Abstract
Pneumococcal meningitis (PM) causes neurological sequelae in up to half of surviving patients. Neuronal damage associated with poor outcome is largely mediated by the inflammatory host response. Dexamethasone (DXM) is used as an adjuvant therapy in adult PM, but its efficacy in the treatment of pneumococcal meningitis in children is controversially discussed. While DXM has previously been shown to enhance hippocampal apoptosis in experimental PM, its impact on hippocampal cell proliferation is not known. This study investigated the impact of DXM on hippocampal proliferation in infant rat PM. Eleven-day-old nursing Wistar rats (n = 90) were intracisternally infected with Streptococcus pneumoniae to induce experimental meningitis. Treatment with DXM or vehicle was started 18 h after infection, concomitantly with antibiotics (ceftriaxone 100 mg/kg of body weight twice a day [b.i.d.]). Clinical parameters were monitored, and the amount of cells with proliferating activity was assessed using in vivo incorporation of bromodeoxyuridine (BrdU) and an in vitro neurosphere culture system at 3 and 4 d postinfection. DXM significantly worsened weight loss and survival. Density of BrdU-positive cells, as an index of cells with proliferating activity, was significantly lower in DXM-treated animals compared to vehicle controls (P < 0.0001). In parallel, DXM reduced neurosphere formation as an index for stem/progenitor cell density compared to vehicle treatment (P = 0.01). Our findings provide clear evidence that DXM exerts an antiproliferative effect on the hippocampus in infant rat PM. We conclude that an impairment of regenerative hippocampal capacity should be taken into account when considering adjuvant DXM in the therapeutic regimen for PM in children.
INTRODUCTION
Pneumococcal meningitis (PM) is the most common form of bacterial meningitis in children and continues to result in substantial morbidity and mortality despite the availability of effective antimicrobial therapy (1).
The pathogenesis and pathophysiology of bacterial meningitis involve a complex interplay between virulence factors of the pathogen and the host immune response. Experimental data suggest that the excessive inflammatory reaction to the invading bacteria is responsible for many of the pathophysiologic consequences of the disease (2–4). Thus, adjunctive corticosteroids, namely, dexamethasone (DXM), have been introduced to the therapeutic regimen in patients with bacterial meningitis with the rationale that down-modulating the immune response would be the key to an improved outcome. The early use of DXM in PM has been shown to improve selected aspects of clinical outcome in adults (5). In children, the effect of adjuvant dexamethasone in bacterial meningitis was evaluated in several studies and meta-analyses with conflicting results. For Haemophilus influenzae meningitis, but not for Streptococcus pneumoniae meningitis, a beneficial effect was found for hearing loss (6). In a large randomized, controlled double-blind trial that included 598 children in resource-poor countries, no difference in survival or neurologic sequelae was found with adjuvant dexamethasone (7). In a more recent study, the only significant effect of dexamethasone was to prevent deafness in children with H. influenzae meningitis without considering the timing between dexamethasone and ceftriaxone administration (8). This finding was challenged by results from another recent study, in which dexamethasone failed to prevent hearing loss regardless of the causative agent or the timing of antibiotics administration (9). A difference in therapy efficacy according to the socioeconomic level, with restricted benefit in adult patients from high-income countries, was suggested in a recent meta-analysis (10). The latest and most complete meta-analysis concluded that a significant beneficial effect of DXM in the management of bacterial meningitis in children is limited to short-term neurological sequelae in high-income countries and to severe hearing loss in H. influenzae-infected patients. In particular, no significant beneficial effect of corticosteroids on S. pneumoniae-infected children was detected (5).
Concerns arise from animal studies documenting a potentially harmful effect of DXM on hippocampal structure and function when applied on the developing brain in PM (11). It has been shown that DXM increases hippocampal injury and reduces learning capacity during experimental PM in infant rats (11). In the same infant rat model, a decrease in some inflammatory parameters was more pronounced in tissue samples than in cerebrospinal fluid (CSF) (12). A direct association of hippocampal damage and learning deficiencies consecutive to bacterial meningitis has not been shown in patients so far (13). The reported hippocampal damage was caused by an increase of apoptosis in the dentate gyrus. A proapoptotic effect of adjuvant DXM was also observed in the dentate gyrus of adult rabbits with PM (14) or with bacterial meningitis induced in the same adult rabbit model by Escherichia coli (15). In addition to an aggravated hippocampal apoptosis, transcriptome analysis suggests that DXM-induced hippocampal damage in PM may be related to a decrease of proneurogenic processes in the infant rat model (12). Of note, the proapoptotic and antiproliferative properties of DXM on embryonic and neonatal rat hippocampal cells have been previously reported outside of the context of PM (16–18). The hippocampus is a site of persistent neurogenesis (19), and an increase in the cellular proliferative capacity that might be involved in the regeneration of PM-induced brain damage has been observed in the hippocampi of infant rats (20), adult mice (21), and even patients who died from meningitis (22). Since the effect of DXM on the proliferation of hippocampal cells in infant PM has not been investigated so far, this study was initiated with the aim to assess the impact of adjuvant DXM on the proliferation of hippocampal cells in infant rat PM using a combined in vivo/in vitro approach.
MATERIALS AND METHODS
Model of experimental PM.
All animal studies were approved by the Animal Care and Experimentation Committee of the Canton of Bern, Switzerland (license BE 100/11) and followed the Swiss national guidelines for the performance of animal experiments. A well-established model of infant rat PM was used (11, 12, 20, 23). Series of 8 to 12 nursing Wistar rat pups of mixed sex with their dam were purchased at postnatal day 6 (Charles River, Sulzfeld, Germany) and were acclimatized for 5 days before infection. Before and during the experiment, animals were housed in a temperature-controlled environment at a 12 h light/dark cycle with food and water provided ad libitum. Nine independent sets of experiments were performed. For each set, equal numbers of subjects were attributed to each experimental group.
Bacterial meningitis was induced by infecting 11-day-old rats by direct intracisternal injection of 10 μl saline solution, which contained log10 5.5 ± 0.3 CFU/ml S. pneumoniae (serotype 3), using a 32-gauge needle (24). At 18 h after infection, CSF was obtained by puncture of the cisterna magna, and 5 μl was cultured quantitatively on agar plates to document successful infection.
Study design.
On the day of the experiments, animals (n = 90) were randomized into two treatment groups (DXM or saline). Treatment was started at 18 h postinfection. DXM (Mephameson; Mepha Pharma, Aesch/BL, Switzerland; 0.7 mg/kg of body weight every 8 h; n = 46) or saline (control group; n = 44) was administered subcutaneously. All animals were treated concomitantly with 100 mg/kg ceftriaxone (Rocephin; Roche Pharma, Reinach, Switzerland), which was administered intraperitoneally twice daily. Treatment was continued until the time of sacrifice. The dosage of DXM (0.7 mg/kg) was in line with previous studies on adjuvant DXM in infant rat PM (11). The higher dose of DXM compared with the recommended dose in human infants (0.15 mg/kg) compensates for the higher clearance rates in infant rats (15). Hence, dosing and therapy duration reflect clinical use in human infants.
A subset of the animals (n = 21 for DXM treated and n = 19 for saline treated) was randomly preassigned in each set of experiments for assessment by the in vitro neurosphere assay and was sacrificed at 72 h after infection by an overdose of intraperitoneal pentobarbital (150 mg/kg; Esconarkon; Streuli & Co. AG, Uznach, Switzerland).
To assess in vivo cell proliferation, the remaining animals (n = 25 for each treatment group) received intraperitoneal bromodeoxyuridine (BrdU) (Sigma; 50 mg/kg of body weight, daily) at days 1 to 3 after infection. The last BrdU injection was scheduled concomitantly with the sacrifice of littermates assigned to the neurosphere assay. Animals were sacrificed 24 h after the last BrdU injection (94 h after infection).
All animals were clinically assessed by using a motor performance score as described previously (11) and by weighing at predetermined time points. Survival was assessed for the whole course of the experiment.
Neurosphere assay.
Thirty-five surviving animals with confirmed meningitis (18 treated with DXM and 17 treated with saline) were assessed by the neurosphere assay. Immediately after sacrifice, animals were perfused with ice-cold phosphate-buffered saline (PBS) via the left cardiac ventricle, and their brains were processed for neurosphere assay as previously described (25). The brains were carefully removed and placed into ice-cold dissection solution consisting of Ca2+- and Mg2+-free Hanks' buffered salt solution (HBSS; Biochrom, Berlin, Germany) supplemented with antibiotic/antimycotic solution (Gibco, Life Technologies) and 6 mg/ml d-glucose. Hippocampi were dissected, and meninges and vascular tissue were removed. Tissue was minced into 1-mm3 pieces, collected in the cold dissection solution, and centrifuged for 5 min at 500 × g. After removal of the supernatant, hippocampal cells were dissociated in 1.5 ml of papain/dispase/DNase (PDD) enzyme mix (1 mg/ml [10 U/ml] papain, 1 mg/ml [0.5 U/ml] dispase II, 0.1 mg/ml [400 U/ml] DNase I, and 12 mM MgSO4 in Ca2+- and Mg2+-free HBSS, all from Sigma) (26). Cells were incubated for 5 min at 37°C and then twice for 10 min at room temperature (RT) with gentle trituration between the incubation steps. Three milliliters of Dulbecco modified Eagle medium (DMEM)/Ham's F-12 were added, and the cell suspension was filtered through a 70-μm cell strainer (BD Biosciences, USA). After centrifugation for 5 min at 500 × g, the supernatant was removed and cells were washed in 10 ml DMEM/Ham's F-12. The washing step was repeated. The pellet was resuspended in 10 ml serum free medium (SFM) consisting of DMEM/Ham's F-12 plus l-glutamine (Biochrom), which was supplemented with 10 mM HEPES, antibiotic/antimycotic solution (Gibco, Life Technologies), and B-27 (Gibco); 20 ng/ml recombinant human fibroblast growth factor (FGF) and human epidermal growth factor (EGF) (Pepro Tech, Rocky Hill, NJ); and 4 μg/ml heparin (Stemcell Technologies, Grenoble, France). Finally, the cell suspension was plated onto two 60-mm culture dishes coated with poly-hydroxyethyl-methacrylate (poly-HEMA) (Sigma P3932-1G) and was maintained for 1 day at 37°C in a 5% CO2 atmosphere.
The next day, cells were harvested, and viable cells were determined by trypan blue exclusion. For each animal, 4 wells of a 24-well poly-HEMA-coated tissue culture test plate were seeded with 104 cells in 0.5 ml of a 0.8 mg/ml collagen (Sigma)-containing SFM medium. The plate was incubated at 37°C for 1 h to allow gel formation. Once the gel had set, 0.5 ml of prewarmed supplemented SFM was added to the top of each gel, and neurospheres were grown for 17 days by replenishing half of the medium (0.25 ml/well) with fresh supplemented SFM every 2 to 3 days
After 17 days, gels were washed with PBS and were incubated at RT for 15 min in paraformaldehyde (PFA) 4% to achieve fixation. After washing twice with PBS for 10 min, the neurosphere-containing gels were transferred to glass slides (Superfrost; Menzel GmbH, Braunschweig, Germany) and were allowed to dry. Gels were stained with cresyl violet, and slides were subsequently mounted with Entellan (Merck, Zug, Switzerland). The number of neurospheres per gel was determined under a light microscope by an investigator blinded to the experimental group. For each animal, 4 gels were assessed, and the mean number of neurospheres was calculated.
BrdU immunohistochemistry.
Thirty-eight surviving animals with confirmed meningitis (15 DXM-treated and 23 saline-treated animals) were sacrificed and perfused via the left cardiac ventricle with ice-cold PBS. Brains were removed and were fixed in methanol-acetic acid (95:5) and processed for paraffin embedding. For each animal, twelve 10-μm-thick coronal sections were sampled at regular intervals throughout the hippocampus and were processed for anti-BrdU immunostaining using a mouse monoclonal primary anti-BrdU antibody (1:50; Dako Schweiz AG, Baar, Switzerland) and a Cy3-conjugated donkey anti-mouse IgG secondary antibody (1:1,000; Jackson ImmunoResearch, Newmarket, United Kingdom). Sections were counterstained with 4′-6-diamidino-2-phenylindol-dihydrochlorid (DAPI; Merck, Darmstadt, Germany) and mounted with Mowiol (Merck, Darmstadt, Germany) containing 2.5% DABCO (Sigma).
Digital image acquisition was performed using a Zeiss Imager M1 fluorescence microscope and a charge-coupled device camera. Using ImageJ software (NIH), the area of the dentate gyrus was determined from the DAPI-stained image and was superimposed on the BrdU image. After calibration, the surface area was assessed, and cell density was determined as the number of BrdU-positive cells/unit area by an investigator who was blinded to the experimental grouping (23).
Statistical analysis.
Statistical analyses were performed using Prism (version 6.02; GraphPad Software, San Diego, CA, USA). Normality tests were performed to verify whether data followed a Gaussian distribution. Normally distributed variables were compared using a two-tailed unpaired t test. Comparisons between non-normally distributed variables were performed by the two-tailed nonparametric Mann-Whitney U test. Post hoc power calculation was determined using freely available calculator software (http://clincalc.com/Stats/Power.aspx).
Survival curves were compared by means of the log rank test. Furthermore, odds ratio and significance were determined using Fisher's exact test. A P value of <0.05 in two-tailed tests was considered statistically significant. Data are indicated as mean ± standard deviation.
RESULTS
Survival.
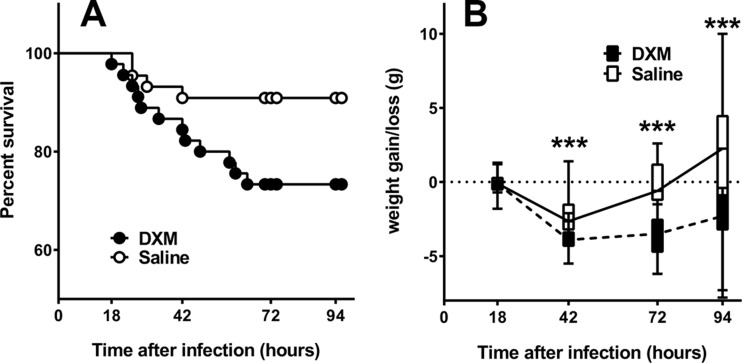
A comparison of the survival of DXM-treated versus saline-treated animals revealed a significant difference (P = 0.04) with a lower survival rate in the DXM group. Of the 45 animals with confirmed meningitis that were treated with DXM, 12 did not survive until the end of the study. Among the 44 animals in the control group, 4 died before the end of the study (Fig. 1A). The odds ratio for DXM was 3.636 [1.071 to 12.34] with a P value of 0.051 (Fisher's exact test).
FIG 1.
(A) Survival proportion of animals with meningitis treated with DXM (n = 45, closed circles) or saline (n = 44, open circle). (B) Time course of weight loss/gain in animals with meningitis treated with DXM (dashed line, hatched boxed) or saline (straight line, open boxes). For each time point, experimental groups are represented by boxes and whiskers in the 10th to 90th percentile. ***, P < 0.001 (Mann-Whitney test).
Clinical parameters.
Except for one subject in the DXM-treated group that was excluded from further analyses, all animals developed meningitis as documented by a combination of positive quantitative bacterial CSF cultures at 18 h after infection, altered clinical score, and weight loss.
There was no significant difference (P = 0.61) between the concentrations of bacteria in the CSF of animals treated with DXM (log10 7.5 ± 0.5 CFU/ml) or with saline (log10 7.5 ± 0.6 CFU/ml) at 18 h postinfection (hpi). No difference in clinical score was detected between the two groups during the course of infection (data not shown).
While there was no significant weight loss difference at 18 h postinfection, the treatment groups differed significantly at later time points (42, 72, and 94 h after infection). At 42 h after infection, weight reduction in DXM-treated animals was significantly more pronounced than that of saline-treated animals (−3.8 ± 0.7 g versus −2.3 ± 1.2 g; P < 0.0001). At 72 h postinfection, the mean weight change in the two groups still indicated a weight loss, which was significantly worse in animals receiving DXM (−3.6 ± 1.3 g versus −0.7 ± 2.3 g; P < 0.0001). The mean weight difference in DXM-treated animals at 94 h postinfection still denoted a weight reduction (−2.6 ± 2.1 g), while a slight increase in weight (1.7 ± 3.8 g) was observed in saline-treated animals (P < 0.001) (Fig. 1B).
Neurosphere assay.
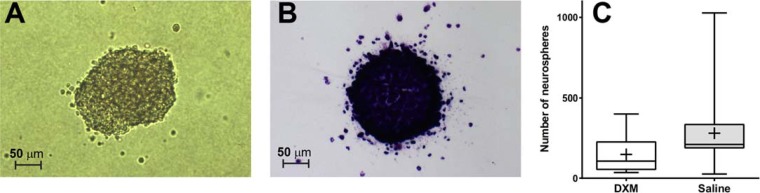
Development of cell aggregate as cell progenies arising from a single stem or progenitor cell was observed in the gel matrix (Fig. 2A) over time. Quantification was performed by drying and staining the gel matrix with cresyl violet (Fig. 2B). The number of neurospheres in animals treated with DXM was significantly lower than that of the saline-treated animals (P < 0.01; post hoc statistical power of 60.3% for α = 0.05). In the DXM group, a mean amount of 132 ± 112 neurospheres/well (n = 16) was determined, whereas the saline-treated group showed a mean number of 284 ± 224 neurospheres/well (n = 16) (Fig. 2C).
FIG 2.
Representative pictures of neurospheres during incubation in collagen gels (A) or after processing and staining with cresyl violet (B). (C) Number of neurosphere/well of a 24-well plate, originating from hippocampal cells isolated from animals with meningitis treated with DXM (open boxes) or saline (gray boxes). Experimental groups are represented by boxes and whiskers in the 5th to 95th percentile, and mean values are represented by a cross.
Density of BrdU-positive cells in the dentate gyrus.
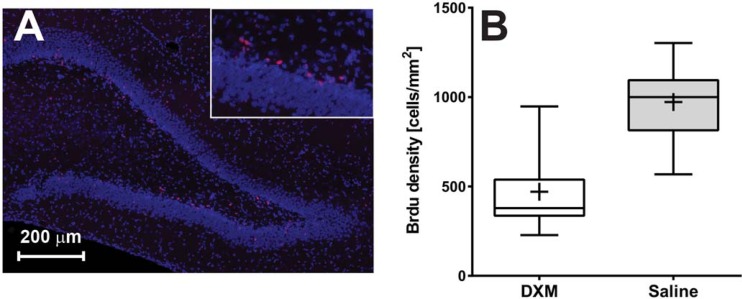
The majority of BrdU-labeled cells were found at the interface between the hilus and the subgranular zone of the dentate gyrus (Fig. 3A). Animals who were administered DXM showed a mean density of 471 ± 230 BrdU-positive cells/mm2 of the dentate gyrus (n = 12). The observed mean cell density in animals receiving saline was 972 ± 202 cells/mm2 (n = 20) (Fig. 3B). The density of BrdU-positive cells in the dentate gyrus was significantly lower in animals receiving DXM than the density in those treated with saline (P < 0.0001; post hoc statistical power of 100% for α = 0.05).
FIG 3.
(A) Representative immunofluorescence picture of the dentate gyrus of an animal with meningitis (blue, DAPI staining; red, anti-BrdU staining). (B) Density of BrdU immunolabeled cells in the dentate gyrus of animals with meningitis treated with DXM (open boxes) or saline (gray boxes), respectively. Experimental groups are represented by boxes and whiskers in the 5th to 95th percentile, and mean values are represented by a cross.
DISCUSSION
The present study in infant rat PM investigated the impact of adjuvant DXM on stem/progenitor cell density and on cells with proliferative capacity in the hippocampus by using a combined in vivo/in vitro approach. We found a decreased density of BrdU-positive cells in the hippocampal dentate gyrus and a decreased amount of cells with proliferating capacity (stem and progenitor cells) by documenting a lower number of neurospheres from hippocampal tissue of animals receiving DXM compared to the vehicle control group. Moreover, DXM treatment worsened clinical parameters as was indicated by a more pronounced weight loss and a lower survival rate. These findings document a DXM-induced decline in the regenerative capacity of the hippocampus in infant PM. While the identity of the proliferative, BrdU-positive cells has not been studied in detail in the present study, evidence from other studies suggests neuronal precursors as the main cell population. An increase in doublecortin- or TUC4-immunopositive cells, two markers for immature neurons, was demonstrated in patients after bacterial meningitis (22). Furthermore, a non-neuronal immune cell identity was largely excluded in adult mice after meningitis (21).
Our findings extend the available literature on the potentially harmful effect of adjuvant DXM on the hippocampus in infant rat PM. While the proapoptotic effect of DXM in the paradigm of PM has been shown previously (11, 14), we hereby provide evidence of an additional negative impact of adjuvant DXM treatment on neuronal regeneration in the developing brain affected by PM. Our findings are in line with the recently reported decrease in proneurogenic signaling at the transcriptome level (12). Moreover, our data are consistent with the results of several investigations that addressed the effect of perinatal glucocorticoid administration on hippocampal neurogenesis in otherwise healthy animals. Neural precursor cells and immature neurons in the hippocampus were shown to be sensitive to the proapoptotic actions of DXM in neonatal rats, leading to significant and sustained reductions in the volume of the dentate gyrus (18). In addition, a one to three day DXM regimen in 4- to 7-day-old rats significantly decreased brain weight, which was associated with a decrease in BrdU-labeled cells in the subpial granular layer (SGL), the subventricular zone (SVZ), and the cortex (16). The same decline in BrdU incorporation following DXM application was also shown in in vitro investigations on embryonic rat neural stem cells (17).
Previous studies have consistently shown an increased proliferation of neural progenitors in response to bacterial meningitis (20–22). The observed anti-regenerative effect of DXM may therefore impair the body's own attempt to alleviate the consequences of PM-induced neuronal damage, thereby increasing rather than reducing disease morbidity. In addition to the net decrease of cells in a proliferative state, we found a more pronounced weight loss and a lower survival rate in DXM-treated animals than in vehicle control animals.
The strengths of this study are the use of a well-characterized experimental model of infant rat PM and the investigation of hippocampal regenerative capacity using a combined in vivo/in vitro approach. We nevertheless acknowledge some limitations. First, the functional impact of the observed anti-regenerative effect was not assessed in the present study. However, the clinical relevance of our findings is supported by our previous study showing reduced learning capacity in DXM-treated infant rats using the same experimental protocol (11). Second, although the applied neurosphere and BrdU-labeling techniques are well-established methods to assess hippocampal cell proliferation, no definitive statement can be made about whether the observed reduction of cells in a proliferative state as documented by the BrdU analysis is due to a reduced amount of precursor cells, as it would be suggested from our previous study (11), an impaired proliferative capacity or these precursors, or a combination of the two. Third, the present study precludes a distinction between direct and indirect effects of dexamethasone with regard to hippocampal regeneration. Evidence from the available literature suggests both. In particular, it has been shown that stimulation of cultured rat neural stem cells with dexamethasone or corticosterone reduced cell proliferation by BrdU labeling, therefore suggesting a direct effect (17). Indirect effect by modulation of the inflammatory reaction is also plausible. We have shown that DXM modulated the inflammatory reaction in the present model, particularly in tissue samples (12). The modulation of the neuro-inflammation, particularly that caused by microglia, and its consequence on neurogenesis is complex (27). While studies of inflammatory meningitis by injection of pneumococcal cell wall (28) or lipopolysaccharides (29) were shown to have a negative impact on neurogenesis, we (20) and others (21) have demonstrated the opposite using models of productive bacterial infections. Consequently, inhibition of inflammation in these different experimental paradigms leads to different results. Furthermore, since the microglial population changes over time with respect to phenotype and cytokine expression, possibly altering its effect from cytotoxic in the acute phase to supportive in the chronic phase (27), differences in the timing of microglial inhibition may lead to the opposite effect on neural proliferation. Our findings of an anti-regenerative effect of DXM in PM are based on an infant rat model reflecting infection during early childhood, which precludes direct applicability to human beings. Thus, a similar negative impact of adjuvant DXM on the hippocampus of children with PM still remains speculative. This study was designed to reflect the situation in patients suffering from long-term sequelae. It should however be mentioned that, in contrast to our experimental evidence, DXM was not shown to significantly influence mortality during childhood PM (5). Clinical research in human beings is needed to assess the possible adverse effects of adjuvant DXM on hippocampal function in pediatric patients with PM. In that context, the meta-analysis of Barrington on preterm infants with postnatal steroids for bronchopulmonary dysplasia demonstrated a very clear increase in neurodevelopmental impairment and led the author to conclude that the use of corticosteroids for this indication should be abandoned (30). In particular, one study showed that a DXM treatment as short as 3 days was still associated with severe adverse outcome (31). In contrast to the beneficial effects of DXM in adult patients with pneumococcal meningitis, which are most likely due to its anti-inflammatory property, the negative impact of this therapy on the regenerative capacity of an immature central nervous system in children may explain differences in the therapeutic efficacy observed between these two distinct patient populations. These findings must be taken into account when evaluating the effect of adjuvant DXM in the therapeutic regimen for PM in children.
In conclusion, the present study demonstrates that DXM decreases the density of proliferating cells, including stem/progenitor cells in the hippocampus of infant rat with PM. This finding further clarifies the underlying mechanisms of the previously shown learning deficiency in infant rats following treatment with adjuvant DXM for PM. Experimental evidence of the harmful effects of adjuvant DXM on hippocampal structure and function suggests that DXM should be used with caution when treating PM in children.
ACKNOWLEDGMENTS
We thank Angela Bühlmann, Kevin Oberson, and Benno Grabscheid for excellent technical assistance.
L.B. and D.G. participated in the design, execution, and evaluation of the present study. S.L.L. participated in the design and evaluation of the study. All authors significantly contributed to manuscript preparation.
Funding Statement
The Swiss National Science Foundation provided funding to S.L.L. under SNF grant 310030_162583. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A, Emerging Infections Programs Network. 2011. Bacterial meningitis in the United States, 1998-2007. N Engl J Med 364:2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 2.Tuomanen E. 1994. Modulation of inflammation in bacterial meningitis. Isr J Med Sci 30:339–341. [PubMed] [Google Scholar]
- 3.Leib SL, Täuber MG. 1999. Pathogenesis of bacterial meningitis. Infect Dis Clin North Am 13:527–548, v–vi. doi: 10.1016/S0891-5520(05)70093-3. [DOI] [PubMed] [Google Scholar]
- 4.Pfister HW, Koedel U, Paul R. 1999. Acute meningitis. Curr Infect Dis Rep 1:153–159. doi: 10.1007/s11908-996-0023-7. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer MC, McIntyre P, Prasad K, van de Beek D. 2013. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 6:CD004405. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, Perez CM. 1997. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA 278:925–931. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux EM, Walsh AL, Forsyth H, Tembo M, Mwenechanya J, Kayira K, Bwanaisa L, Njobvu A, Rogerson S, Malenga G. 2002. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 360:211–218. doi: 10.1016/S0140-6736(02)09458-8. [DOI] [PubMed] [Google Scholar]
- 8.Peltola H, Roine I, Fernandez J, Zavala I, Ayala SG, Mata AG, Arbo A, Bologna R, Mino G, Goyo J, Lopez E, de Andrade SD, Sarna S. 2007. Adjuvant glycerol and/or dexamethasone to improve the outcomes of childhood bacterial meningitis: a prospective, randomized, double-blind, placebo-controlled trial. Clin Infect Dis 45:1277–1286. doi: 10.1086/522534. [DOI] [PubMed] [Google Scholar]
- 9.Peltola H, Roine I, Fernandez J, Gonzalez Mata A, Zavala I, Gonzalez Ayala S, Arbo A, Bologna R, Goyo J, Lopez E, Mino G, Dourado de Andrade S, Sarna S, Jauhiainen T. 2010. Hearing impairment in childhood bacterial meningitis is little relieved by dexamethasone or glycerol. Pediatrics 125:e1–e8. doi: 10.1542/peds.2009-0395. [DOI] [PubMed] [Google Scholar]
- 10.Assiri AM, Alasmari FA, Zimmerman VA, Baddour LM, Erwin PJ, Tleyjeh IM. 2009. Corticosteroid administration and outcome of adolescents and adults with acute bacterial meningitis: a meta-analysis. Mayo Clin Proc 84:403–409. doi: 10.1016/S0025-6196(11)60558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leib SL, Heimgartner C, Bifrare YD, Loeffler JM, Täuber MG. 2003. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr Res 54:353–357. doi: 10.1203/01.PDR.0000079185.67878.72. [DOI] [PubMed] [Google Scholar]
- 12.Blaser C, Wittwer M, Grandgirard D, Leib SL. 2011. Adjunctive dexamethasone affects the expression of genes related to inflammation, neurogenesis and apoptosis in infant rat pneumococcal meningitis. PLoS One 6(3):e17840. doi: 10.1371/journal.pone.0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jonge RC, Swart JF, Koomen I, Rombouts SA, Gemke RJ, Barkhof F, van Furth AM. 2008. No structural cerebral differences between children with a history of bacterial meningitis and healthy siblings. Acta Paediatr 97:1390–1396. [DOI] [PubMed] [Google Scholar]
- 14.Zysk G, Bruck W, Gerber J, Bruck Y, Prange HW, Nau R. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol 55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Spreer A, Gerber J, Hanssen M, Schindler S, Hermann C, Lange P, Eiffert H, Nau R. 2006. Dexamethasone increases hippocampal neuronal apoptosis in a rabbit model of Escherichia coli meningitis. Pediatr Res 60:210–215. doi: 10.1203/01.pdr.0000227553.47378.9f. [DOI] [PubMed] [Google Scholar]
- 16.Kanagawa T, Tomimatsu T, Hayashi S, Shioji M, Fukuda H, Shimoya K, Murata Y. 2006. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am J Obstet Gynecol 194:231–238. doi: 10.1016/j.ajog.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. 2006. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci 26:5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF. 2010. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol 67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]
- 19.Altman J, Das GD. 1967. Postnatal neurogenesis in the guinea-pig. Nature 214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- 20.Wittwer M, Grandgirard D, Rohrbach J, Leib SL. 2010. Tracking the transcriptional host response from the acute to the regenerative phase of experimental pneumococcal meningitis. BMC Infect Dis 10:176. doi: 10.1186/1471-2334-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tauber SC, Stadelmann C, Spreer A, Bruck W, Nau R, Gerber J. 2005. Increased expression of BDNF and proliferation of dentate granule cells after bacterial meningitis. J Neuropathol Exp Neurol 64:806–815. doi: 10.1097/01.jnen.0000178853.21799.88. [DOI] [PubMed] [Google Scholar]
- 22.Gerber J, Tauber SC, Armbrecht I, Schmidt H, Bruck W, Nau R. 2009. Increased neuronal proliferation in human bacterial meningitis. Neurology 73:1026–1032. doi: 10.1212/WNL.0b013e3181b9c892. [DOI] [PubMed] [Google Scholar]
- 23.Grandgirard D, Bifrare YD, Pleasure SJ, Kummer J, Leib SL, Täuber MG. 2007. Pneumococcal meningitis induces apoptosis in recently postmitotic immature neurons in the dentate gyrus of neonatal rats. Dev Neurosci 29:134–142. doi: 10.1159/000096218. [DOI] [PubMed] [Google Scholar]
- 24.Leib SL, Leppert D, Clements J, Täuber MG. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 68:615–620. doi: 10.1128/IAI.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofer S, Grandgirard D, Burri D, Frohlich TK, Leib SL. 2011. Bacterial meningitis impairs hippocampal neurogenesis. J Neuropathol Exp Neurol 70:890–899. doi: 10.1097/NEN.0b013e3182303f31. [DOI] [PubMed] [Google Scholar]
- 26.Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M, Kaesbauer J, Uyanik G, Klucken J, Karl C, Tebbing J, Svendsen C, Weidner N, Kuhn HG, Winkler J, Aigner L. 2003. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest 83:949–962. doi: 10.1097/01.LAB.0000075556.74231.A5. [DOI] [PubMed] [Google Scholar]
- 27.Ekdahl CT, Kokaia Z, Lindvall O. 2009. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann O, Mahrhofer C, Rueter N, Freyer D, Bert B, Fink H, Weber JR. 2007. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect Immun 75:4289–4297. doi: 10.1128/IAI.01679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. 2003. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrington KJ. 2001. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, Yurman S, Dolfin T, Kogan A, Dollberg S, Arbel E, Goldberg M, Gur I, Naor N, Sirota L, Mogilner S, Zaritsky A, Barak M, Gottfried E. 2000. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 83:F177–F181. doi: 10.1136/fn.83.3.F177. [DOI] [PMC free article] [PubMed] [Google Scholar]