Abstract
Doripenem is a broad-spectrum parenteral carbapenem with enhanced activity against Pseudomonas aeruginosa. While the initial dosing recommendation for renally competent patients and patients undergoing continuous renal replacement therapy (cRRT) was 500 mg every 8 h (q8h), the dose for renally competent patients was updated to 1 g q8h in June 2012. There are no updated data for the dosing of patients on continuous renal replacement therapy. The original dosing regimen for cRRT patients was based on nonseptic patients, while newer publications chose comparatively low target concentrations for a carbapenem. Thus, there is an urgent need for updated recommendations for dosing during cRRT. In the trial presented here, we included 13 oliguric septic patients undergoing cRRT in an intensive care setting. Five patients each were treated with hemodiafiltration or hemodialysis, while three patients received hemofiltration treatment. All patients received 1 g doripenem every 8 h. Doripenem concentrations in the plasma and ultrafiltrate were measured over 48 h. The mean hemofilter clearance was 36.53 ml/min, and the mean volume of distribution was 59.26 liters. The steady-state trough levels were found at 8.5 mg/liter, with no considerable accumulation. Based on pharmacokinetic and pharmacodynamic considerations, we propose a regimen of 1 g q8h, which may be combined with a loading dose of 1.5 to 2 g for critically ill patients. (This study has been registered with EudraCT under registration no. 2009-018010-18 and at ClinicalTrials.gov under registration no. NCT02018939.)
INTRODUCTION
Doripenem is a broad-spectrum parenteral carbapenem developed in 1997. It is active against a broad spectrum of bacteria, including streptococci, methicillin-susceptible staphylococci, Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter species, and Bacteroides fragilis. Compared to other carbapenem antimicrobials, doripenem features increased activity against Pseudomonas aeruginosa, making it a useful tool for the treatment of critically ill cystic fibrosis or burn patients (1, 2). The initial dosing recommendation for renally competent patients was 500 mg every 8 h (q8h). Based on this regimen, a pharmacokinetic (PK) trial with otherwise healthy patients on continuous renal replacement therapy (cRRT) was performed by Cirillo et al. in 2011, showing reduced elimination of doripenem for patients on continuous renal replacement therapy relative to renally competent patients (3). These data were supported by a publication by Roberts et al. in September 2014 (4). In June 2012, the European Medicines Agency published the results of a review of the drug and concluded that for renally competent patients, an increased dosage of 1,000 mg q8h should be employed (5). The trial presented here characterizes the pharmacokinetic profile of 1,000 mg doripenem q8h for 13 critically ill patients receiving continuous venovenous renal replacement therapy.
Continuous renal replacement therapies play a vital role in the fluid management of critically ill patients suffering from renal failure. Two methods are used most widely: continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodialysis (CVVHD). While CVVH is usually used for base-acid correction in acidotic septic patients and is more and more replaced by a combination of both methods (continuous venovenous hemodiafiltration [CVVHDF]), CVVHD is used mainly for the purpose of fluid management.
MATERIALS AND METHODS
Patient eligibility.
The trial followed an open-label prospective design. Patients were included if the treating physicians had decided to initiate treatment with doripenem. Only sedated and intubated patients 18 years old or older and in need of intensive care unit (ICU) treatment and renal replacement therapy were deemed eligible. Exclusion criteria included a urine production of >500 ml/24 h and therapy with probenecid or valproic acid.
The trial was approved by the independent ethics committee of the Medical University of Vienna (vote number 1057/2009) and the Austrian Federal Office for Safety in Health Care. It was registered with EudraCT (registration no. 2009-018010-18) and clinicaltrials.gov (registration no. NCT02018939).
Drug administration and sampling.
All patients received 1,000 mg of doripenem (Doribax 500 mg; Janssen-Cilag, Vienna, Austria) every 8 h via a central venous line different from the one used for cRRT. After reconstitution, the drug solution was diluted in 100 ml normal saline and was infused with 200 ml/h.
Blood and dialysate samples were drawn from the arterial (input), venous (output), and effluent dialysate ports of the dialysis machine before the first administration of doripenem and at 0.5, 1, 2, 3.5, 7, 8, 9, 16, 17, 24, 24.5, 25, 26, 27.5, 31, 32, 33, 40, 41, and 48 h following the start of the first doripenem infusion. For doripenem administrations 2, 3, 5, and 6, additional sampling 30 min after the end of the infusion was performed in order to assess peak plasma levels post-drug equilibration. Blood samples were centrifuged and the plasma supernatant immediately stored at −80°C.
Drug assay.
Frozen patient plasma samples were thawed at room temperature and were centrifuged at 13,000 × g for 5 min. After the addition of 200 μl of methanol to 100 μl of plasma, the samples were centrifuged (at 13,000 × g for 5 min), and 80 μl of the clear supernatant was injected onto the high-performance liquid chromatography (HPLC) column. Diafiltrate samples (10 μl) were injected onto the column without any prior precipitation procedure. Doripenem concentrations were determined using a Dionex “UltiMate 3000” system (Dionex Corp., Sunnyvale, CA) with UV detection at 298 nm. Chromatographic separation was carried out at 35°C on a Hypersil BDS C18 column (particle size, 5 μm; length, 250 mm; inside diameter [i.d.], 4.6 mm; Thermo Fisher Scientific, Inc., Waltham, MA), preceded by a Hypersil BDS C18 precolumn (particle size, 5 μm; length, 10 mm; i.d., 4.6 mm). The mobile phase consisted of 0.1% acetic acid-methanol (90:10, vol/vol) at a flow rate of 1.0 ml/min. Linear calibration curves were calculated from the peak areas of doripenem compared with the external standard by spiking drug-free human plasma and diafiltrate with standard solutions of doripenem to obtain a concentration range of 0.01 to 10 mg/liter (average correlation coefficients, >0.99). The limit of detection (LOD) for doripenem in the plasma and microdialysate was 0.05 mg/liter, and the limit of quantification (LOQ) was 0.01 mg/liter. The coefficients of accuracy and precision for this compound were <8.7%.
Pharmacokinetic analysis.
The methods of pharmacokinetic analysis using commercially available software (Kinetica, version 3.0; InnaPhase, Philadelphia, PA, USA) have been described previously (6, 7). The area under the concentration-time curve (AUC) was determined by noncompartmental analysis using the trapezoidal rule. The elimination half-life (t1/2β) was calculated as ln2/kel, where kel (elimination rate constant) is the slope of the decreasing part of the concentration-time curve. The total-body clearance (Cltot) was calculated as dose/AUC. The volume of distribution (V) was calculated as Cltot/kel. The filter clearance was calculated on the basis of pre- and postfilter concentrations. In CVVH and CVVHDF, the postfilter concentrations were corrected for postdilution. Calculations for pre- and postfilter clearance for all patients, as well as for sieving/saturation coefficient-based clearance for CVVHD (ClHD) and CVVH (ClHF), were performed using previously published formulae (8). Sieving coefficient-based clearance for CVVHDF (ClHDF) was calculated using a derived formula.
The sieving or saturation coefficient-based formulae are as follows:
with terms defined as follows:
The hemofilter inlet/outlet-based formula is as follows:
with terms defined as follows:
where UFR is the ultrafiltration rate during hemofiltration, Sc is the sieving/saturation coefficient, QD is the dialysate flow, SRpre or SRpost is the pre- or postfilter substitution rate, BFR is the blood flow rate, CPL is the concentration of doripenem in plasma measured at the hemofilter inlet, CUF is the concentration of doripenem in the ultrafiltrate/dialysate, and FRR is the fluid removal rate during hemodialysis.
Compared to the commonly used formula, the formulae described above allow for correct clearance calculation in CVVH(D)F settings with high pre- and/or postdilution rates, which would otherwise increase the calculated clearance over the actual clearance.
Population pharmacokinetic model.
Arterial doripenem concentration-time profiles were analyzed using the nonlinear mixed-effects modeling software NONMEM, version 7.3, with the integrated first-order conditional estimation (FOCE) method (Icon Development Solutions, Ellicott City, MD, USA). Perl-speaks-NONMEM (PsN), version 3.7.6, was used to perform bootstrap analysis, prediction-corrected visual predictive checks, and covariate analysis.
A linear two-compartment model parameterized by clearance (Cl), central volume of distribution (V1), peripheral volume of distribution (V2), and intercompartmental clearance (Q) adequately described the doripenem pharmacokinetics. Between-subject variability was estimated for Cl, V1, and V2 and was assumed to be log-normally distributed. Residual variability was described using a combined-error model with a proportional and an additive component. Model selection was based on the comparison of objective function values, evaluation of goodness-of-fit plots, and visual predictive checks. Ninety-five percent confidence intervals for all pharmacokinetic parameters were obtained via a nonparametric bootstrap with 1,000 replicates. Furthermore, the potential covariates body weight (expressed in kilograms), body mass index (BMI; calculated as the body weight [in kilograms] divided by the square of height [in meters]), and gender were investigated on the model parameters Cl, V1, and V2 in a stepwise manner by forward inclusion (P = 0.05) and backward elimination (P = 0.01). Since none of the covariates tested showed a statistically or clinically significant effect on the respective pharmacokinetic parameters, no covariates were included in the final model.
Monte Carlo simulations.
The final model was used to perform Monte Carlo simulations for three different dose levels: 500 mg/8 h, 750 mg/8 h, and 1,000 mg/8 h. Each simulated data set contained 1,000 patients who were treated continuously for 7 days. On the basis of previous publications on PK/pharmacodynamic (PD) parameters for beta-lactams, recommendations for critically ill patients, and the EUCAST breakpoints for Pseudomonas aeruginosa as a model pathogen, we set a minimum doripenem concentration of 8 mg/liter as the target concentration. Since beta-lactam concentrations of at least 4× MIC are recommended, this concentration would result in sufficient exposure of a doripenem-susceptible Pseudomonas aeruginosa strain with a MIC of 2 mg/liter for the entire dosing interval (9, 10).
RESULTS
Between August 2011 and June 2013, a total of 14 sedated and intubated patients receiving antimicrobial treatment with 1 g doripenem q8h and in need of continuous renal replacement therapy, with a maximum residual urine volume of 500 ml/24 h, were included in this trial. One screening failure occurred (patient 12) due to a residual urine volume of >500 ml/24 h. The concentrations of doripenem in plasma measured for patient 11 were exceptionally low due to accidental storage at −20°C instead of −80°C. Thus, the data from patient 11 had to be excluded from the calculation of average values. The demographic parameters for the patients may be found in Table 1.
TABLE 1.
Demographic table displaying individual data for each patient
| Screening no. | Sex | Age (yr) | Body wt (kg) | BMI (kg/m2) | Infectiona | Pathogenb | Survival (daysc) | Dose (mg/kg)d |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | 89 | 27.47 | Endocarditis of the aortic and mitral valves; aspiration pneumonia | Enterococcus faecalis | 16 | 11.24 |
| 2 | M | 65 | 77 | 26.03 | Acute pancreatitis and peritonitis | VRE | Alive | 12.99 |
| 3 | M | 76 | 90 | 27.78 | ARDS | Alive | 11.11 | |
| 4 | M | 65 | 98 | 25.25 | Septic shock | 9 | 10.20 | |
| 5 | M | 79 | 75 | 24.49 | Postoperative sepsis following bio-AVR | Alive | 13.33 | |
| 6 | M | 72 | 160 | 52.24 | Infected hemorrhage of the psoas muscle; putrid bronchial secretion | 158 | 6.25 | |
| 7 | F | 55 | 70 | 24.22 | Streptococcus pneumoniae sepsis after splenectomy | S. pneumoniae | Alive | 14.29 |
| 8 | M | 73 | 85 | 24.84 | Pancreatitis, tuberculosis | 85 | 11.76 | |
| 9 | M | 65 | 100 | 30.86 | E. coli sepsis following SBP | Escherichia coli | 12 | 10.00 |
| 10 | M | 77 | 60 | 19.37 | S. pneumoniae sepsis | S. pneumoniae | 12 | 16.67 |
| 11 | M | 42 | 120 | 37.04 | Pneumonia | 13 | 8.33 | |
| 13 | M | 62 | 102 | 32.93 | Endocarditis of the aortic valve | Staphylococcus aureus | 6 | 9.80 |
| 14 | M | 56 | 75 | 23.15 | Septic shock, pleural empyema | E. coli | 5 | 13.33 |
| Mean ± SD | 66.9 ± 10.2 | 92.4 ± 24.8 | 28.9 ± 8.0 | 11.5 ± 2.6 |
ARDS, adult respiratory distress syndrome; bio-AVR, aortic valve replacement with a bioprosthetic valve; SBP, spontaneous bacterial peritonitis.
VRE, vancomycin-resistant enterococcus.
Days after inclusion in the trial.
Each patient received a doripenem dose of 1,000 mg q8h.
Two polysulfone dialyzers were used: a Fresenius (Vienna, Austria) AV100S dialyzer with a membrane area of 1.8 m2 and an ultrafiltration coefficient (kUF) of 48 ml/h · mm Hg, for five patients receiving CVVHD, and a Fresenius AV 600 dialyzer with a membrane area of 1.4 m2 and a kUF of 40 ml/h · mm Hg, for two patients receiving CVVH. For five patients receiving CVVHDF and one patient receiving CVVH, an AN69 dialyzer (ST150 Set; Gambro, Vienna, Austria) with a membrane area of 1.5 m2 and a kUF of 37.5 ml/h · mm Hg was used. Blood flow rates during the first dosing interval were stable, with an average of 127 ml/min. Individual dialysis parameters during the first dosing interval (hours 0 to 8) may be found in Table 2.
TABLE 2.
cRRT parameters of the individual patientsa
| Screening no. | cRRT modeb | Capillaryc | Qb (ml/min) | QD (ml/h) | QUF (ml/h) | Qs_pre (ml/h) | Qs_post (ml/h) | Hkt |
|---|---|---|---|---|---|---|---|---|
| 1 | HDF | ST150 | 180 | 1,000 | 100 | 0 | 500 | 26.3 |
| 2 | Citrate HD | AV 1000S | 100 | 2,000 | 26.8 | |||
| 3 | Citrate HD | AV 1000S | 100 | 2,000 | 28.4 | |||
| 4 | Citrate HD | AV 1000S | 100 | 2,000 | 26.7 | |||
| 5 | HDF | ST150 | 200 | 1,000 | 0 | 0 | 1,000 | 28.7 |
| 6 | Citrate HD | AV 1000S | 120 | 2,000 | 31.2 | |||
| 7 | HF | AV 600 | 180d | 50d | 0d | 2,400d | 22.3 | |
| 8 | Citrate HD | AV 1000S | 100 | 2,000 | 150 | 28.6 | ||
| 9 | HDF | ST150 | 100 | 1,000 | 0 | 0 | 500 | 31.6 |
| 10 | Citrate HDF | ST150 | 100 | 1,000 | 0 | 0 | 500 | 28.6 |
| 11 | HF | AV 600 | 150 | 150 | 3,200 | 0 | 28.8 | |
| 13 | HF | ST150 | 150 | 0 | 1,100 | 1,100 | 30.4 | |
| 14 | Citrate HDF | ST150 | 100 | 900 | 0 | 1,000 | 900 | 25.2 |
Qb, blood flow, QD, dialysate flow; QUF, ultrafiltration flow; Qs_pre, predilution rate; Qs_post, postdilution rate; Hkt, hematocrit.
HD, hemodialysis; HF, hemofiltration; HDF, hemodiafiltration.
AV 1000S, Fresenius AV 1000S dialyzer with a polysulfone membrane; AV 600, Fresenius AV 600 dialyzer with a polysulfone membrane; ST150, Gambro ST150 dialyzer with an AN69 membrane.
HD was stopped at 2 and 3.5 h following the start of the first doripenem infusion.
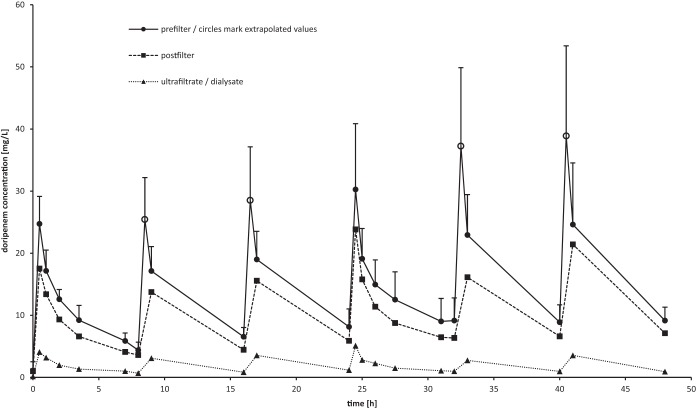
The mean of each patient's maximum plasma doripenem level at the arterial port was 30.2 mg/liter (±10.6 mg/liter) (n = 10) directly after the fourth administration of doripenem (time point, 24.5 h). The maximum extrapolated plasma doripenem level at the arterial port was found at 40.5 h (38.9 ± 15.9 mg/liter) (n = 6). The mean maximum plasma doripenem level 1 h after doripenem administration was 24.6 mg/liter (±10.6 mg/liter) (n = 6). The mean concentrations of doripenem in the plasma and ultrafiltrate over time may be found in Fig. 1.
FIG 1.
Mean pre- and postfilter concentrations of doripenem in plasma and mean concentrations in the ultrafiltrate (n = 12). Prefilter values at 8.5, 16.5, 32.5, and 40.5 h were extrapolated (marked by open circles).
The mean area under the concentration-time curve of the first dosing interval (AUC0–8) was calculated as 78.6 mg · h/liter (±10.3 mg · h/liter) (n = 12), and the mean volume of distribution (V) amounted to 59.3 liters (±26.5 liters) (n = 12). Correction of the volume of distribution for body weight resulted in a mean V of 0.70 liter/kg (±0.36 liter/kg) (n = 12); however, since patient 2 exhibited an exceptionally high V, calculating the median may be more appropriate (Vmedian, 0.62 liters/kg [interquartile range {IQR}, 0.24]) (n = 12). The mean total body clearance was 8.1 liters/h (±1.8 liters/h) (n = 12). Finally, the mean half-life of doripenem during continuous renal replacement therapy was determined as 5.4 h (±2.8 h) (n = 12). The mean hemofilter clearance, calculated from pre- and postfilter plasma doripenem concentrations, was 36.1 ml/min (±14.3 ml/min) (n = 12). Clearance values derived from the sieving or saturation coefficient were calculated as 5.2 ml/min (±2 ml/min) (n = 12). When sorted by cRRT mode, the clearance values were 5.5 ml/min (±1.8 ml/min) (n = 2) for CVVH, 5.6 ml/min (±2.3 ml/min) (n = 5) for CVVHD, and 4.68 ml/min (±1.43 ml/min) (n = 5) for CVVHDF. The mean sieving/saturation coefficient was 0.150 (±0.053) (n = 12). An overview of all pharmacokinetic parameters may be found in Table 3.
TABLE 3.
Pharmacokinetic resultsa
| Screening no. | AUC0–8 (mg · h/liter) prefilter | Cltot (liters/h) | Clpre-post filter (ml/min) | Sc | ClSc (ml/min) |
V |
t1/2β (h) | |
|---|---|---|---|---|---|---|---|---|
| Total (liters) | Corrected for body wt (liters/kg) | |||||||
| 1 | 71.96 | 9.53 | 23.26 | 0.123 | 3.32 | 57.75 | 0.65 | 4.19 |
| 2 | 58.65 | 7.42 | 30.61 | 0.146 | 5.09 | 135.90 | 1.76 | 12.69 |
| 3 | 67.50 | 8.93 | 35.75 | 0.272 | 9.41 | 76.82 | 0.85 | 5.96 |
| 4 | 89.89 | 9.00 | 26.61 | 0.184 | 6.26 | 41.56 | 0.42 | 3.20 |
| 5 | 81.58 | 7.62 | 33.12 | 0.195 | 6.50 | 54.37 | 0.72 | 4.94 |
| 6 | 84.32 | 6.93 | 47.23 | 0.153 | 5.09 | 41.55 | 0.26 | 4.15 |
| 7 | 95.28 | 7.53 | 69.65 | 0.168 | 7.26 | 40.87 | 0.58 | 3.76 |
| 8 | 87.59 | 5.14 | 29.97 | 0.064 | 2.30 | 59.22 | 0.70 | 7.98 |
| 9 | 81.64 | 8.24 | 24.24 | 0.104 | 2.97 | 51.15 | 0.51 | 4.30 |
| 10 | 71.95 | 11.31 | 20.70 | 0.176 | 4.51 | 51.71 | 0.86 | 3.13 |
| 11 | —c | —c | 42.06 | 0.139 | 7.66 | —c | —c | —c |
| 13 | 79.02 | 5.53 | 57.98 | 0.089 | 3.63 | 61.22 | 0.60 | 7.67 |
| 14 | 73.62 | 9.73 | 33.63 | 0.128 | 6.12 | 39.01 | 0.52 | 2.78 |
| Mean ± SD | ||||||||
| All patientsb | 78.58 ± 10.32 | 8.07 ± 1.77 | 36.06 ± 14.27 | 0.150 ± 0.053 | 5.20 ± 1.95 | 59.26 ± 26.47 | 0.70 ± 0.36 | 5.39 ± 2.84 |
| Patients on CVVH (n = 3) | 87.15 ± 8.13 | 6.53 ± 1.00 | 63.82 ± 5.83 | 0.129 ± 0.033 | 6.45 ± 1.81 | 51.05 ± 10.18 | 0.59 ± 0.01 | 5.72 ± 1.96 |
| Patients on CVVHD (n = 5) | 77.59 ± 12.31 | 7.48 ± 1.43 | 34.03 ± 7.22 | 0.164 ± 0.067 | 5.63 ± 2.30 | 71.01 ± 34.98 | 0.80 ± 0.52 | 6.80 ± 3.37 |
| Patients on CVVHDF (n = 5) | 76.15 ± 4.50 | 9.29 ± 1.28 | 26.99 ± 5.34 | 0.145 ± 0.035 | 4.68 ± 1.43 | 50.80 ± 6.34 | 0.65 ± 0.13 | 3.87 ± 0.80 |
Prefilter, measured in the blood before it enters the filter (concentrations are as found in the patient); Cltot, total body clearance; Clpre-post filter, clearance calculated by the hemofilter inlet/outlet-based formula described in Materials and Methods; Sc, sieving/saturation coefficient; ClSc, clearance calculation based on the sieving coefficient. The dose for each patient was 1 g doripenem q8h.
Except for patient 11, whose values were excluded from the calculation of means.
—, values for patient 11 have been excluded from calculation of means (absolute concentrations too low due to storage at -20°C). The same error may affect calculated values as the clearance and sieving coefficient values, which are given due to the fact that they are calculated as relative values.
Population pharmacokinetic model.
A two-compartment model with linear elimination was best for describing the pharmacokinetics of doripenem in plasma, which is consistent with findings from previous population pharmacokinetic studies (4, 11). The estimated pharmacokinetic parameters, as well as the bootstrap results, are shown in Table 4. A full covariate analysis for body weight (in kilograms), body mass index (in kilograms per square meter), and gender was performed, but no statistically significant effects on the pharmacokinetic parameters Cl, V1, and V2 could be found.
TABLE 4.
Population pharmacokinetic parameter estimatesa
| Parameter | Estimate | RSE (%) | IIV (%) | Bootstrap valueb (n = 1,000) |
|---|---|---|---|---|
| Cltot (liters/h) | 9.79 | 15 | 50.5 | 9.96 (7.92–13.2) |
| V1 (liters) | 43.5 | 21 | 64.8 | 43.43 (30.3–64.3) |
| Q (liters/h) | 27.1 | 15 | 0 FIXc | 30.75 (21.8–53.8) |
| V2 (liters) | 29.5 | 24 | 81.8 | 30.35 (16.2–44.2) |
| Residual error | ||||
| Proportional | 0.23 | 11 | 0.22 (0.15–0.27) | |
| Additive | 0.89 | 32 | 0.99 (0.6–1.86) |
RSE, relative standard error; IIV, interindividual variability.
Given as mean (95% confidence interval).
0 FIX, IIV could not be estimated by the model and was set to 0 in the final model.
Simulations.
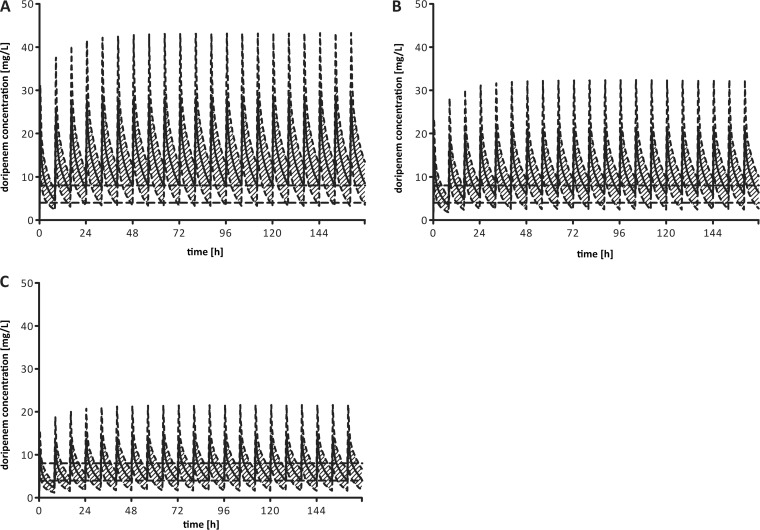
The simulations conducted show that the proposed dose level of 1,000 mg every 8 h is superior to lower doses for reaching the desired plasma doripenem concentration of 8 mg/liter. Although mean trough concentrations in all dosing regimens exceeded 4 mg/liter during steady state, the individual outcomes were highly variable (Fig. 2).
FIG 2.
Simulated concentration-time profiles (means ± standard deviations) based on 1,000 hypothetical patients receiving a doripenem dose of 1,000 mg/8 h (A), 750 mg/8 h (B), or 500 mg/8 h (C) continuously over 7 days. The horizontal dashed lines represent the desired concentrations of 4 mg/liter and 8 mg/liter in plasma.
A dose of 500 mg/8 h seems insufficient for the treatment of patients infected with pathogens with MICs above 0.5 to 1 mg/liter. Only 39.5% of the simulated patients showed trough concentrations that were constantly above the lower threshold (4 mg/liter) during steady state. Increasing the dose to 750 mg/8 h led to more-consistent results. Still, plasma doripenem concentrations systematically fell below the minimum value in 32% of the simulated patients. To treat organisms that require doripenem levels above 8 mg/liter for at least 60 to 80% of the dosing interval, 1,000 mg/8 h seems to be the most appropriate choice. Simulations show that mean trough levels above 8 mg/liter can be sustained with this dosing regimen. However, due to the high interindividual variability, plasma doripenem concentrations still fell below 8 mg/liter, but not lower than 4 mg/liter, for 60.5% of the simulated patients. Furthermore, concentration drops below 4 mg/liter during steady state could be observed in 15.2% of the cases.
DISCUSSION
The mean hemofilter clearance rates observed in our trial slightly exceeded those reported previously by Cirillo et al. and Roberts et al. (3, 4). Like Roberts et al., we found increased total-body clearance and volume of distribution in comparison to the values reported by Cirillo et al. (3, 4). The sieving coefficients observed in our study differed dramatically from those reported by Cirillo et al. A possible explanation for the difference between prefilter/postfilter clearance and sieving coefficient-dependent clearance could be adsorption of doripenem to the filter membrane.
The prefilter/postfilter clearance values found in our trial are within the range of values reported previously for imipenem (36 ± 13 ml/min with CVVH and 57 ± 32 ml/min with CVVHDF) and meropenem (49 ± 8.3 ml/min with CVVH), although the membrane sizes in our trial were considerably larger than those in the previous studies (12, 13). Our data show an uncharacteristically low clearance for CVVHDF patients, which may be attributed to the larger membrane size and higher membrane kUF employed for the CVVHF and CVVHD groups. Additionally, the dialysate flow rate (mean values, 2,000 ±0 ml/h [n = 5] for CVVHD and 980 ± 40 ml/h [n = 5] for CVVHDF [P < 0.001]) and the substitution rate (mean values, 2,600 ± 374 ml/h [n = 3] for CVVH and 878 ±500 ml/h [n = 5] for CVVHDF [P = 0.0069]) differed significantly between the CVVHDF group and the other groups. When the ultrafiltration rate, dialysate flow rate, and substitution rate are summed, the differences between the CVVH and CVVHDF groups are reduced to a trend—mainly due to the large standard deviation within the CVVHDF group—while the differences between the CVVH and CVVHD groups continue to be statistically significant (values, 2,792 ± 398 ml/h [n = 3] for CVVH, 2,095 ± 90 ml/h [n = 5] for CVVHD, and 1,927 ± 499 ml/h [n = 5] for CVVHDF; P, 0.017 for CVVH versus CVVHD, 0.53 for CVVHD versus CVVHDF, and 0.069 for CVVHDF versus CVVH). Together with the membranes used, these flow rate differences may help to explain the clearance values found. We emphasize this observation, because the usual expectation for beta-lactam antimicrobials would be quite the opposite: CVVHDF clearance values should be higher than CVVHD or CVVH clearance values. However, this is true only if the same flow rates and membrane materials are chosen. Since we allowed physicians to perform cRRT as they deemed fit, our findings show that if intensive care physicians choose different membranes and flow rates for different cRRT techniques, as is common practice, clearance values may differ to large extents from the values predicted in the literature. This trial, however, is limited by the limited number of subjects available in each group. Given the standard deviations observed, differences in clearance values between the three techniques employed may be overestimated in the current work.
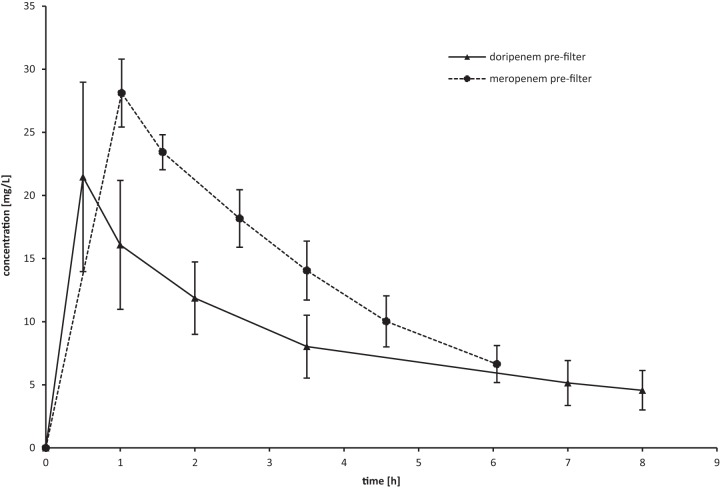
The mean doripenem Cmax (maximum concentration in serum) of 24.7 mg/ml and Cmin 8 h (minimum concentration in serum at 8 h) of 4.6 mg/liter during the first dosing interval were comparable to the mean Cmax of 28.1 mg/liter and Cmin 6 h of 6.6 mg/liter reported previously for a single 1-g dose of meropenem (Fig. 3), while no literature with comparable dosing is available for imipenem (13). Although on the basis of the mean peak values shown in Fig. 1, an accumulation of doripenem may be suspected, Monte Carlo simulations showed no evidence of doripenem accumulation after a steady state was reached (after the first four administrations) with a 1-g q8h regimen (see Fig. 2).
FIG 3.
Comparison of mean prefilter carbapenem concentrations during the first dosing interval (1 g doripenem versus 1 g meropenem) during continuous renal replacement therapy (13).
Given the fact that doripenem may be chosen for its enhanced killing of Pseudomonas spp., which are considered susceptible to doripenem up to a MIC of 2 mg/liter, and the fact that for optimal killing, beta-lactam antimicrobials should be dosed at four times the MIC for the targeted organism for at least 60 to 80% of the dosing interval, a doripenem concentration of 8 mg/liter for 80% of the dosing interval seems desirable (14, 15). This level is reached for 57.8% of all simulated patients following a 1-g q8h regimen, while only 12.9% of all simulated patients following a 500-mg q8h regimen reach this goal. The minimal PK target of 4 mg/liter for 60% of the dosing interval is reached by 73.2% of all patients receiving 500 mg doripenem q8h and 97.8% of all patients on a 1-g q8h dose.
Based on the assumption that doripenem will be used for critically ill patients, early attainment of PK goals to improve treatment efficacy seems desirable (9, 16, 17). If early killing of Pseudomonas spp. is intended, levels above 4 times the MIC for 100% of the first dosing interval should be reached (9, 10). If the 1-g q8h regimen investigated is followed, 39.5% of the patients will reach a trough level of 8 mg/liter at the end of each dosing interval. To attain sufficient doripenem exposure during the first dosing interval, we suggest the administration of an initial “loading dose” of 20.4 mg/kg of body weight, which is equal to 1.5 g doripenem for patients with a body weight around 75 kg and 2 g doripenem for patients with a body weight above 100 kg. These values were calculated from the median observed apparent volume of distribution of 0.62 liter/kg and the steady-state kinetics, where a mean peak value of 32.9 mg/liter (±12.1 mg/liter) (n = 36) and a mean trough of 8.8 mg/liter (±3.0 mg/liter) (n = 34) is reached. The correction for body weight is based on the observation that in our trial, the steady-state peak levels correlated inversely with the body weight of the patients and on previous reports that a higher BMI may require higher beta-lactam doses, as shown by Chen et al. for ertapenem (18, 19).
When the pharmacokinetic parameters found in previous trials are compared (Table 5), great variation in the values can be observed, with a trend to longer half-lives for doripenem (13, 15). cRRT modalities differ widely, making dosage recommendations difficult. In our view, the broad therapeutic index of beta-lactams favors higher dosing, especially for ICU patients, providing a safety margin for more-effective RRT modalities and thus benefiting the critically ill patient.
TABLE 5.
Pharmacokinetic parameters of carbapenems reported in previous trials
| Carbapenem and publication | Cltot (liters/h) |
V |
t1/2β (h) | Cmax 0–8h (mg/liter) | Ctrough (mg/liter) | ClcRRT (liters/h) | |
|---|---|---|---|---|---|---|---|
| Total (liters) | Corrected for body wt (liters/kg) | ||||||
| Meropenem | |||||||
| Valtonen et al. (20) | 4.8–7.5 | 3.27–4.72 | |||||
| Thalhammer et al. (13) | 8.6 ± 1.2 | 29.5 ± 2.7 | 0.36 ± 0.1 | 2.3 ± 0.4 | 28.1 ± 2.5 | 6.6 ± 1.5 | 2.98 ± 0.5 |
| Isla et al. (21) | 9.0–63.9 | 0.4–1.3 | 1.5–3.7 | 30.4–43.3 | 1.0–7.7 | 1.0–1.9 | |
| Imipenem | |||||||
| Afshartous et al (15) | 5.3 ± 0.8 | 33.1 | 1.9–2.1 | ||||
| Fish et al. (12) | 8.7–10.7 | 35.2 ± 15.2 | 0.37 ± 0.10 | 1.5–2.0 | 15.6–17.8 | 1.1–1.4 | 2.2–3.4 |
| Doripenem | |||||||
| Cirillo et al. (3) | 4.9–6.0 | 28.2–29.6 | 0.297–0.343 | 3.87–4.24 | 18.9–24.1 | 1.3–1.5 | |
| Roberts et al. (4) | 4.46 | 38.0 | 1.34–1.84 | ||||
ACKNOWLEDGMENTS
We thank the staff of the intensive care units of the General Hospital of Vienna for their continued support.
Funding Statement
This research received no specific grant from any funding agency in the commercial or not-for-profit sector. This work was supported by internal funding from the Medical University of Vienna and the General Hospital of Vienna. F. Thalhammer reports grants from Janssen-Cilag during the conduct of the study. All other authors report no conflicts of interest.
REFERENCES
- 1.Drzewiecki A, Bulanda M, Talaga K, Sodo A, Adamski P. 2012. Comparison of in vitro activity of doripenem, imipenem and meropenem against clinical isolates of Enterobacteriaceae, Pseudomonas and Acinetobacter in Southern Poland. Pol Przegl Chir 84:449–453. doi: 10.2478/v10035-012-0076-2. [DOI] [PubMed] [Google Scholar]
- 2.Hojabri Z, Ahangarzadeh Rezaee M, Nahaei MR, Soroush MH, Ghojazadeh M, Pirzadeh T, Davodi M, Ghazi M, Bigverdi R, Pajand O, Aghazadeh M. 2013. Comparison of in vitro activity of doripenem versus old carbapenems against Pseudomonas aeruginosa clinical isolates from both CF and burn patients. Adv Pharm Bull 3:121–125. doi: 10.5681/apb.2013.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirillo I, Vaccaro N, Balis D, Redman R, Matzke GR. 2011. Influence of continuous venovenous hemofiltration and continuous venovenous hemodiafiltration on the disposition of doripenem. Antimicrob Agents Chemother 55:1187–1193. doi: 10.1128/AAC.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts JA, Udy AA, Bulitta JB, Stuart J, Jarrett P, Starr T, Lassig-Smith M, Roberts NA, Dunlop R, Hayashi Y, Wallis SC, Lipman J. 2014. Doripenem population pharmacokinetics and dosing requirements for critically ill patients receiving continuous venovenous haemodiafiltration. J Antimicrob Chemother 69:2508–2516. doi: 10.1093/jac/dku177. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency. 22 June 2012. European Medicines Agency advises doctors treating patients with nosocomial pneumonia with Doribax. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 6.Thalhammer F, Schmaldienst S, Elmenyawi I, Atteneder M, Burgmann H, Hollenstein U, Georgopoulos A, Graninger W, Putz D, Rosenkranz AR, Mayer G, Hörl WH, Breyer S. 1996. Multiple-dose pharmacokinetics of cefpirome in long-term hemodialysis with high-flux membranes. Clin Pharmacol Ther 60:645–650. doi: 10.1016/S0009-9236(96)90212-X. [DOI] [PubMed] [Google Scholar]
- 7.Meyer B, Ahmed el Gendy S, Delle Karth G, Locker GJ, Heinz G, Jaeger W, Thalhammer F. 2003. How to calculate clearance of highly protein-bound drugs during continuous venovenous hemofiltration demonstrated with flucloxacillin. Kidney Blood Press Res 26:135–140. doi: 10.1159/000070997. [DOI] [PubMed] [Google Scholar]
- 8.Weiler S, Seger C, Pfisterer H, Stienecke E, Stippler F, Welte R, Joannidis M, Griesmacher A, Bellmann R. 2013. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother 57:4053–4057. doi: 10.1128/AAC.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taccone FS, Laterre P-F, Dugernier T, Spapen H, Delattre I, Wittebole X, Backer DD, Layeux B, Wallemacq P, Vincent J-L, Jacobs F. 2010. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14:R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalda-Molina R, Dokoumetzidis A, Charkoftaki G, Dimaraki E, Margetis K, Archontaki H, Markantonis S, Boutos N, Sakas D, Vryonis E, Skoutelis A, Valsami G. 2012. Pharmacokinetics of doripenem in CSF of patients with non-inflamed meninges. J Antimicrob Chemother 67:1722–1729. doi: 10.1093/jac/dks106. [DOI] [PubMed] [Google Scholar]
- 12.Fish DN, Teitelbaum I, Abraham E. 2005. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 49:2421–2428. doi: 10.1128/AAC.49.6.2421-2428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thalhammer F, Schenk P, Burgmann H, El Menyawi I, Hollenstein UM, Rosenkranz AR, Sunder-Plassmann G, Breyer S, Ratheiser K. 1998. Single-dose pharmacokinetics of meropenem during continuous venovenous hemofiltration. Antimicrob Agents Chemother 42:2417–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. 2014. Breakpoint tables for interpretation of MICs and zone diameters, version 4.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_4.0.pdf.
- 15.Afshartous D, Bauer SR, Connor MJ, Aduroja OA, Amde M, Salem C, Groszek JJ, Fissell WH. 2014. Pharmacokinetics and pharmacodynamics of imipenem and meropenem in critically ill patients treated with continuous venovenous hemodialysis. Am J Kidney Dis 63:170–171. doi: 10.1053/j.ajkd.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie C. 2011. Antibiotic dosing in critical illness. J Antimicrob Chemother 66(Suppl 2):ii25–ii31. doi: 10.1093/jac/dkq516. [DOI] [PubMed] [Google Scholar]
- 17.Pinder M, Bellomo R, Lipman J. 2002. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care 30:134–144. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS. 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob Agents Chemother 50:1222–1227. doi: 10.1128/AAC.50.4.1222-1227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley MJ, Abernethy DR, Greenblatt DJ. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Valtonen M, Tiula E, Backman JT, Neuvonen PJ. 2000. Elimination of meropenem during continuous veno-venous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother 45:701–704. doi: 10.1093/jac/45.5.701. [DOI] [PubMed] [Google Scholar]
- 21.Isla A, Maynar J, Sánchez-Izquierdo JA, Gascón AR, Arzuaga A, Corral E, Pedraz JL. 2005. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol 45:1294–1304. doi: 10.1177/0091270005280583. [DOI] [PubMed] [Google Scholar]