately, the use of IPT and ITN remains low throughout Africa.20
Studies have reported IPT and/or ITN may reduce the Ab response to placental-binding IE or VAR2CSA in primigravidae and multigravidae,21–25 but many questions remain. In particular, a limited understanding exists about how these interventions affect the quality and specificity of the anti-VAR2CSA Ab response acquired by primigravidae and if multigravidae, who acquired immunity to PM in previous pregnancies, maintain their immunity.
If protective Abs fail to develop in primigravid women or rapidly wane in multigravidae, then the complications associated with malaria during pregnancy would occur in subsequent pregnancies if IPT or ITN were no longer used. Questions related to protective immunity are difficult to address, since the standard of care is for all pregnant women to have access to the IPT and ITN, making it unethical to withhold these treatments. Therefore, answers to these questions can only be obtained indirectly. Recently, we reported that 1) high Ab levels to full-length VAR2CSA (FV2), 2) having ≥ 35% of high avidity Abs to FV2 (i.e., ≥ 35% of Abs to FV2 remain bound after treatment with 3M NH4SCN), and 3) Abs to multiple Duffy antigen-binding ligand (DBL) domains and variants from midpregnancy were associated with absence of PM.26,27 More recently, Ndam and others reported that high Ab levels to N-terminal domains, for example, DBL1 + 2 and DBL3, are surrogate markers for protection.28 Currently, these immune parameters are the best available indicators of protection.
The studies reported herein followed two cohorts of pregnant women throughout pregnancy residing in the same rural Cameroonian villages before (2001–2004) and after (2008–2013) implementation of the government policy recommending use of IPT and ITN for pregnant women. Between the two studies, a number of public health changes also took place in Cameroon that reduced transmission of malaria. As a result, entomological inoculation rates dropped from 0.77 to 0.34 infectious bites per person per night between the two studies (J. Bigoga, unpublished data). Thus, pregnant women in the first study received ∼210 infectious bites during pregnancy, whereas women in the second study would have received ∼93 infectious bites if they have not used IPT and ITN. The results reported herein describe Ab responses to VAR2CSA, including Ab acquisition, maintenance, amount, repertoire of multiple DBL domains and variants recognized, and avidity for FV2 in pregnant women, before and after IPT. The level and quality of Ab to VAR2CSA were reduced in primigravidae, including a reduction in Ab repertoire and lower proportion of high avidity Abs, and a less mature response was observed in some multigravid women.
Materials and Methods
Institutional review board approvals.
Clinical data and archival plasma samples collected between 2001 and 2004 were obtained “before” implementation of the government policy recommending use of IPT and ITN for pregnant women. The study was approved by the Ethics Committee, Ministry of Health, Cameroon, and the Institutional Review Board (IRB), Georgetown University. The use of coded plasma samples and clinical information in this study was exempt from human subject research by the Committee on Human Studies, University of Hawaii. A second study was conducted between 2008 and 2013, immediately after IPT was implemented. This study was approved by the National Ethics Committee, Cameroon, and the IRB at the University of Hawaii. All women and men participating in these studies gave written informed consent.
Study population.
Both studies took place at prenatal clinics in the rural villages of Ngali II and Ntouessong, Cameroon, where P. falciparum is perennial.29 Between 2001 and 2004, pregnant women were enrolled during the first trimester, followed monthly until delivery, and treated for malaria if they became slide positive for malaria. Details of the study have been described previously.29 This study included 92 women, 42 of whom were followed monthly throughout pregnancy and an additional 50 women enrolled at delivery, and women in this study did not use antimalarial preventive measures. In 2008, a second prospective longitudinal study was initiated after the government policy on IPT and ITN was implemented. Women in this cohort (N = 147) were enrolled during their first prenatal visit. At their first visit after quickening, the women took 1,500 mg sulfadoxine and 75 mg pyrimethamine (SP) and most women were issued long-lasting ITN. Thus, the majority of women in the second study used both IPT and ITN. Some women enrolled too late in the third trimester to receive benefit from IPT or ITN (N = 30), whereas the other 117 women received one to three doses of SP based on date of enrollment and were protected from malaria for 4–23 weeks. Women were monitored monthly by blood smears for parasites. Table 1 provides information on the women enrolled. The human immunodeficiency virus (HIV) status of the individual women is unknown, but HIV prevalence was estimated to be 5–9.9% at the time the studies were conducted.30 Blood samples from sympatric males (N = 58) residing in Ngali II were used as negative controls in the serological assays.
Table 1.
Characteristics of women enrolled before and after IPT
| Primigravidae | Multigravidae | |||||
|---|---|---|---|---|---|---|
| Before (2001–2004) | After (2008–2013) | P value | Before (2001–2004) | After (2008–2013) | P value | |
| No. of women | 18 | 37 | 74 | 110 | ||
| Age in years (mean ± SD) | 18.1 ± 2.3 | 19.9 ± 5.9 | 0.21 | 27.2 ± 6.4 | 26.5 ± 6.8 | 0.54 |
| Gravidity (mean ± SD) | 1 | 1 | 3.8 ± 2.4 | 3.6 ± 2.2 | 0.58 | |
| Secundigravidae (%) | 16.0% | 23.6% | 0.27 | |||
| Gravidity 3–5 (%) | 51.4% | 45.5% | 0.46 | |||
| Gravidity 6–13 (%) | 32.4% | 30.9% | 0.87 | |||
| Peripheral malaria positive at enrollment by blood smear | 69% | 45% | 0.19 | 31% | 22% | 0.33 |
| Weeks enrolled in the study (mean ± SD) | 19.6 ± 6 | 17.1 ± 7 | 0.7 | 22.2 ± 7 | 19.2 ± 7 | 0.6 |
| Weeks on IPT (mean ± SD) | 0 | 12.1 ± 6.7 | 0 | 12.8 ± 6.3 | ||
| No. of SP doses (mean ± SD) | 0 | 1.7 ± 0.8 | 0 | 1.8 ± 0.6 | ||
| Prevalence of women who used bed nets | 0 | 66% | 0 | 69% | ||
| Peripheral blood smear positive at least one time during the study | 77% | 60% | 0.32 | 68% | 36% | 0.0046 |
| Peripheral blood smear positive more than once during the study | 69% | 26% | 0.0085 | 39% | 9% | 0.0003 |
| Peripheral blood smear malaria positive at delivery* | 35% (N = 17) | 25% (N = 16) | 0.71 | 22% (N = 72) | 4% (N = 45) | 0.009 |
| Percent placental malaria positive at delivery by impression smears* | 75% (N = 12) | 25% (N = 16) | 0.02 | 52% (N = 65) | 13% (N = 45) | < 0.0001 |
IPT = intermittent preventive treatment; SD = standard deviation; SP = sulfadoxine–pyrimethamine. Bold values in the table represent those that were significant (i.e. P < 0.05).
Based on available placental samples (number shown in parentheses).
Detection of P. falciparum infections by microscopy and histology.
Plasmodium falciparum infections were detected by microscopy and polymerase chain reaction.31 Routine thick and thin blood smears were prepared from peripheral blood samples collected during pregnancy and at delivery, stained with Giemsa, and examined for IE. At delivery, blood from the intervillous space and placental biopsies was collected and examined for IE. A woman was considered to have PM if IE were detected in either blood smears of intervillous space blood, impression smears of villous tissue, or histological sections of the placenta.
VAR2CSA antigens.
The panel of recombinant proteins used has been described previously27 and included FV2, DBL1 + 2, DBL2, DBL3, DBL4, DBL5, and DBL6 of the FCR3 strains expressed in baculovirus-transfected insect cells at the University of Copenhagen, Denmark; DBL1, DBL3, DBL4, DBL5, and DBL6 for the 7G8 and 3D7 strains expressed in Pichia pastoris at Seattle Biomedical Research Institute (Seattle, WA)32; and DBL3 from A4 strain expressed in Escherichia coli at National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). Detailed information for each recombinant protein, including construct boundaries is listed in Supplemental Table 1. In addition, the following nonpregnancy-associated antigens were used: recombinant AMA-1 3D7, MSP-142 3D7, and MSP-2 3D7 (provided by the Malaria Vaccine Development Branch, NIAID, NIH).
Multianalyte platform assay.
Ab levels to the panel of VAR2CSA recombinant proteins were measured in samples collected during the second and third trimesters and at delivery, including N = 175 samples from women in the first study and N = 523 samples from women in the second study. Each antigen was covalently coupled at optimal concentrations (1 μg for DBL domains, 3 μg for FV2) to 1 million SeroMap beads (Luminex, Austin, TX), as previously described.27,33 Antigen-coupled beads were pooled and tested to confirm that competition among the antigens did not occur. The serological assay was performed as described previously.26,27,33 In brief, 50 μL of antigen-coupled microspheres (2,000 microspheres pertest) were incubated with 50 μL of a 1:100 dilution of plasma in PBS-1% BSA (phosphate-buffered saline containing 1% bovine serum albumin) for 1 hour at 25°C with rotation. The beads were washed twice with PBS-0.05% Tween20 and once with PBS-1% BSA. Then, 100 μL secondary Abs (R-Phycoerythrin-conjugated, AffiniPure F(ab')2 Fragment, Goat Anti-Human IgG Fc Fragment Specific [Jackson Immunoresearch, West Grove, PA]) diluted to 2 μg/mL in PBS-1% BSA was added to each well and incubated for 1 hour. Microspheres were washed, resuspended in 100 μL PBS-1% BSA, and the microsphere suspension was analyzed using a Liquichip L100 reader (Qiagen, Valencia, CA). The reader was programmed to analyze a minimum 100 beads per spectral address, DD Gate 7500-15000. Results are expressed as median fluorescence intensity (MFI). Controls were run on each plate and included three different pools of positive plasma from eight Cameroonian multigravidae with high Ab levels to VAR2CSA and pools of negative plasma from 40 Americans (U.S. control) who never traveled to malaria-endemic areas. The cutoff for positivity for FV2 and each DBL domain was based on results from 58 sympatric males. Mean Ab levels plus two standard deviations (SDs) of U.S. control was used as a cutoff for seropositivity to AMA1, MSP1, and MSP2.
Determining the breadth of the VAR2CSA Ab response.
Using the mean + 2SD based on 58 Cameroonian males as the cutoff, the number of DBL variants that Abs of a woman recognized was counted, providing a score of 0–14 variants.27 A woman was considered Ab positive for a particular DBL domain if she had Abs to one or more variants of that domain (breadth of DBL domains was 0–6).
Avidity to the FV2.
Samples collected at delivery or late in the third trimester were screened in the avidity assay from FV2-seropositive women as previously described.34 In brief, plasma was diluted 1:300, 1:1,000 and 1:3,000 in 1% BSA-PBS, and 50 μL of diluted plasma was added to 50 μL of FV2-coupled microspheres (2,000 microspheres per test). After incubation for 1 hour, beads were washed and half of the paired beads were resuspended in 100 μL of 3M NH4SCN in 1% BSA-PBS and in 100 μL of 1% BSA-PBS. After 30 minutes, the beads were washed, incubated with secondary Abs for 1 hour, washed, and analyzed by Liquichip L100 as described above. Avidity was determined for each dilution using the formula: (MFI obtained from wells incubated with salt)/(MFI obtained from corresponding control wells) × 100, and the results were averaged for the three dilutions.
Statistical analysis.
For the demographics presented in Table 1, continuous variables are summarized by means and SDs, while categorical variables are summarized by frequencies and percentages. Continuous variables are compared using a two-sample t test with unequal variances, whereas categorical variables are compared with Fisher's exact test. Generalized additive mixed models with penalized B-splines estimated the nonlinear trend of MFI across gestation before and after IPT separately for primigravidae and multigravidae with a random intercept accounting for correlation within subjects.35 Because of multiple comparisons of interest in the analyses, the false discovery rate36 was controlled at 5% separately for each primigravidae and multigravidae analysis for the six DBL domains (FCR3 strains). The false discover rate was controlled only for the DBL domains, since variants of the domains shared high sequence homology. The statistical analysis was performed in GraphPad Prism 6 (La Jolla, CA), STATA12 (College Station, TX), and R v3.1.2 (Vienna, Austria).
Results
Description of study participants.
Women enrolled before and after IPT were similar with respect to age, gravidity, and prevalence of peripheral malaria at enrollment (Table 1). None of the women enrolled between 2001 and 2004 used prophylaxis or ITN; whereas, 80% of the women in 2008–2013 received one to three doses of SP for an average of 12.6 weeks (range: 4–23 weeks) and 68% used ITN. Malaria infections were significantly reduced in the IPT group, including number of multigravid women blood smear positive > 1 times during pregnancy (P = 0.0003), blood smear positive at delivery (P = 0.009), and percent PM positive (P < 0.0001). Clearly, malaria was significantly reduced in this central African country after implementation of IPT and other protective methods.
The 147 women in the IPT study reported they had not taken SP or used ITN before enrollment. Thus, women who enrolled very late in pregnancy received only zero to one doses of SP; whereas, those who enrolled earlier received two to three doses (Table 2). Among 147 women, 30 enrolled too late to receive IPT or use ITN. Accordingly, a comparison between women in the two studies who did not receive SP (i.e., evaluate the possible influence of reduced malaria transmission), as well as the influence of zero to three SP doses on Abs to VAR2CSA, could be made. The 117 women who received one, two, and three doses of SP should have been protected from malaria during the last 9, 14, and 20 weeks of pregnancy, respectively (Table 2). As a result, PM was reduced at delivery (Table 1).
Table 2.
Number of doses of IPT
| Primigravidae (enrolled: N = 37) | Multigravidae (enrolled: N = 110) | ||||
|---|---|---|---|---|---|
| No. of SP doses | n | Weeks on SP* | No. of SP doses | n | Weeks on SP |
| 0 | 6 | 0 | 0 | 24 | 0 |
| 1 | 10 | 8.5 (7, 15) | 1 | 21 | 9 (6, 13) |
| 2 | 10 | 13.5 (11, 17) | 2 | 52 | 14.5 (11, 19) |
| 3 | 7 | 20 (13, 22) | 3 | 13 | 20 (16, 23) |
IPT = intermittent preventive treatment; SP = sulfadoxine–pyrimethamine.
Median weeks (25th, 75th percentile are indicated in parentheses).
Data for four women were not available.
Ab levels to FV2 during pregnancy.
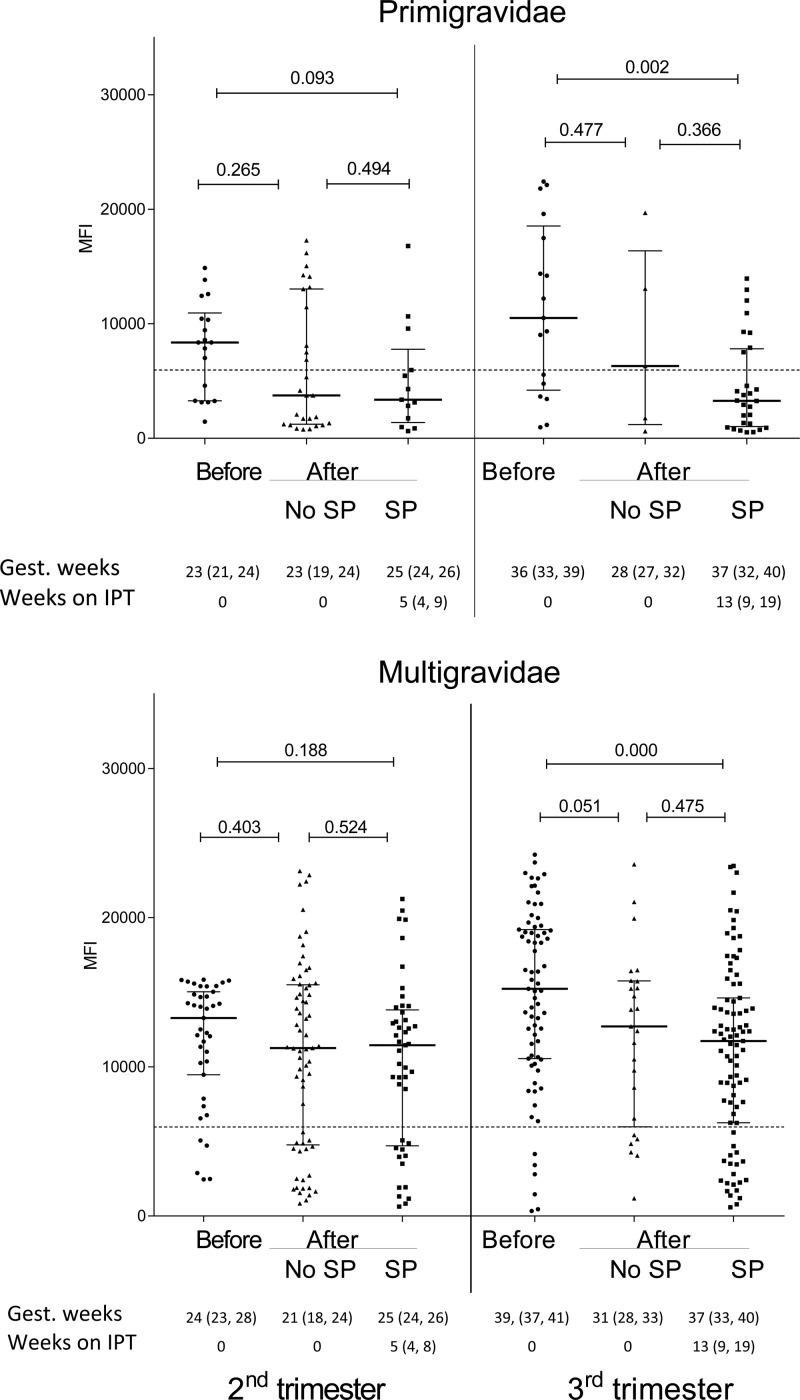
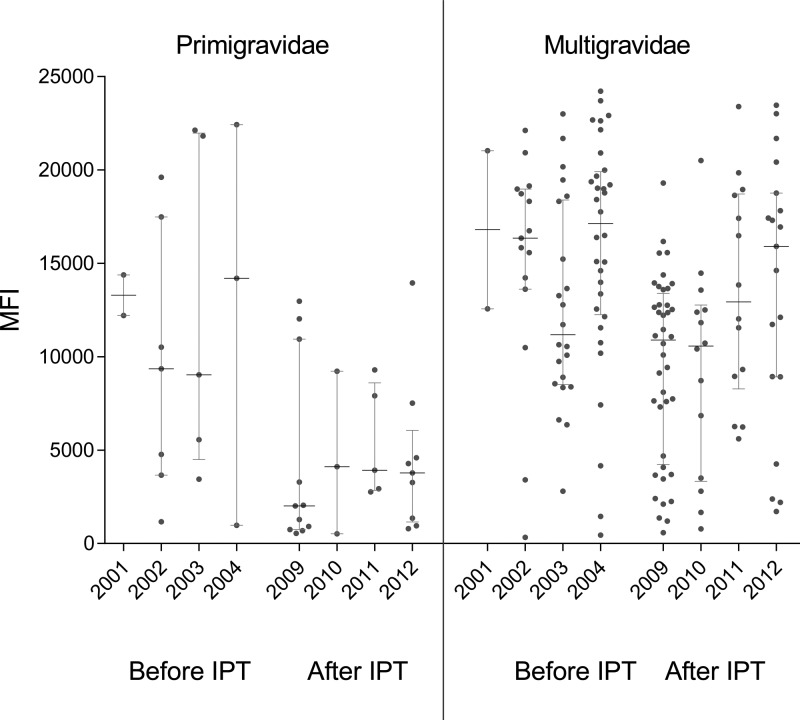
Ab levels to FV2 were compared among pregnant women 1) in the first study (N = 92), 2) in the second study who did not use SP (N = 30), and 3) women who took one to three doses of SP (N = 117) (Figure 1 ). No significant differences in Ab levels to FV2 were observed in the second trimester between primigravidae and multigravidae enrolled in the two studies, suggesting that short-term use of SP (median: 5 weeks, interquartile range [IQR]: 4, 8) did not influence Ab levels. On the other hand, during the third trimester Ab levels to FV2 were significantly lower in primigravidae (P = 0.002) and multigravidae (P = 0.0002) receiving SP for ∼13 weeks (median: 13 weeks, IQR: 9, 19) compared with before IPT. Thus, taking SP throughout the second and third trimesters resulted in lower Ab levels to FV2 at delivery. Data in Figure 1 show that Ab levels to FV2 tended to be lower in women who did not take SP in the second study (2008–2013) compared with the earlier study (2001–2004), but the difference was not statistically significant. Figure 2 shows that Ab levels to FV2 at delivery declined between 2004 and 2008. Thus, the combined intervention strategy resulted in women in the rural villages having lower Ab levels to FV2.
Figure 1.
Antibody (Ab) levels to full-length VAR2CSA during the second and third trimesters. Ab levels are shown as median (25th, 75th interquartile range) to FV2. Blue dotted line represents cutoff for seropositivity (5,964 MFI). A two-sample t test with unequal variances was used to compare the groups. Information on length of gestation (median and 25th, 75th percentile weeks) and length on SP (weeks) is provided below each graph. IPT = intermittent preventive treatment; MFI = median fluorescence intensity; SP = sulfadoxine–pyrimethamine.
Figure 2.
Antibody (Ab) levels to full-length VAR2CSA (FV2) at delivery before and after intermittent preventive treatment (IPT). Ab levels to FV2 in median fluorescence intensity (MFI) are shown for women enrolled before IPT (2001–2004) and after IPT who used SP (2009–2012). Data for 2008 and 2013 are not shown because data for only a few months at the beginning and end of the study were available. Horizontal lines represent median and 25th, 75th interquartile range.
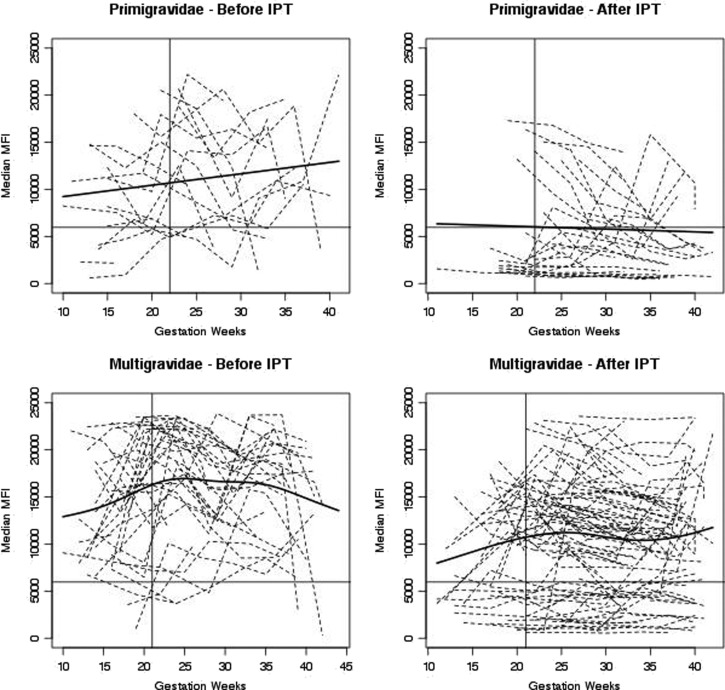
To further assess this point, the Ab response to FV2 of individual women is shown in Figure 3 with nonlinear trend lines superimposed. As expected, variation in the Ab response was observed among the women. Ab levels rose in primigravidae before IPT (upward linear trend line) during pregnancy as they became infected with P. falciparum, whereas Abs were either not produced or declined (downward trend line) in primigravidae protected by SP. Before IPT, essentially all primigravidae produce Abs to FV2 by delivery, but most primigravidae taking SP lacked Abs to FV2 at delivery. Thus, IPT had a significant impact on the immune response to VAR2CSA. Among multigravidae, the overall trend before IPT was for significant boosting to occur during the first half of pregnancy, with maximal levels reached by midpregnancy (∼24–25 weeks) and remaining high thereafter. Essentially, all multigravidae had FV2 Abs at delivery. In contrast, lower levels of Abs were reached by midpregnancy in women taking SP, with levels modulating thereafter. Approximately one-third of the multigravidae taking SP were Ab-negative at delivery. Thus, a significant decrease in prevalence of Abs to FV2 at delivery was observed in women receiving IPT (P = 0.03).
Figure 3.
Antibody (Ab) levels to full-length VAR2CSA throughout pregnancy in individual women. Each line represents an individual woman. Solid line on the y axis represents cutoff for seropositivity (5,964 median fluorescence intensity [MFI]) based on Ab levels in males. The nonlinear trend lines were estimated with generalized additive mixed models with penalized B-spines. Vertical solid lines indicate the time point when half the women started taking sulfadoxine–pyrimethamine (22 weeks for primigravidae and 21 weeks for multigravidae).
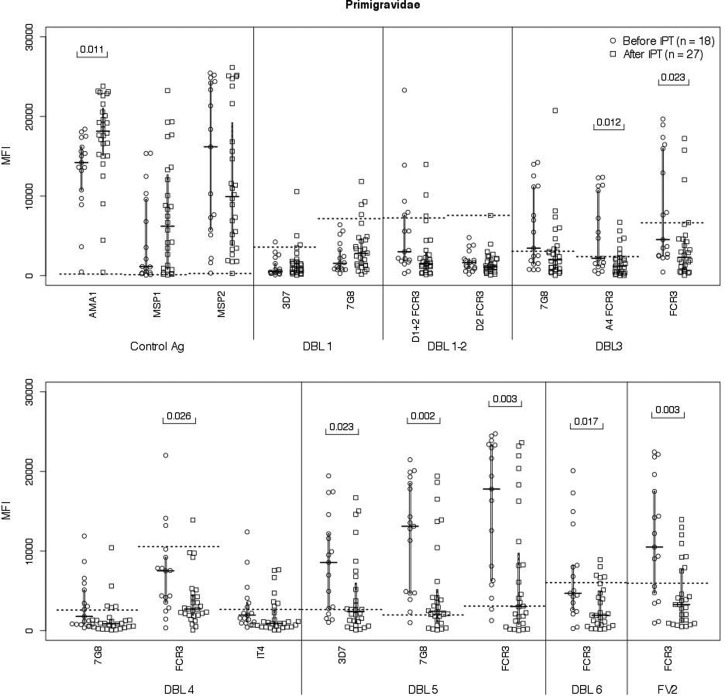
Abs to VAR2CSA DBL domains in primigravidae.
Since primigravidae receiving one to three doses of SP had significantly lower Ab levels to FV2, the study investigated if Ab levels were reduced to all, or only a few, DBL domains within FV2 (Figure 4 ). Overall, Ab levels were lower to the DBL3 and the C-terminal region, that is, DBL4–6. After controlling for multiple comparisons, Ab responses to these domains remained significant. The number of primigravidae per group was limited, so the effect of number of doses or length of antimalarial coverage could not be determined. SP did not affect Ab levels or prevalence to AMA1, MSP1, and MSP2, as 96–100% of primigravidae remained Ab positive (Table 3).
Figure 4.
Antibodies (Abs) to Duffy antigen-binding ligand (DBL) domains in primigravidae at delivery. Ab levels at delivery (or last visit during the third trimester) were measured in primigravidae before (N = 18) and after (N = 27) intermittent preventive treatment (IPT) (median fluorescence intensity [MFI], interquartile range). All women in the IPT group received at least one dose of sulfadoxine–pyrimethamine. Statistical analysis: two-sample t test with unequal variances. After taking multiple comparisons into consideration for the six DBL domains, the differences remained significantly reduced.
Table 3.
Percentage of Ab-positive women at delivery
| Primigravidae | Multigravidae | |||||
|---|---|---|---|---|---|---|
| Before IPT, N = 18 | After IPT, N = 28† | P* | Before IPT, N = 73 | After IPT, N = 86† | P* | |
| AMA1 3D7 | 94 | 100 | 0.390 | 99 | 99 | 1.000 |
| MSP1 3D7 | 89 | 96 | 0.552 | 99 | 99 | 1.000 |
| MSP2 3D7 | 89 | 96 | 0.552 | 97 | 98 | 1.000 |
| DBL1 3D7 | 0 | 7 | 0.513 | 11 | 32 | 0.002‡ |
| DBL1 7G8 | 0 | 11 | 0.270 | 0 | 18 | < 0.0001‡ |
| DBL1 + 2 FCR3 | 17 | 7 | 0.366 | 27 | 25 | 0.857 |
| DBL2 FCR3 | 0 | 0 | – | 10 | 9 | 1.000 |
| DBL3 7G8 | 44 | 21 | 0.115 | 79 | 64 | 0.053 |
| DBL3 A4 FCR3 | 44 | 18 | 0.092 | 73 | 57 | 0.049 |
| DBL3 FCR3 | 33 | 11 | 0.124 | 45 | 38 | 0.421 |
| DBL4 7G8 | 33 | 7 | 0.042 | 70 | 51 | 0.016 |
| DBL4 FCR3 | 17 | 4 | 0.284 | 32 | 30 | 0.864 |
| DBL4 IT4 | 22 | 18 | 0.721 | 55 | 40 | 0.081 |
| DBL5 3D7 | 61 | 32 | 0.072 | 88 | 79 | 0.205 |
| DBL5 7G8 | 83 | 50 | 0.030 | 95 | 85 | 0.071 |
| DBL5 FCR3 | 78 | 36 | 0.007 | 92 | 84 | 0.156 |
| DBL6 FCR3 | 22 | 4 | 0.069 | 56 | 26 | 0.000 |
| FV2 FCR3 | 61 | 29 | 0.037 | 88 | 74 | 0.030 |
Ab = antibody; DBL = Duffy antigen-binding ligand; FV2 = full-length VAR2CSA; IPT = intermittent preventive treatment. Bold values represent those that are significant (P < 0.05).
Fisher's exact t test. Cutoff s for seropositivity for each FV2-associated antigen was based on mean + 2SD of Ab levels in sympatric males; cutoffs for merozoite antigens were the mean + 2SD of Ab data for U.S. adults. Bolded P values indicate domains that were significant after controlling for false discovery rate at 5%.
Percentage calculated based on the delivery or last samples in the third trimester for women who had taken sulfadoxine–pyrimethamine.
Prevalence increased.
Ab levels to DBL domains in multigravidae.
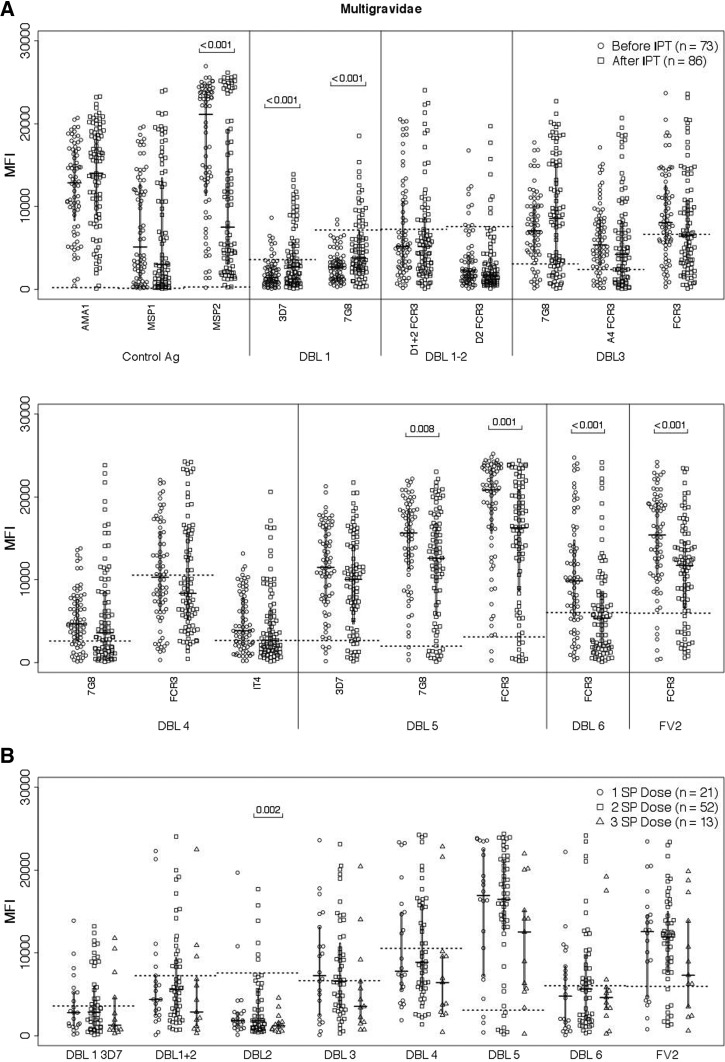
Ab levels were reduced in multigravidae taking IPT, not only to FV2, but also DBL5 (7G8: P = 0.008 and FCR3: P = 0.01) and DBL6 (FCR3: P < 0.001), even after controlling for multiple comparisons (Figure 5 ). As a result, the Ab prevalence at delivery to DBL6 was lower (FCR3: P < 0.0001) (Table 3). Interestingly, Ab levels were significantly increased to both strains of DBL1 in women receiving SP, whereas Abs to MSP2 declined. Overall, Ab levels of women receiving three doses of SP tended to be lower to multiple domains, no significant difference in Ab levels to FV2 or the any of the DBL domains was observed at delivery among multigravidae receiving one, two, or three doses of SP.
Figure 5.
Antibody (Ab) levels to Duffy antigen-binding ligand (DBL) domains in multigravidae at delivery. (A) Ab levels to DBL domains (median fluorescence intensity [MFI], interquartile range) were measured in 73 women before intermittent preventive treatment (IPT) and in 86 women who received at least one dose of sulfadoxine–pyrimethamine (SP). (B) Influence of number of SP doses on Ab levels (median MFI, interquartile range) to DBL domains (FCR3 strain unless otherwise specified) were measured at delivery. Statistical analysis: two-sample t test with unequal variances. After taking multiple comparisons into account for DBL domains, the differences remained significant.
Ab responses associated with absence of PM.
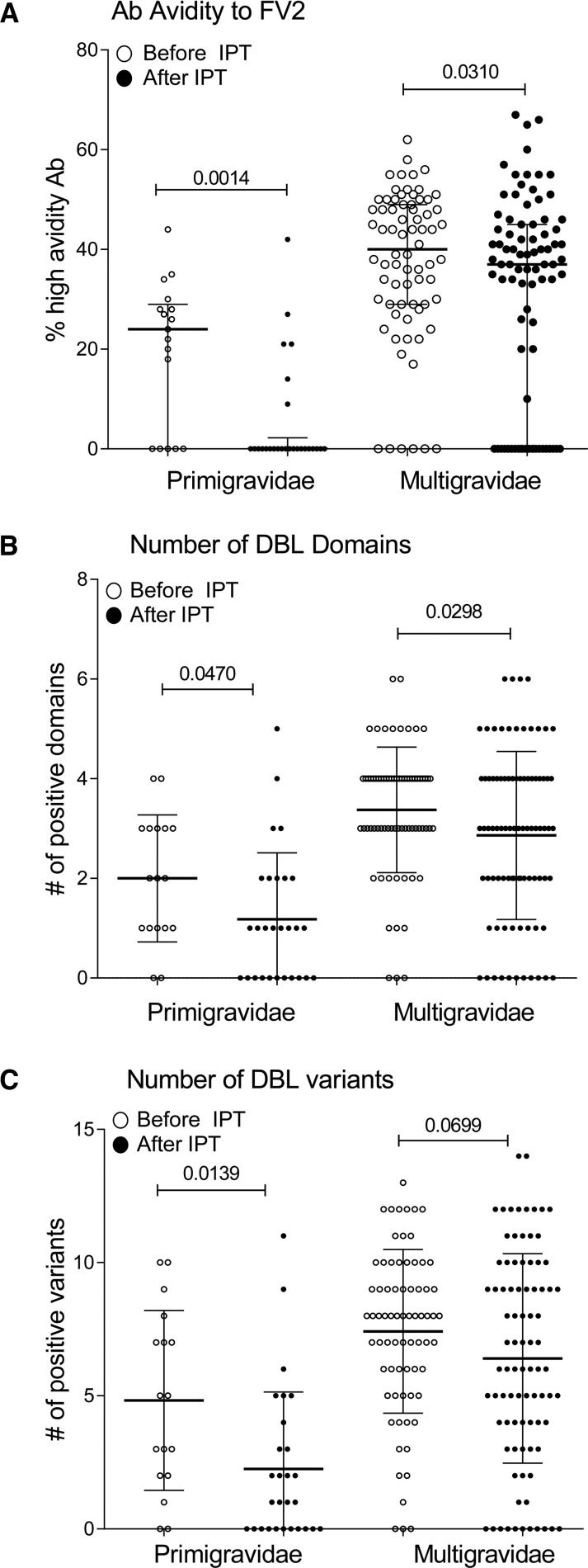
Because the proportion of high avidity Ab to FV2 and number of DBL domains and variants recognized were reported to be associated with absence of PM, the influence of IPT on these immune parameters was assessed (Figure 6 ). Both primigravidae and multigravidae taking SP had a lower proportion of high avidity Ab to FV2 at delivery (P = 0.0014 and 0.031, respectively) (Figure 6). In addition, the Ab repertoire for different DBL domains and geographic variants was also reduced in these women.
Figure 6.
Comparison of immune responses associated with absence of placental malaria. Plasma samples collected at or near delivery were used in assays to measure (A) percent high avidity of antibody (Ab) to full-length VAR2CSA (FV2) (i.e., percent of Abs that remain bound to FV2 in the presence of 3M NH4SCN), (B) number of Duffy antigen-binding ligand (DBL) domains that Abs of women recognized, and (C) number of variants recognized in women enrolled before and after intermittent preventive treatment (IPT) who took one to three doses of sulfadoxine–pyrimethamine . Statistical analysis: two-sample t test with unequal variances. Horizontal lines represent median values and bars represent interquartile range.
Discussion
The aim of this study was to evaluate the influence of implementation of IPT, ITN, and other preventive measures on the Ab response to VAR2CSA, including Ab acquisition, maintenance, amount, repertoire of multiple DBL domains and variants recognized, and proportion of high avidity Ab to FV2 in primigravid and multigravid women. Previous studies evaluating the influence of IPT measured Abs that bound to the surface of CSA-binding IE15,16,23,25,34 or DBL524,34 in cohorts of women consisting of either primigravidae alone or a combination of primigravidae and multigravidae. Thus, the direct influence of IPT on multigravidae has not been assessed. Prior studies reported that Ab levels either decreased23,24 or remained unchanged25,35 in women receiving IPT. An early study found that chloroquine prophylaxis reduced acquisition of Abs to the surface of CSA-binding IE in primigravidae.15 Thus, additional studies on the impact of IPT were needed, especially in multigravidae. In addition, the impact of IPT might change across different geographic conditions and deserved further investigation.
Prevention of malaria during pregnancy includes use of IPT, ITN and effective case management. IPT implementation began in 2004 in Cameroon, with the goal of pregnant women taking three doses of SP between the 16th and 36th week of pregnancy.37 In this study, women had access to IPT and 69% used ITN, thus the decline in malaria prevalence during pregnancy and PM at delivery, as well as changes in Ab response to VAR2CSA, resulted from the combined effect of IPT and ITN. In parallel, a general reduction of malaria transmission occurred in the rural village of Ngali II and Ntouessong, from 0.7 to 0.34 infectious mosquito bites per night. Since many of the women receiving IPT also used ITN, it is not possible to determine how frequently they became infected with malaria during pregnancy. However, 45% of primigravidae and 22% of multigravidae were blood smear positive at enrollment; 60% of primigravidae and 36% of multigravidae were blood smear positive at least once during pregnancy; and 25% and 13% of primigravid and multigravid women, respectively, had PM in this high-transmission setting (Table 1). These data suggest that exposure to malaria remained high and the initial recommendation of three doses of SP was inadequate to prevent malaria both in early and throughout pregnancy.
This is the first study to report the influence of IPT on Ab levels to the FV2 in longitudinal cohort of primigravidae. Similar to previous studies that measured Abs to the surface of CSA-binding IE,21 reduced exposure to malaria in primigravidae resulted in reduced Ab acquisition to VAR2CSA (Figures 1–3). After the first dose of SP, a general decline in Abs to VAR2CSA in primigravidae was observed, suggesting that only short-lived plasma cells had been produced. Overall, 71% of primigravidae were Ab negative at delivery. This is in contrast with the situation before IPT where, by midpregnancy, primigravidae had Abs to FV2 and only 39% were seronegative to FV2 at delivery.
Multigravidae taking SP had a more complex depiction. Overall, ∼70% of multigravidae were Ab positive to FV2 on enrollment, showing that Abs had either persisted since the last pregnancy or were produced early in the current pregnancy through activation of memory B cells. Accordingly, a woman's immune status before starting SP treatment (first or early second trimester) in the current pregnancy is a major component in determining her immune status in subsequent pregnancies. Two distinct subgroups of multigravidae were seen in this high-transmission setting, namely, low and high responders (Figures 1 and 3). Among the low responders, the majority of women were seronegative at delivery and more likely to have had only one to two prior pregnancies. In contrast, the high responders were more likely to be gravidity 4 and above. Women in low- and high-responder groups tended to maintain Ab levels to FV2 throughout pregnancy, with some women exhibiting decreased and a few having increased Ab levels by the time of delivery. Women with increased Ab levels probably became infected with P. falciparum when SP levels declined below effective levels because of a delay in taking SP. A recent study in Cameroon38 showed that clinical malaria was associated with long spacing between SP doses. In our study, malaria was not always detected in blood smears when Ab levels increased, suggesting that the women rapidly cleared their infections or had submicroscopic infections. These results support the conclusion of Aitken and others.23 that undetectable submicroscopic infections in women receiving SP might lead to Ab boosting. Prior to IPT, a pronounced boosting occurred before 20–22 weeks of gestation in multigravidae, which allowed low responders to seroconvert before delivery. This boosting effect during the second trimester was prevented by the first dose of SP that was received around the 21st week of gestation (Figure 3). As a result, 26% of multigravidae were seronegative at delivery (Table 3). Overall, the small number of multigravidae who received three doses of SP tended to have lower Ab levels to VAR2CSA domains (Figure 5).
Researchers continue to search for reliable correlates of protection from PM. Various VAR2CSA domains have received attention as they elicit Abs associated with inhibition of binding: ID1-ID2a, DBL1, DBL1-DBL2, DBL3, DBL4, DBL5, DBL628,39–44 and opsonic phagocytosis: DBL2, DBL3, DBL545 activity. Having high Ab levels to FV2, > 35% of Abs to FV2 with high avidity, and broad DBL repertoire early in pregnancy were also associated with absence of PM at delivery.26,27 And, finally, high Ab levels to DBL1 + 2 and DBL3 were reported to be surrogates of protection from PM.28 Thus, the above Ab properties were assessed. CSA minimal binding site of VAR2CSA, ID1-ID2a, was not included in the study, since the Ab responses of Cameroonian women does not appear to be pregnancy specific.34 Results reported herein show that the VAR2CSA Ab response in primigravidae on IPT were poor, as most primigravidae lacked high Ab levels to FV2 (Figures 1 and 3); Abs to C-terminal DBL domains, especially DBL5; high avidity Ab to FV2; and recognized only few domains and/or variants (Figure 6). These data suggest that primigravidae on IPT do not have immunological protection from PM at delivery and will be compromised during future pregnancies. In contrast, many multigravidae taking SP maintained solid Ab response to VAR2CSA that were likely boosted before taking SP during the current pregnancy. However, even among multigravidae, some women, especially those with two to three prior pregnancy, had significantly reduced Ab levels to FV2 (Figure 1), DBL5, and DBL6 (Figure 5) and lower proportion of high avidity Ab (Figure 6) and breadth of repertoire (Figure 6). Future studies need to address the impact of IPT on additional Ab responses that are associated with absence of PM, such as inhibition of binding and phagocytosis.46 Longitudinal data from individual multigravidae in this study suggest that lower Ab levels in women using IPT most likely resulted from lack of boosting and fine tuning of the VAR2CSA response during the second/third trimester and not from a short-lived Ab response.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to J. Smith at the Seattle Biomedical Research Institute for providing recombinant malaria proteins expressed in Pichia pastoris, and K. Singh and C. Long at NIAID, NIH, for providing DBL3 (A4). We acknowledge the contributions of A. Kayatani who played an important role in developing the Ab avidity assay. Importantly, we thank the entire Malaria Research Team at the Biotechnology Center, University of Yaoundé I, Cameroon, for their outstanding work and the women and their families who participated in the studies.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial support: The work was supported by grants from NIAID, NIH, UO1AI43888 (pre-intervention samples) and RO1AI071160 (post-intervention samples) (Diane Wallace Taylor, Rose G. F. Leke) and FP7/2007-2013 grant agreement no. 200889 (STOPPAM) (Ali Salanti), IMPM (Rose G. F. Leke).
Authors' addresses: Anna Babakhanyan, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH, E-mail: axb784@case.edu. Yeung L. Tutterrow, Naveen Bobbili, Andrew Wey, and Diane Wallace Taylor, Department of Tropical Medicine, Medical Microbiology and Pharmacology, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HI, E-mails: yltutterrow@gmail.com, bobbili@hawaii.edu, awey@hawaii.edu, and dwtaylor@hawaii.edu. Ali Salanti, Centre for Medical Parasitology, Department of Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark, and Department of Infectious Diseases, Copenhagen University Hospital, Copenhagen, Denmark, E-mail: salanti@sund.ku.dk. Josephine Fogako and Rose G. F. Leke, The Biotechnology Center, Faculty of Medicine and Biomedical Research, University of Yaoundé I, Yaoundé, Cameroon, E-mail: roseleke@yahoo.com.
References
- 1.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 3.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 4.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:636S–645S. doi: 10.1093/jn/131.2.636S. discussion 646S–648S. [DOI] [PubMed] [Google Scholar]
- 5.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 6.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 7.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, Faure G, Baron B, Ramboarina S, Singh SK, Belrhali H, England P, Lewit-Bentley A, Scherf A, Bentley GA, Gamain B. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci USA. 2010;107:4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira MU, da Silva Nunes M, Wunderlich G. Antigenic diversity and immune evasion by malaria parasites. Clin Diagn Lab Immunol. 2004;11:987–995. doi: 10.1128/CDLI.11.6.987-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newbold C, Craig A, Kyes S, Rowe A, Fernandez-Reyes D, Fagan T. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int J Parasitol. 1999;29:927–937. doi: 10.1016/s0020-7519(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 11.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 13.Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, Dahlback M, Bernasconi NL, Fried M, John D, Duffy PE, Salanti A, Lanzavecchia A, Lim CT, Ndam NT, Higgins MK, Hviid L. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol. 2010;185:7553–7561. doi: 10.4049/jimmunol.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184:618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 16.O'Neil-Dunne I, Achur RN, Agbor-Enoh ST, Valiyaveettil M, Naik RS, Ockenhouse CF, Zhou A, Megnekou R, Leke R, Taylor DW, Gowda DC. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun. 2001;69:7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, Simpson JA, Rogerson SJ. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis. 2009;200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region. Geneva, Switzerland: World Health Organization; 2004. AFR/MAL/04/01. [Google Scholar]
- 19.WHO . Updated WHO Policy Recommendation: Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva, Switzerland: WHO Press; 2012. [Google Scholar]
- 20.van Eijk AM, Hill J, Larsen DA, Webster J, Steketee RW, Eisele TP, ter Kuile FO. Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11. Lancet Infect Dis. 2013;13:1029–1042. doi: 10.1016/S1473-3099(13)70199-3. [DOI] [PubMed] [Google Scholar]
- 21.Staalsoe T, Shulman CE, Dorman EK, Kawuondo K, Marsh K, Hviid L. Intermittent preventive sulfadoxine-pyrimethamine treatment of primigravidae reduces levels of plasma immunoglobulin G, which protects against pregnancy-associated Plasmodium falciparum malaria. Infect Immun. 2004;72:5027–5030. doi: 10.1128/IAI.72.9.5027-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aitken EH, Mbewe B, Luntamo M, Kulmala T, Beeson JG, Ashorn P, Rogerson SJ. Antibody to P. falciparum in pregnancy varies with intermittent preventive treatment regime and bed net use. PLoS One. 2012;7:e29874. doi: 10.1371/journal.pone.0029874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aitken EH, Mbewe B, Luntamo M, Maleta K, Kulmala T, Friso MJ, Fowkes FJ, Beeson JG, Ashorn P, Rogerson SJ. Antibodies to chondroitin sulfate A-binding infected erythrocytes: dynamics and protection during pregnancy in women receiving intermittent preventive treatment. J Infect Dis. 2010;201:1316–1325. doi: 10.1086/651578. [DOI] [PubMed] [Google Scholar]
- 24.Diouf I, Tine RC, Ndiaye JL, Sylla K, Faye B, Mengue ML, Faye O, Dieng Y, Gaye A, Gaye O. Effect of intermittent presumptive treatment with sulfadoxine-pyrimethamine on the acquisition of anti-VAR2CSA antibodies in pregnant women living in a hypoendemic area in Senegal [in French] Bull Soc Pathol Exot. 2011;104:277–283. doi: 10.1007/s13149-011-0153-5. [DOI] [PubMed] [Google Scholar]
- 25.Serra-Casas E, Menendez C, Bardaji A, Quinto L, Dobano C, Sigauque B, Jimenez A, Mandomando I, Chauhan VS, Chitnis CE, Alonso PL, Mayor A. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J Infect Dis. 2010;201:123–131. doi: 10.1086/648595. [DOI] [PubMed] [Google Scholar]
- 26.Tutterrow YL, Salanti A, Avril M, Smith JD, Pagano IS, Ako S, Fogako J, Leke RG, Taylor DW. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One. 2012;7:e40049. doi: 10.1371/journal.pone.0040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tutterrow YL, Avril M, Singh K, Long CA, Leke RJ, Sama G, Salanti A, Smith JD, Leke RG, Taylor DW. High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission. Infect Immun. 2012;80:1479–1490. doi: 10.1128/IAI.00071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, Fievet N, Massougbodji A, Luty AJ, Deloron P. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis. 2015;21:813–823. doi: 10.3201/eid2105.141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leke RF, Bioga JD, Zhou J, Fouda GG, Leke RJ, Tchinda V, Megnekou R, Fogako J, Sama G, Gwanmesia P, Bomback G, Nama C, Diouf A, Bobbili N, Taylor DW. Longitudinal studies of Plasmodium falciparum malaria in pregnant women living in a rural Cameroonian village with high perennial transmission. Am J Trop Med Hyg. 2010;83:996–1004. doi: 10.4269/ajtmh.2010.10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . Summary Country Profile for HIV/AIDS Treatment Scale-Up. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 31.Snounou G, Pinheiro L, Goncalves A, Fonseca L, Dias F, Brown KN, do Rosario VE. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;87:649–653. doi: 10.1016/0035-9203(93)90274-t. [DOI] [PubMed] [Google Scholar]
- 32.Avril M, Hathaway MJ, Cartwright MM, Gose SO, Narum DL, Smith JD. Optimizing expression of the pregnancy malaria vaccine candidate, VAR2CSA in Pichia pastoris. Malar J. 2009;8:143. doi: 10.1186/1475-2875-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fouda GG, Leke RF, Long C, Druilhe P, Zhou A, Taylor DW, Johnson AH. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin Vaccine Immunol. 2006;13:1307–1313. doi: 10.1128/CVI.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babakhanyan A, Leke RG, Salanti A, Bobbili N, Gwanmesia P, Leke RJ, Quakyi IA, Chen JJ, Taylor DW. The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site. PLoS One. 2014;9:e88173. doi: 10.1371/journal.pone.0088173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood S. Generalized Additive Models: An Introduction with R. Chapman and Hall/CRC Press; 2006. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:298–300. [Google Scholar]
- 37.Tonga C, Kimbi HK, Anchang-Kimbi JK, Nyabeyeu HN, Bissemou ZB, Lehman LG. Malaria risk factors in women on intermittent preventive treatment at delivery and their effects on pregnancy outcome in Sanaga-Maritime, Cameroon. PLoS One. 2013;8:e65876. doi: 10.1371/journal.pone.0065876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mbu RE, Takang WA, Fouedjio HJ, Fouelifack FY, Tumasang FN, Tonye R. Clinical malaria among pregnant women on combined insecticide treated nets (ITNs) and intermittent preventive treatment (IPTp) with sulphadoxine-pyrimethamine in Yaounde, Cameroon. BMC Womens Health. 2014;14:68. doi: 10.1186/1472-6874-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salanti A, Resende M, Ditlev SB, Pinto VV, Dahlback M, Andersen G, Manczak T, Theander TG, Nielsen MA. Several domains from VAR2CSA can induce Plasmodium falciparum adhesion-blocking antibodies. Malar J. 2010;9:11. doi: 10.1186/1475-2875-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen MA, Pinto VV, Resende M, Dahlback M, Ditlev SB, Theander TG, Salanti A. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect Immun. 2009;77:2482–2487. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, Resende M, Agerbaek MO, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem. 2012;287:23332–23345. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez P, Viebig NK, Dechavanne S, Lepolard C, Gysin J, Scherf A, Gamain B. Var2CSA DBL6-epsilon domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites. Malar J. 2008;7:170. doi: 10.1186/1475-2875-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magistrado PA, Minja D, Doritchamou J, Ndam NT, John D, Schmiegelow C, Massougbodji A, Dahlback M, Ditlev SB, Pinto VV, Resende M, Lusingu J, Theander TG, Salanti A, Nielsen MA. High efficacy of anti DBL4varepsilon-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine. 2011;29:437–443. doi: 10.1016/j.vaccine.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 44.Fried M, Avril M, Chaturvedi R, Fernandez P, Lograsso J, Narum D, Nielsen MA, Oleinikov AV, Resende M, Salanti A, Saveria T, Williamson K, Dicko A, Scherf A, Smith JD, Theander TG, Duffy PE. Multilaboratory approach to preclinical evaluation of vaccine immunogens for placental malaria. Infect Immun. 2013;81:487–495. doi: 10.1128/IAI.01106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert LH, Bullock JL, Cook ST, Miura K, Garboczi DN, Diakite M, Fairhurst RM, Singh K, Long CA. Antigen reversal identifies targets of opsonizing IgGs against pregnancy-associated malaria. Infect Immun. 2014;82:4842–4853. doi: 10.1128/IAI.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teo A, Hasang W, Randall LM, Unger HW, Siba PM, Mueller I, Brown GV, Rogerson SJ. Malaria preventive therapy in pregnancy and its potential impact on immunity to malaria in an area of declining transmission. Malar J. 2015;14:215–223. doi: 10.1186/s12936-015-0736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.