Abstract
Colistin is increasingly used as a last option for the treatment of severe infections due to Gram-negative bacteria in critically ill patients requiring intermittent hemodialysis (HD) for acute renal failure. Our objective was to characterize the pharmacokinetics (PK) of colistin and its prodrug colistin methanesulfonate (CMS) in this population and to suggest dosing regimen recommendations. Eight intensive care unit (ICU) patients who were under intermittent HD and who were treated by CMS (Colimycine) were included. Blood samples were collected between two consecutive HD sessions. CMS and colistin concentrations were measured by a specific chromatographic assay and were analyzed using a PK population approach (Monolix software). Monte Carlo simulations were conducted to predict the probability of target attainment (PTA). CMS nonrenal clearance was increased in ICU-HD patients. Compared with that of ICU patients included in the same clinical trial but with preserved renal function, colistin exposure was increased by 3-fold in ICU-HD patients. This is probably because a greater fraction of the CMS converted into colistin. To maintain colistin plasma concentrations high enough (>3 mg/liter) for high PTA values (area under the concentration-time curve for the free, unbound fraction of a drug [fAUC]/MIC of >10 and fAUC/MIC of >50 for systemic and lung infections, respectively), at least for MICs lower than 1.5 mg/liter (nonpulmonary infection) or 0.5 mg/liter (pulmonary infection), the dosing regimen of CMS should be 1.5 million international units (MIU) twice daily on non-HD days. HD should be conducted at the end of a dosing interval, and a supplemental dose of 1.5 MIU should be administered after the HD session (i.e., total of 4.5 MIU for HD days). This study has confirmed and complemented previously published data and suggests an a priori clear and easy to follow dosing strategy for CMS in ICU-HD patients.
INTRODUCTION
Over the last several years, colistin (polymyxin E) has been increasingly used as a last option for the treatment of infections caused by multidrug-resistant Gram-negative bacteria, such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae (1, 2), for which the mortality rate has increased (3).
Colistin is administered as a prodrug, colistin methanesulfonate (CMS), which is mostly excreted unchanged in urine (70%) and is partly converted to colistin (30% at most), whereas renal excretion of colistin is negligible (4). As a result, in patients with renal failure, a greater fraction of the CMS dose may be converted into colistin (5); therefore, all other things being equal, colistin plasma concentrations at steady state should increase. The elimination of colistin is nonrenal—it undergoes extensive renal tubular reabsorption—and nonbiliary by unknown mechanism (1).
It has recently been shown that in intensive care unit (ICU) patients, colistin plasma concentrations at steady state are mostly governed by renal function and that creatinine clearance can be used for dosing regimen adaptation (5, 6). Studies have also been conducted in critical care patients with acute renal failure requiring intermittent hemodialysis (HD) (5, 7–9). However, focus was placed on CMS and colistin clearance during HD rather than between two consecutive HD sessions. CMS is produced by fermentation that results in a mixture of approximately 30 compounds, which may be partially converted into intermediates before administration and which explains that CMS and colistin pharmacokinetics (PK) may vary with the CMS brand (5, 6, 10). Therefore, the aim of this study was to characterize CMS and colistin PK in ICU patients with end-stage renal failure and to compare these results with those previously obtained from ICU patients with preserved renal function (6) and from healthy volunteers (4) receiving the same CMS brand.
MATERIALS AND METHODS
Study population.
This population PK study was conducted in two sites: University Hospital of Poitiers, France, and Hôpital Lariboisière, Paris, France. The study protocol was approved by the local ethics committee (CPP Ouest III, approval number 09.02.01) and by French national authorities (National Security Agency of Medicines and Health Products [ANSM] number 2009-009578-28). Informed consent was obtained from all patients or their relatives. Patients were eligible for enrollment in the study if they were between 18 and 85 years of age, receiving CMS as part of their treatment according to dosage regimens freely chosen by physicians, and under intermittent HD for acute renal failure. Patients were not eligible if they had received colistin for 7 days prior to the study. At study onset, the following data were collected: age, sex, weight, diagnosis on admission, serum urea, serum creatinine, and simplified acute physiology score (SAPS II).
Hemodialysis.
Intermittent hemodialysis sessions (Gambro AK 200) were typically performed over 4 h every 2 days. Hemodialysis conditions were standard with the blood flow setting at 300 ml/min and dialysis effluent at 500 ml/min, using a 1.6 m2 B3 polymethylmethacrylate membrane (Toray industries, Tokyo, Japan).
CMS administration.
Each patient received CMS (Colimycine; Sanofi-Aventis, Paris, France) as a 1-h infusion every 8 h according to dosage regimens freely chosen by physicians. The solutions were prepared just before administration by dissolution of CMS in 50 ml of saline solution. Some patients also received CMS as an aerosol, after dissolution in 10 ml of saline, and nebulization over 30 min with a vibrating-mesh nebulizer (Aeroneb Pro; Aerogen, Galway, France).
Sample collection.
Venous blood samples (n = 90) were collected between hemodialysis sessions. Blood samples were collected immediately before and at 0.5, 1, 2, 3, and 8 h after the beginning of the first infusion and at various times following consecutive administrations. Samples were immediately centrifuged (3,000 × g for 10 min) at 4°C, and plasma was stored at −20°C until analysis.
Determination of CMS and colistin concentrations in plasma.
CMS and colistin concentrations were measured as previously described (4, 6, 7, 11) with a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay with a limit of quantification of 0.04 mg/liter for CMS and colistin.
Population PK modeling.
CMS and colistin plasma concentrations were analyzed simultaneously using a nonlinear mixed-effect model, with Monolix version 4.3.2 (Lixoft). A previously reported PK model (6, 12) was fitted to the data. CMS pharmacokinetics was described using a one-compartment model. CMS renal clearance (CLRCMS) was fixed at 0, i.e., the fraction of CMS excreted unchanged in urine was supposed to be equal to zero, whereas clearance equal to nonrenal clearance (CLNRCMS) and volume of distribution (VCMS) were estimated. A one-compartment model was also used for colistin pharmacokinetics, but only apparent volume of distribution (Vcol/fm) and apparent total clearance (CLcol/fm) can be estimated, where fm corresponds to the unknown fraction of CMS nonrenally cleared that was eventually converted into colistin (5). The clearances of CMS and colistin due to hemodialysis sessions were set to values previously determined experimentally with the same brand of CMS and the same hemodialysis apparatus, i.e., a CLHD,CMS of 90 ml/min and a CLHD,COL of 137 ml/min (7). The contribution of aerosol cotreatment on plasma CMS and colistin concentrations was taken into consideration by fixing the relevant pharmacokinetic parameter values previously estimated as independent estimates (12). Considering this model for aerosol administration of CMS, about 9% of the dose reached the systemic compartments, of which 1.4% was presystemically transformed into colistin. The typical PK parameters of the population and the interindividual variability (IIV) (assuming a log-normal distribution) were estimated. The between-occasion variability was not estimated because of the lack of data. The residual variability was modeled as combined (additive and proportional) for CMS and colistin plasma concentrations (6). Differences of objective function values (OFV) were used to discriminate between different models, with a reduction of at least 10.83 (corresponding to a P value of <0.001 for 1 degree of freedom) required to choose the more complex model. Because the number of HD patients was limited (n = 8), a full exploration of the correlation between PK parameters and covariates was not conducted. Only the covariates previously detected for ICU patients from the same clinical study but who did not require HD were tested (6). Covariate model building was performed in a stepwise fashion with forward inclusion (P < 0.05) and backward deletion (P < 0.01). The covariates evaluated included body weight, age, creatinine clearance, serum urea concentration, and temperature. Plasma concentrations below the limit of quantification were handled by the Beal M3 method (13). (The control stream for the final model can be found in the supplemental material.) Model performance was assessed by visual inspection of diagnostic plots (normalized prediction distribution errors [NPDE] had to be normally distributed with a mean of 0 and a standard deviation of 1), the evaluation of the residual error, and the precision of parameter estimates.
Simulations.
Three types of simulations were conducted.
First, CMS and colistin PK were compared between ICU patients requiring intermittent HD, ICU patients with preserved renal function, and virtual ICU patients with the same PK as the latter but with no renal clearance of CMS. CMS and colistin concentrations were simulated after a single 3-million-international-unit (MIU) dose of CMS in the following 3 types of patients over a 48-h period without HD session: a typical ICU-HD patient using PK parameter values estimated in the present study, a typical ICU patient with preserved renal function (ICU-85 patient) using PK parameter values previously estimated by us for creatinine clearance (CLCR) equal to 85 ml/min (and therefore a CLRCMS of 68.5 ml/min) (6), and a virtual ICU patient (ICU-00 patient) with the same PK parameter values as those in the previous group (6) (except for CLCR) and therefore a CMS renal clearance fixed at zero (CLCR = CLRCMS = 0 ml/min).
Second, the effect of HD sessions on colistin plasma concentrations was assessed by simulating 4-h HD sessions with different values of CMS and colistin HD clearances reported in the literature, i.e., 94.8, 90, and 94.8 ml/min for CMS and 56.7, 137, and 66.5 ml/min for colistin, respectively (5, 7, 8). For this simulation, PK parameter values—except HD clearances—were fixed to estimates of the present analysis, and the dosage regimen consisted of repeated administrations of CMS at 1.5 MIU every 12 h (q12h) and a supplemental reloading dose of 1.5 MIU administered along with the scheduled 1.5-MIU dose just after the HD session.
Third, the probability of target attainment (PTA) with the usual recommended dosage regimen was assessed. A 1,000-patient Monte Carlo simulation was carried out using Berkeley Madonna (version 8.3.18; University of California) to evaluate the PTA in a typical ICU-HD patient with twice-daily administrations of 1.5 MIU of CMS. The pharmacokinetic/pharmacodynamic (PK/PD) target was defined as a free area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC of >15 (nonpulmonary infection) or 50 (pulmonary infection) (14) assuming 50% protein binding (15). PTAs were evaluated for each dosing regimen across a range of MICs from 0.125 to 2 mg/liter for 24-h periods of time including no HD sessions.
RESULTS
A total of 2 women and 6 men were enrolled. Their demographic, clinical, and biological data are summarized in Table 1. The median first dose was 1.5 MIU (range from 0.4 to 9 MIU), and the median maintenance dose was 0.5 MIU every 8 h (q8h) (0.4 to 2 MIU q8h) (1 MIU is equivalent to 80 mg of CMS sodium) (16). Seventeen out of 24 PK were assessed on a day without HD, and 7 PK parameters were assessed on a day with HD (4 before and 3 after the HD session). Four patients received CMS as an aerosol—one of them received a single aerosol of 2 MIU 8 h before the intravenous (i.v.) infusion with PK assessment and the three others received 1 or 1.5 MIU of CMS as multiple aerosol treatments administered every 8 h. The delay between aerosol and the start of i.v. infusion for PK assessment was variable (range, 0 to 6 h). Measured plasma concentrations of CMS and colistin are shown on Fig. 1. Twelve CMS concentrations and 9 colistin concentrations were below the limit of quantification. Goodness of data fit plots were satisfactory with unbiased individual fits (not shown). NPDE values were <2, and no obvious bias was observed versus time (Fig. 2). No covariate was included in the model due to nonsignificant decreases of the objective function values. The residual variability (Table 2) was moderate for CMS plasma concentrations (48% proportional and 0.11 mg/liter additive) and low for colistin plasma concentrations (15% proportional and 0.13 mg/liter additive). (Visual predictive check and observed-versus-predicted concentrations for CMS and colistin can be found in the supplemental material.)
TABLE 1.
Demographic and clinical characteristics of ICU-HD patientsa
| ID no. | Genderb | Age (yr) | Weight (kg) | Serum creatinine (μmol/liter)c | SAPS II score | Loading dose (MIU) | Maintenance dose (MIU q8h) | Receiving aerosol |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 71 | 83 | 172 | 60 | 2 | 0.5 | Yes |
| 2 | M | 62 | 100 | 470 | 49 | 2 | 2 | No |
| 3 | M | 36 | 58 | 304 | 39 | 1 | 1 | No |
| 4 | M | 39 | 88 | 375 | 63 | 0.5 | 0.5 | No |
| 5 | F | 82 | 80 | 329 | 70 | 0.4 | 0.4 | Yes |
| 6 | M | 73 | 80 | 223 | 55 | 9 | 0.5 | Yes |
| 7 | F | 63 | 52 | 296 | 75 | 0.8 | 0.5 | Yes |
| 8 | M | 66 | 75 | 315 | 42 | 2 | 2 | No |
| Median | 65 | 80 | 310 | 58 | 1.5 | 0.5 |
Table provides values at baseline.
M, male; F, female.
Eighty-four micromoles per liter of serum creatinine corresponds to 1 mg/dl.
FIG 1.
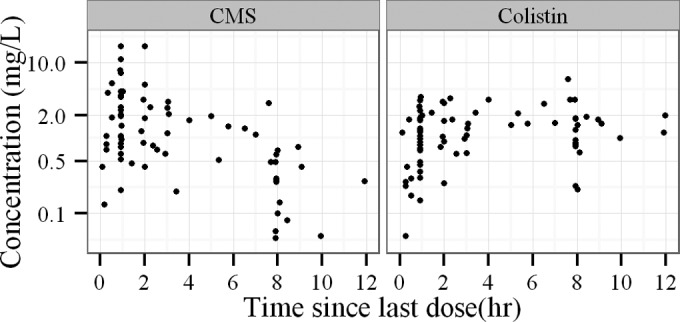
Plasma concentrations of CMS (left) and colistin (right) measured in ICU-HD patients. Times are relative to the last dose.
FIG 2.
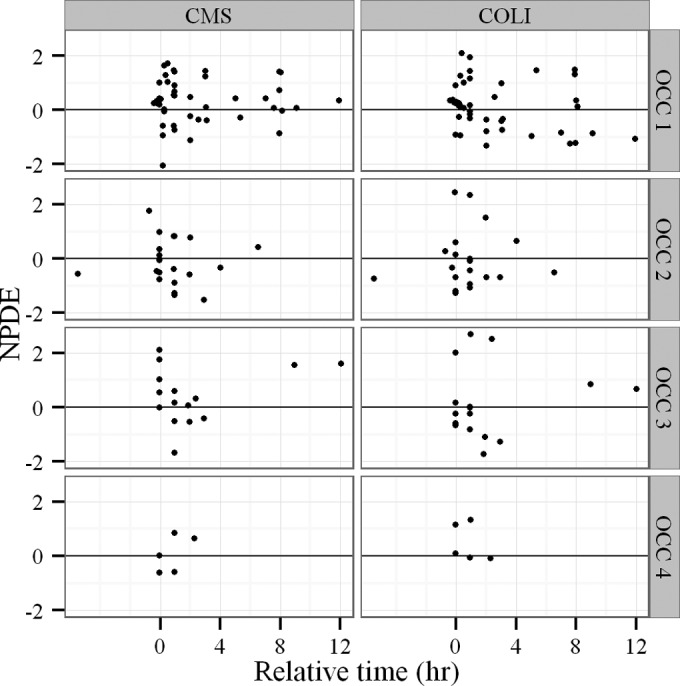
Normalized prediction distribution errors (NPDE) as a function of time from previous administration for CMS (left) and colistin (COLI) (right) plasma concentrations. Results are presented for each visit with a PK assessment (OCC, occasion).
TABLE 2.
Population pharmacokinetic parameters of ICU-HD patients compared with those of ICU-85 patients (6)
| Parameter | Volume of distribution or clearance | ICU-HD patients |
ICU-85 (6)a |
||||
|---|---|---|---|---|---|---|---|
| Typical value (% RSE) | IIV, % CVb (RSE, %) | Residual Errors |
Typical value (% RSE) | IIV, % CV (% RSE) | |||
| Proportional % CV (% RSE) | Additive (mg/liter) (% RSE) | ||||||
| CMS | |||||||
| VCMS (liters) | Volume of distribution of CMS | 21 (13) | 24 (45) | 48 (12) | 0.11 (28) | 15.7 (7) | 44 (14) |
| CLRCMS (ml/min) | Renal clearance of CMS | 0 (fixed) | 0 (fixed) | 68.5 (12) | 72 (11) | ||
| CLNRCMS (ml/min) | Nonrenal clearance of CMS | 113 (14) | 31 (35) | 43.7 (11) | 42 (18) | ||
| Colistin | |||||||
| Vcol/fm (L) | Apparent volume of distribution of colistin | 28.3 (18) | 42 (36) | 15 (24) | 0.13 (27) | 10.2 (16) | 81 (15) |
| CLcol/fm (ml/min) | Apparent clearance of colistin | 33.3 (16) | 42 (29) | 37.7 (10) | 37 (15) | ||
| Renal replacement | |||||||
| CLHDCMS (ml/min) | Dialysis clearance of CMS | 90 (fixed) | |||||
| CLHDCOL (ml/min) | Dialysis clearance of colistin | 137 (fixed) | |||||
Typical value for a patient of 70 kg with a creatinine clearance of 85 ml/min.
CV, coefficient of variation.
Estimated PK parameter values in ICU-HD patients are presented in Table 2 and are compared with those obtained in ICU-85 patients (6). The typical CLNRCMS value in these ICU-HD patients was estimated at 113 ml/min, and the typical CMS volume of distribution was estimated at 21 liters, with a resulting elimination half-life equal to 2.1 h. Typical colistin apparent clearance (CLcol/fm) was estimated at 37.7 ml/min, and the typical apparent volume of distribution (Vcol/fm) was estimated at 28 liters with a corresponding colistin half-life equal to 9.8 h. The precision of the parameter estimates (expressed as relative standard error [RSE] in Table 2) was good (<45%).
Figure 3 shows CMS and colistin plasma concentration-time profiles simulated after a single dose of CMS (3 MIU) in the three predefined populations. The CMS plasma concentration profiles simulated in a typical ICU-HD patient and in a typical ICU-85 patient were virtually superimposed, and accordingly, corresponding area under the concentration-time curve from 0 h to infinity (AUC0–∞) values were almost identical (33.0 mg · h/liter and 33.25 mg · h/liter, respectively) (Fig. 3, left). However, CMS systemic exposure was noticeably increased by almost 2.5-fold in the virtual ICU-00 patient with a corresponding AUC0–∞ value of 85.4 mg · h/liter (Fig. 3, left).
FIG 3.
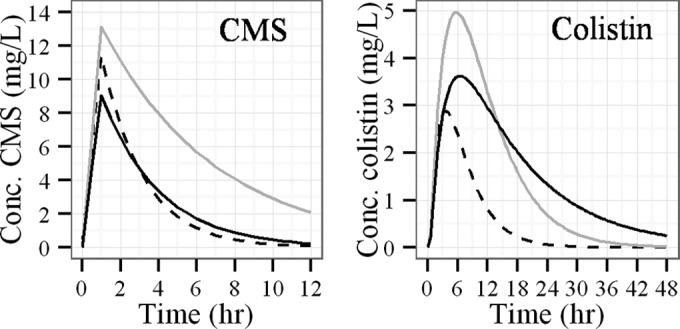
Plasma concentration-time profiles of CMS (left) and colistin (right) after single-dose administration of CMS (3 MIU), predicted from PK parameter values corresponding to a typical ICU-HD patient (black full line), a typical ICU-85 patient (black dashed line), and a virtual ICU-00 patient (gray full line).
Different observations were made for colistin. In particular, the predicted AUC0–∞ value in a typical ICU-HD patient (79.4 mg · h/liter) was about three times higher than that in a typical ICU-85 patient (27.6 mg · h/liter) and was close to the value simulated for the virtual ICU-00 individual (70.8 mg · h/liter). Although colistin exposure was comparable in ICU-HD and in ICU-00 patients, concentration profiles differed with a lower maximum concentration of drug in plasma (Cmax) in HD patients (3.6 versus 4.9 mg/liter). This is likely due to a larger volume of distribution, which also leads to a longer half-life (Fig. 3, right).
Simulations suggest that after multiple administrations of CMS at 1.5 MIU q12h in an ICU-HD patient, plasma colistin concentrations at steady state should fluctuate between 3 and 4 mg/liter. However, at the end of HD sessions, colistin concentrations drop to 1 to 1.5 mg/liter depending on the HD clearance used for simulation. Administration of a 3-MIU reloading dose of CMS instead of the regular 1.5 MIU dose after each HD session reduces the time necessary to return to steady state (Fig. 4). However, with these colistin concentration profiles, a high PTA should be obtained for bacteria with an MIC of ≤1.5 mg/liter for nonpulmonary infections and for bacteria with an MIC of ≤0.5 mg/liter for pulmonary infections (Fig. 5).
FIG 4.
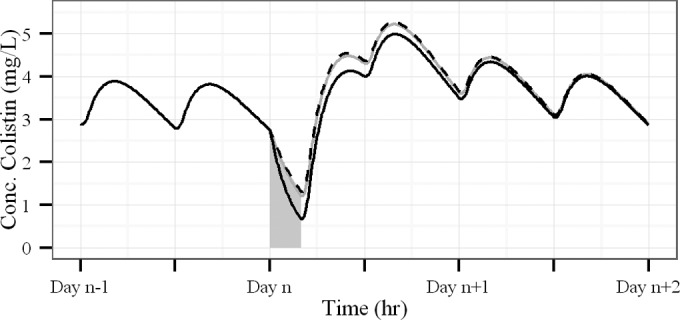
Simulations of colistin plasma concentration-time profiles in ICU-HD patients receiving a 4-hour HD session on day n and dosed with 1.5 MIU q12h of CMS. The CMS dose planned before the HD session was postponed to immediately after the session and was 3 MIU. Curves were simulated with different values of CMS and colistin HD clearances reported in the literature, i.e., 94.8, 90, and 94.8 ml/min for CMS and 56.7, 137, and 66.5 ml/min for colistin (solid grey line, solid black line, and dashed black line), respectively (5, 7, 8). PK parameter values—except HD clearances—were fixed to estimates of the present analysis.
FIG 5.
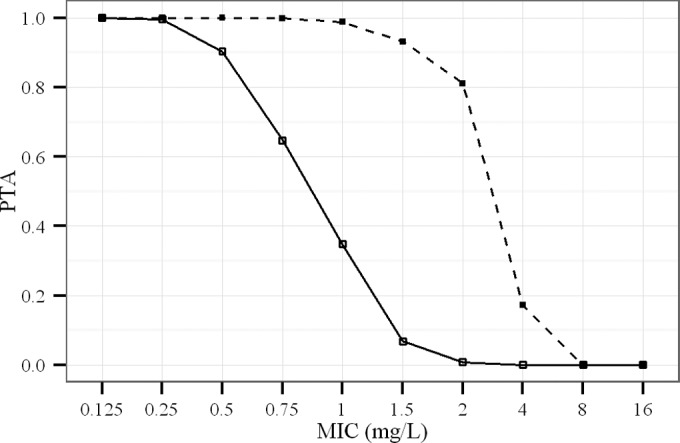
Probability of target attainment (PTA) of colistin in ICU-HD patients dosed with 1.5 MIU q12h of CMS on days without HD for nonpulmonary (dashed line) and pulmonary (solid line) infections.
DISCUSSION
This study allowed comparisons between CMS and colistin PK in ICU patients with preserved renal function (ICU-85) and those with acute renal disease requiring intermittent HD (ICU-HD). However, in order to evaluate whether ICU-85 and ICU-HD patients present the same CMS and colistin PK parameter values (except for CMS renal excretion), simulations were also carried out in virtual patients (ICU-00) using the PK parameters of ICU-85 patients (6), except for a CLRCMS value set to 0 ml/min. This will be discussed first, and the effect of HD on CMS and colistin PK will then be considered to provide practical dosing regimen recommendations in ICU-HD patients.
As can be seen in Fig. 3, the AUC of CMS is 2-fold lower for ICU-HD patients than that for ICU-00 patients, which means that ICU-HD patients cannot be considered as just ICU-85 patients without renal clearance of CMS. The CMS volume of distribution was moderately increased in ICU-HD patients compared with that in ICU-85 patients (Table 2), but, surprisingly, total clearance in ICU-HD patients (typical value, 113 ml/min) was virtually the same as that in ICU-85 patients (typical value, 112 ml/min). This result suggests that CMS nonrenal clearance (CLNRCMS) is increased by approximately 2-fold (from 43.7 ml/min to 113 ml/min) in HD patients, which was not seen in previous studies (5, 8). Accordingly, CMS concentrations versus time profiles in typical ICU-HD and ICU-85 patients are virtually superimposed (Fig. 3, left). Yet, because the exact mechanism of CMS nonrenal clearance remains unknown, it is difficult to assess the origin of this increased CLNRCMS in ICU-HD patients. Noticeably, because CMS PK parameters were estimated between HD sessions, artifacts such as drug adsorption onto HD membranes must be ruled out. Other methodological issues seem unlikely since the same procedures were used by the same group across these various studies (4, 6, 7). Among the various potential explanations, it can be hypothesized that endogenous substances that accumulate in plasma between HD sessions perhaps increase the hydrolysis of CMS occurring at a physiologic pH and eventually lead to colistin (17, 18). However, it should also be noted that measured CMS concentrations correspond to the sum of various methanesulfonate derivative intermediates (i.e., all five or only a part of the primary amine group of colistin is methanesulfonated in CMS) and are eventually converted into colistin (19). Therefore, CMS PK parameters, including CLNRCMS, correspond to apparent parameters that must be carefully interpreted.
More importantly for dosing regimen optimization, it should be noted that colistin exposure, or average colistin concentration at steady state, is determined by its rate of formation and its rate of elimination. The rate of formation depends on the fraction of the CMS dose that is eventually converted into colistin and not on CLNRCMS. In ICU patients with acute renal failure, the renal excretion of CMS between HD sessions is very low. As a consequence, most CMS is available for colistin formation (1 − fe = 100%), whereas for ICU patients with preserved renal function, about 61% of CMS is excreted unchanged in urine (1 − fe = 39%) (6). The estimation of the apparent clearance of colistin in ICU-HD patients (CLcol/fm, 33.3 ml/min) was comparable to that previously estimated in ICU-85 patients (CLcol/fm, 37.7 ml/min) in the same conditions. Therefore, the almost 3 times greater AUC of colistin in ICU-HD patients compared with that in ICU-85 patients (Fig. 3) is very likely due to the abolished CMS renal excretion in ICU-HD patients. Colistin elimination half-life is about 3 times longer in ICU-HD patients than in ICU-85 patients (9.8 h versus 3.1 h), but this is apparently mostly due to an increased volume of distribution (Table 2) of several potential origins that need to be explored but among which might be a decreased binding on alpha-1-acid glycoprotein (15, 20) or a disease-related increased distribution within tissues, e.g., increased vascular permeability due to sepsis, edema formation, or fluid administration (21).
A CMS dosing regimen in ICU-HD patients must take into account the consequences of renal failure on colistin formation and elimination but also on CMS and colistin removal during HD. Although this specific aspect was not investigated during the present study, we have previously shown that CMS and colistin were efficiently cleared during HD (7). Because HD clearance may vary with the type of HD system and membrane, we have used CMS and colistin HD clearance values previously determined in a similar setting (7). Our estimate of CMS HD clearance (90 ml/min or 5.4 liters/h) is fully consistent with values reported by Garonzik et al. (5) and by Jitmuang et al. (8), and it predicts that 90% of the CMS is cleared during a 4-h HD session. However, our estimate of colistin HD clearance (137 ml/min or 8.22 liters/h) differs somewhat from values previously reported (5, 8). These differences may be due to different methods of determination (modeling, amount recovered in dialysate, or difference of concentrations in pre and postmembrane plasma samples) or different hemodialysis characteristics. From our estimate of colistin HD clearance, it can be estimated that 76% of the colistin present in the body is cleared during an HD session, whereas only approximately 55% is cleared according to colistin HD clearance estimates by Garonzik et al. (5) or Jitmuang et al. (8), which has few impacts on colistin concentration-time profiles (Fig. 4). Therefore, considering the current knowledge about CMS and colistin clearance during intermittent HD, it seems appropriate not to administer CMS before HD but rather immediately after HD sessions.
From our results, we have determined a dosing regimen that should allow typical colistin plasma concentrations of about 3 to 4 mg/liter (Fig. 4). Considering the large interindividual variability, higher dosing regimens might result in toxicities for many patients, and in this case, it is recommended to monitor plasma concentrations and toxicity signs. An appropriate maintenance dosing regimen may be 3 MIU/day on days without HD session (Fig. 4), which is comparable with the recommendations of Garonzik et al. (5). On days with an HD session, a post-HD reloading CMS dose may be 3 MIU, leading to 4.5 MIU administered on days with HD. This dosing regimen, which corresponds to a 50% increase in daily dose on days with HD compared with days without HD, is consistent with that of Garonzik et al., suggesting the addition of 30% to 50% to the daily maintenance on days with a HD session (5). However, because colistin PK reported for ICU patients vary between studies (5, 6, 10), particularly concerning the half-life of colistin (from 3 h to 14 h), the extrapolation of our recommendations to other populations/countries or brands should be considered with caution.
Two different targets were used for nonpulmonary infections (area under the concentration-time curve for the free, unbound fraction of a drug [fAUC]/MIC, >15) and for pulmonary infections (fAUC/MIC, >50) (14). The protein binding of colistin in patients was fixed at 50% as for ICU patients who do not require intermittent HD (15). The protein binding of drugs may actually be altered in patients with acute renal failure (21); however, consequences on the unbound fraction (fu) are much more likely for compounds presenting extensive protein binding. To our knowledge, there is no report that the fu of CMS or colistin is modified in patients with altered renal function. Furthermore, the hypothetical effect of acute renal failure on colistin protein binding should also be balanced by interpatient variability, which has not been documented yet. Therefore, the 50% value used for PTA calculations seems reasonable. PTA estimates suggest that by providing an MIC of <1.5 mg/liter for nonpulmonary infections and an MIC of <0.5 mg/liter for pulmonary infections, the proposed dosing regimen should provide satisfactory success rates. Among the pathogens most frequently encountered in the ICU, A. baumannii and P. aeruginosa have epidemiological cutoff values (ECOFF) for MICs of 2 and 4 mg/liter, respectively (http://mic.eucast.org/Eucast2/SearchController/search.jsp?action=init). The ECOFF for K. pneumoniae was not determined, with MICs greater than 4 mg/liter for 4% of the strains. Therefore, some pathogens might not be covered by the proposed regimen, particularly in the case of pulmonary infection. In this case, combinations, aerosol administration, or an increase of dose can be considered. As higher doses may result in toxic exposure, the appearance of renal and neurological toxicities should be particularly monitored.
In conclusion, an unexpected and difficult-to-explain increase of CMS nonrenal clearance between HD sessions, compensating for its abolished renal clearance, was evidenced for the first time in HD patients. Moreover, because in ICU patients with acute renal failure the renal excretion of CMS is abolished, more colistin is formed and plasma concentrations are increased. These findings were subsequently used to conduct PK/PD simulations, which show that a dosing regimen with 1.5 MIU of CMS given twice daily, except for a supplemental reloading dose of 1.5 MIU administered along with the scheduled 1.5-MIU dose just after the HD session (i.e., total of 4.5 MIU on HD days), would allow for the maintenance of colistin plasma concentrations between 3 and 4 mg/liter. These concentrations should be sufficient to obtain high PTA values, at least for bacteria with MICs of ≤1.5 mg/liter (fAUC/MIC, >15) for nonpulmonary infections and 0.5 mg/liter (fAUC/MIC, >50) for pulmonary infections, in the absence of combination with another antibiotic. This dosing regimen seems a priori to be the best initial choice for ICU-HD patients.
Supplementary Material
ACKNOWLEDGMENTS
M. Jacobs was supported by a doctoral fellowship from the University of Poitiers and the Conseil Régional de Poitou-Charentes.
We do not have any financial, commercial, or proprietary interest in any drug, device, or equipment mentioned in this article.
Funding Statement
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01868-15.
REFERENCES
- 1.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 2.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 3.Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. 2013. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect 66:401–414. doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 89:875–879. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 5.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregoire N, Mimoz O, Megarbane B, Comets E, Chatelier D, Lasocki S, Gauzit R, Balayn D, Gobin P, Marchand S, Couet W. 2014. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob Agents Chemother 58:7324–7330. doi: 10.1128/AAC.03508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchand S, Frat JP, Petitpas F, Lemaitre F, Gobin P, Robert R, Mimoz O, Couet W. 2010. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother 65:1836–1837. doi: 10.1093/jac/dkq185. [DOI] [PubMed] [Google Scholar]
- 8.Jitmuang A, Nation RL, Koomanachai P, Chen G, Lee HJ, Wasuwattakul S, Sritippayawan S, Li J, Thamlikitkul V, Landersdorfer CB. 2015. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: implications for dosing. J Antimicrob Chemother 70:1804–1811. [DOI] [PubMed] [Google Scholar]
- 9.Luque S, Sorli L, Li J, Collado S, Barbosa F, Berenguer N, Horcajada JP, Grau S. 2014. Effective removal of colistin methanesulphonate and formed colistin during intermittent haemodialysis in a patient infected by polymyxin-only-susceptible Pseudomonas aeruginosa. J Chemother 26:122–124. doi: 10.1179/1973947813Y.0000000104. [DOI] [PubMed] [Google Scholar]
- 10.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. 2010. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography tandem mass spectrometry. Antimicrob Agents Chemother 54:1941–1948. doi: 10.1128/AAC.01367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boisson M, Jacobs M, Gregoire N, Gobin P, Marchand S, Couet W, Mimoz O. 2014. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 58:7331–7339. doi: 10.1128/AAC.03510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/A:1012299115260. [DOI] [PubMed] [Google Scholar]
- 14.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate (CMS) in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2014. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis 58:139–141. doi: 10.1093/cid/cit680. [DOI] [PubMed] [Google Scholar]
- 17.Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. 2008. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother 52:3047–3051. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J. 2013. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68:2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azad MA, Huang JX, Cooper MA, Roberts KD, Thompson PE, Nation RL, Li J, Velkov T. 2012. Structure-activity relationships for the binding of polymyxins with human alpha-1-acid glycoprotein. Biochem Pharmacol 84:278–291. doi: 10.1016/j.bcp.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blot SI, Pea F, Lipman J. 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


