Abstract
This study examined the activity of the novel antimicrobial combination ceftazidime-avibactam against Enterobacteriaceae exhibiting different outer membrane permeability profiles, specifically with or without porins and with or without expression of the main efflux pump (AcrAB-TolC). The addition of the outer membrane permeabilizer polymyxin B nonapeptide increased the antibacterial activities of avibactam alone, ceftazidime alone, and ceftazidime-avibactam against the characterized clinical isolates of Escherichia coli, Enterobacter aerogenes, and Klebsiella pneumoniae. This enhancement of activities was mainly due to increased passive penetration of compounds since inhibition of efflux by the addition of phenylalanine-arginine β-naphthylamide affected the MICs minimally. OmpF (OmpK35) or OmpC (OmpK36) pores were not the major route by which avibactam crossed the outer membranes of E. coli and K. pneumoniae. In contrast, Omp35 and Omp36 allowed diffusion of avibactam across the outer membrane of E. aerogenes, although other diffusion channels for avibactam were also present in that species. It was clear that outer membrane permeability and outer membrane pore-forming proteins play a key role in the activity of ceftazidime-avibactam. Nevertheless, the MICs of ceftazidime-avibactam (with 4 mg/liter avibactam) against the ceftazidime-resistant clinical isolates of the three species of Enterobacteriaceae studied were ≤8 mg/liter, regardless of outer membrane permeability changes resulting from an absence of defined porin proteins or upregulation of efflux.
INTRODUCTION
The worldwide dissemination of resistant bacteria has severely reduced the efficacy of our antibiotic arsenal and consequently contributes to increasing frequency of therapeutic failure (1–3). For bacterial pathogens, changing expression of transporters and efflux mechanisms directly alters the intracellular concentrations of antibiotics (4, 5), and mutations that decrease permeability or increase efflux contribute to multidrug resistance (6, 7).
These bacterial envelope adaptations act jointly with β-lactamase enzymes that inactivate β-lactam antibiotics in the periplasm. Consequently, several β-lactamase inhibitors are used in combination with β-lactams (e.g., piperacillin-tazobactam or amoxicillin-clavulanic acid) to restore β-lactam activity by inhibiting β-lactamases (8, 9). However, to penetrate the outer membrane, both β-lactams and β-lactamase inhibitors are understood to diffuse through porin-mediated channels, and a decrease in porin expression potentially impairs the penetration of both (6, 10–12). In addition, just as β-lactams are recognized and expelled by efflux pumps (7, 13), physicochemically similar β-lactamase inhibitors may also be recognized and pumped by efflux mechanisms (7, 14).
Avibactam is a first-in-class synthetic, non-β-lactam β-lactamase inhibitor with a novel [3.2.1]-diazabicyclooctane chemical scaffold (15). It inhibits Ambler class A and C β-lactamases, as well as some class D enzymes, with a unique covalent and reversible mechanism (16, 17). Paired with the antipseudomonal cephalosporin ceftazidime, avibactam restores the antibacterial activity of ceftazidime against some strains of Enterobacteriaceae and Pseudomonas aeruginosa that express the above β-lactamases (9).
Few published studies have examined the effect of changes in the Gram-negative outer membrane barrier on the activity of ceftazidime-avibactam in β-lactamase-producing strains: physiological studies have focused on measuring the MICs against isolates characterized by their complements of β-lactamase (bla) genes (18–29).
Recent data (presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy [58]) suggested that avibactam can restore the β-lactam activity in various Gram-negative bacteria irrespective of porin expression. There is a theoretical and practical concern that avibactam may be affected by changes in porin expression and number and by efflux mechanisms (6, 11) and, owing to the avibactam structure (molecular weight, hydrophilicity, and charges), it would also be expected to pass through the outer membrane barrier via aqueous channels (30). The present study has investigated this further using a set of porin-active, porin-deficient, and efflux-active, efflux-deficient Enterobacteriaceae, in combination with polymyxin B nonapeptide, a chemical modulator of Gram-negative bacterial outer membrane permeability, and phenylalanine-arginine β-naphthylamine (PAβN), an efflux pump inhibitor.
(This work was presented in part as an abstract at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014 [31].)
MATERIALS AND METHODS
Antibacterials.
Chloramphenicol, nalidixic acid, norfloxacin, ciprofloxacin, polymyxin B nonapeptide (PMBN), imipenem, ertapenem, meropenem, piperacillin, phenylalanine-arginine β-naphthylamine (PAβN), ceftazidime, aztreonam, ceftaroline, and avibactam were used to assess the antibiotic susceptibility of the various isolates. Different combinations (e.g., ceftazidime-avibactam, ceftazidime plus PMBN, and ceftazidime-avibactam plus PMBN) were assayed during the study. Meropenem, avibactam, and ceftaroline were provided by AstraZeneca. Other compounds were acquired commercially from Sigma except for ciprofloxacin, which was from Fluka.
Bacterial strains.
In order to investigate the involvement of the outer membrane and porins in the susceptibility to the ceftazidime-avibactam combination, several Escherichia coli, Enterobacter aerogenes, and Klebsiella pneumoniae clinical isolates and ATCC strains were selected based on their porin and efflux pump profiles and their antibiotic susceptibilities (Table 1; Fig. 1).
TABLE 1.
Bacterial strains used in this studya
| Strain | Detected porinb |
Efflux component (AcrAB) | Enzyme(s) detectedc | |
|---|---|---|---|---|
| OmpF | OmpC | |||
| E. coli | ||||
| AG100 | + | + | + | AmpC(b) |
| ARS100d | − | − | ND | AmpC(b) |
| ARS108d | − | − | + | AmpC(b), CTX-M-15 |
| ARS144d | + | + | + | AmpC(b), CTX-M-15, DHA-1 |
| ARS150d | + | + | + | AmpC(b), CTX-M-15, TEM-1 |
| ARS237d | + | + | + | AmpC(b), CTX-M-14, TEM-1 |
| ARS273d | − | + | ND | AmpC(b), CTX-M-15 |
| ARS301d | + | + | + | CTX-M-15 |
| E. aerogenes | Omp35 | Omp36 | ||
| ATCC 15038 | + | + | + | AmpC(b) |
| EA2e | + | + | + | AmpC(d), TEM-24 |
| EA3e | − | + (loop 3 mutant) | + | AmpC(d), TEM-24 |
| EA5e | − | − | + | AmpC(d), TEM-24 |
| EA27e | − | − | +++ | AmpC(d), TEM-24 |
| EA117e | − | − (weak signal) | + | AmpC(d), TEM-24 |
| EA DFJ85e | + | + | + | AmpC(d), TEM-24 |
| EA DFJ46e | − | − | + | ND |
| K. pneumoniae | OmpK35 | OmpK36 | ||
| ATCC 11296 | + | + | +/− | ND |
| KP45e | + | + | + | ND |
| KP55e | − | − | + | ESBL (TEM-3) |
| KP63e | − | − | + | ESBL (TEM-3) |
| KP74e | − | + | + | ESBL (TEM-3) |
| KP80e | − | + | + | ESBL (TEM-3) |
| KP89e | − | + | + | ND |
Except for AG100, ATCC 15038, and ATCC 11296, these are previously characterized clinical isolates of E. coli, E. aerogenes, and K. pneumoniae.
Porins and efflux components were identified by Western blot-immunodetection (OmpC or OmpF, AcrAB, TolC): −, no signal (whatever the medium used); +, signal detected; +++, AcrAB overproduction. ND, not determined.
AmpC(b), AmpC basal; AmpC(d), derepressed.
Clinical isolates from the laboratory of J. P. Lavigne, CHU Nîmes (32).
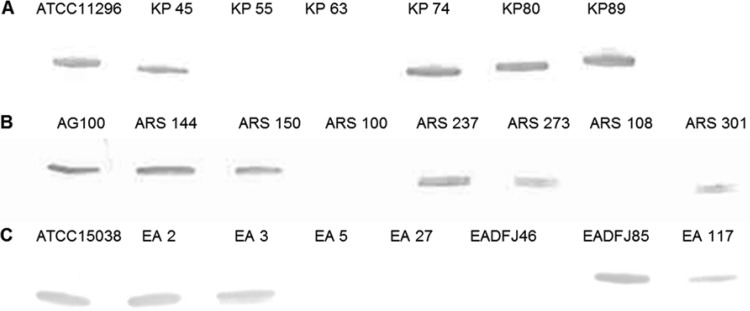
FIG 1.
Immunodetection by Western blotting of porins in various bacterial strains grown in MHII broth. Detection was carried out with a mix of anti-OmpF antiserum and anti-OmpC antiserum. (A) K. pneumoniae strains; (B) E. coli strains; (C) E. aerogenes strains. Only the relevant part of the gel is shown.
MIC determination.
The MIC values of the antibiotics were determined by the microdilution method (CLSI; http://clsi.org) in liquid Mueller-Hinton II (MHII) medium in microplates with an inoculum of 106 CFU in 200 μl of broth containing 2-fold serial dilutions of each antibiotic in the absence or presence of the chemosensitizers/modulators PMBN and PAβN) used at 1/10 of their respective MICs in order to avoid their direct inhibition of growth as previously described (37, 38). Permeabilization, blocking of efflux pumps, and isolates with different levels of porin or efflux expression were used to assess the involvement of the membrane barrier in antibiotic activity (38). Various combinations of ceftazidime and avibactam concentrations (avibactam at 1 to 4 mg/liter) were studied. The MIC values were read after 18 h of incubation at 37°C. Experiments were performed in triplicate for each antibiotic, each strain, and each condition. The resulting mean values are presented in the tables.
The MIC values of ceftazidime with or without avibactam were also determined by microdilution in nutrient broth (NB) and NB plus 20% sorbitol 20% (wt/vol) (NBS). Using specific growth conditions (NB and NBS) (39), the balance between OmpF/OmpC porin expression (OmpK35/OmpK36, Omp35/Omp36) levels was modulated and used to assess the effect of the primary porin type on the ceftazidime-avibactam activity. The MICs were determined in independent triplicate experiments. The osmolalities of the different media were determined using an osmometer apparatus according to the manufacturer's instructions (Gonotec GmbH, Berlin, Germany): MHII, 330 ± 6 mosmol/kg; NB, 46 ± 3 mosmol/kg; NBS, 1,290 ± 23 mosmol/kg.
Determination of membrane protein expression.
Immunodetection of porins and efflux pump components was performed using total bacterial pellets to determine the level of expression of membrane transporters (33, 40). Briefly, exponential-phase bacteria were grown in Luria-Bertani broth, and samples corresponding to similar cell concentrations were pelleted and solubilized, as previously described (33). The total bacterial proteins were separated on SDS-PAGE (final 10% acrylamide-0.27% bis-acrylamide), and the gels were stained with Coomassie brilliant blue R-250 to check the various protein samples (33, 40). In the second step, the corresponding gels were electrotransferred onto a nitrocellulose membrane in transfer buffer (20 mM Tris, 150 mM glycine, 20% isopropanol, 0.05% sodium dodecyl sulfate) using standardized amounts of protein samples. An initial blocking step was performed overnight at 4°C with Tris buffer (50 mM Tris-HCl, 150 mM NaCl [pH 8]) containing skimmed milk powder (10%). The nitrocellulose membranes were then incubated in Tris buffer containing skimmed milk powder (10%) and Triton X-100 (0.2%) for 2 h at room temperature in the presence of specific antisera. The primary antibodies were raised against E. coli proteins and used at the following final dilutions: 1:5,000, OmpF; 1:5,000, OmpC; 1:10,000, AcrA; 1:2,000, AcrB; and 1:2,000, TolC. These antibodies are able to recognize E. coli, E. aerogenes, and K. pneumoniae membrane proteins (33–41). Antigen-antibody complexes was detected with alkaline phosphatase-conjugated goat anti-rabbit antibodies. NBT-BCIP (nitroblue tetrazolium blue chloride-5-bromo-4-chloro-3-indolylphosphate disodium salt) was applied according to the manufacturer's instructions (Roche Molecular Biochemicals). In order to check for the presence of a correct L3 internal loop that defines the eyelet constriction determining the conductance and properties of the porin channel (6), we also used the anti-L3 antibody previously described (40). Experiments were performed in duplicate for each antibody incubation.
Nitrocefin assay for measurement of β-lactamase.
β-Lactamase activity with nitrocefin as the substrate was determined by measuring the product of nitrocefin (Oxoid) hydrolysis at 486 nm (ε = 20,500 M−1 cm−1). Strains growing in the exponential phase were collected, pelleted, and resuspended in water before sonic disruption. The working solution of nitrocefin was prepared by dissolving 1 mg of nitrocefin in 1.9 ml of phosphate buffer (0.1 M [pH 7]). The working solution of nitrocefin was then diluted 2.5-fold in phosphate buffer A (0.06 M Na2HPO4, 0.04 M NaH2PO4 [pH 7]). Assays were performed on the sonicated supernatants. First, the enzymatic activity corresponding to the increase in the hydrolysis product of chromogenic β-lactam nitrocefin (Oxoid) was recorded (spectrophotometer, Infinite 200 PRO; Tecan) at 486 nm during 2 h of incubation at 37°C in the presence of nitrocefin and 5 to 50 μl of sonicated extract in phosphate buffer. Specific activity was defined as activity unit per minute per milligram of protein determined using the initial near-linear slope of the curve and with protein measured by the bicinchoninic acid (BCA) method in the sample. In the text, “nitrocefinase” corresponds to “nitrocefin hydrolysis.”
Ceftazidime hydrolysis.
The hydrolysis of ceftazidime was monitored at 260 nm (ε = 10,500 M−1 cm−1) (42) in the absence or the presence of avibactam. Strains growing in the exponential phase were collected and resuspended in water before sonication (duty cycles, 50%). The working solutions of ceftazidime or avibactam were prepared in water. The enzymatic activity was monitored at 37°C (spectrophotometer, Infinite 200 PRO) using 5 to 50 μl of sonicated suspension in the presence of ceftazidime (0.1 mM) in phosphate buffer B (0.1 M Na2HPO4-0.1 M NaH2PO4 [pH 7]) with and without avibactam at 1 mg/liter or 4 mg/liter. The enzymatic unit of activity was defined as micromoles of compound (nitrocefin or ceftazidime) hydrolyzed per minute per milligram of protein (determined in bacterial lysate) at 37°C. In the text, “ceftazidimase” corresponds to “ceftazidime hydrolysis.”
RESULTS
The MICs of ceftazidime, avibactam, ceftazidime-avibactam, and selected antibacterial agents of other classes are shown in Table 2 for E. coli, in Table 3 for E. aerogenes, and in Table 4 for K. pneumoniae. Three concentrations of avibactam (1, 2, and 4 mg/liter) were assayed in order to obtain comparative ceftazidime, avibactam, and ceftazidime-avibactam MIC measurements against outer membrane permeabilized cells, some of which were more susceptible to the intrinsic antibacterial activity of avibactam at 4 mg/liter when rendered more permeable. MICs were also measured in the presence of the membrane permeabilizer, PMBN (Tables 2, 3, and 4). Low concentrations (1/10 MIC) of PMBN were used to avoid any growth inhibition effect of the molecule (38). The MICs of additional antibacterial agents are shown to illustrate the types of resistance associated with each cellular phenotype and genotype and also to document the degree of multiresistance in the clinical isolates. In the following, we first analyze the results of measuring the MICs of avibactam alone, then of ceftazidime alone, and then of combined ceftazidime and avibactam.
TABLE 2.
Antibiotic susceptibilities of E. coli strains, combination CAZ-AVI, and effect of PMBN
| Strain | MIC (mg/liter)a |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHL | PAβN | NOR | NAL | CIP | PIP | AVI | PMBN | CAZ | CAZ + AVI 1 | CAZ + AVI 2 | CAZ + AVI 4 | CAZ + PMBN | AVI + PMBN | CAZ + PMBN + AVI 1 | CAZ + PMBN + AVI 2 | CAZ + PMBN + AVI 4 | CPT | IPM | MEM | ATM | ERT | |
| AG100 | 8 | 512 | 0.125 | 4 | 0.03 | 2 | 16 | 512 | 0.5 | 0.25 | 0.25 | 0.25 | 0.03 | 8 | 0.03 | 0.03 | ND | 0.125 | 0.125 | 0.02 | 0.125 | 0.02 |
| ARS100 | 256 | 512 | 1,024 | >1,024 | 64 | >1,024 | 128 | >1,024 | 1 | 0.5 | 0.5 | 0.25 | 0.125 | 8 | 0.06 | 0.03 | 0.03 | 32 | 0.25 | 0.06 | 8 | 2 |
| ARS108 | 512 | 256 | 1,024 | >1,024 | 256 | >1,024 | 32 | 1,024 | 32 | 1 | 0.5 | 0.25 | 2 | 8 | 0.03 | 0.03 | 0.03 | >128 | 0.25 | 0.25 | >128 | 4 |
| ARS144 | 32 | 256 | 512 | >1,024 | 128 | >1,024 | 8 | 1,024 | 1,024 | 8 | 8 | 2 | 32 | 8 | 0.5 | 0.5 | 0.5 | >128 | 0.25 | 0.06 | >128 | 0.5 |
| ARS150 | 8 | 256 | >1,024 | >1,024 | 512 | 1,024 | 8 | 1,024 | 512 | 2 | 1 | 2 | 4 | 8 | 0.125 | 0.02 | ND | >128 | 0.5 | 0.06 | >128 | 2 |
| ARS237 | 64 | 256 | 512 | >1,024 | 128 | 1,024 | 32 | 1,024 | 128 | 2 | 1 | 0.06 | 2 | 8 | 0.03 | 0.03 | ND | >128 | 0.125 | 0.5 | >128 | 4 |
| ARS273 | 1,024 | 512 | 512 | >1,024 | 64 | >1,024 | 8 | 512 | 128 | 32 | 1 | 0.25 | 8 | 4 | 0.03 | 0.02 | ND | >128 | 0.25 | 1 | >128 | 8 |
| ARS301 | 16 | 512 | 512 | >1,024 | 256 | >1,024 | 16 | 1,024 | 2,048 | 32 | 16 | 8 | 32 | 16 | 0.5 | 0.5 | ND | >128 | 0.25 | 0.03 | >128 | 2 |
CHL, chloramphenicol; PAβN, phenylalanine-arginine β-naphthylamide; NOR, norfloxacin; NAL, nalidixic acid; CIP, ciprofloxacin; PIP, piperacillin; AVI, avibactam (1, 2, or 4 mg/liter); PMBN, polymyxin B nonapeptide (used at MIC/10 for combination); CAZ, ceftazidime; CPT, ceftaroline; IPM, imipenem; MEM, meropenem; ATM, aztreonam; ERT, ertapenem; ND, not determined because of intrinsic susceptibility preventing the reading of a reliable endpoint.
TABLE 3.
Antibiotic susceptibilities of E. aerogenes strains, combination CAZ-AVI, and effect of PMBN
| Strain | MIC (mg/liter)a |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHL | PAβN | NOR | NAL | CIP | PIP | AVI | PMBN | CAZ | CAZ + AVI 1 | CAZ + AVI 2 | CAZ + AVI 4 | CAZ + PMBN | AVI + PMBN | CAZ + PMBN + AVI 1 | CAZ + PMBN + AVI 2 | CAZ + PMBN + AVI 4 | CPT | IPM | MEM | ATM | ERT | |
| ATCC 15038 | 4 | 1,024 | 0.125 | 4 | 0.125 | 2 | 8 | 256 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 8 | 0.06 | 0.06 | ND | 0.125 | 0.125 | 0.02 | 0.03 | 0.01 |
| EA2 | 512 | 1,024 | 64 | >1,024 | 32 | 16 | 4 | 256 | 256 | 2 | 1 | ND | 32 | 4 | 0.5 | 0.125 | ND | 8 | 0.25 | 0.01 | 64 | 0.25 |
| EA3 | 512 | 1,024 | 64 | >1,024 | 32 | 128 | 64 | 1,024 | 1,024 | 16 | 8 | 2 | 64 | 16 | 2 | 0.5 | 0.25 | 32 | 0.5 | 0.03 | 128 | 0.5 |
| EA5 | 256 | 1,024 | 64 | >1,024 | 16 | 128 | 64 | 1,024 | 1,024 | 64 | 16 | 8 | 128 | 16 | 1 | 0.5 | 0.125 | 32 | 4 | 0.5 | 128 | 8 |
| EA27 | 512 | 1,024 | 64 | >1,024 | 16 | 128 | 256 | 512 | 1,024 | 64 | 64 | 4 | 32 | 8 | 0.5 | 0.03 | 0.03 | 64 | 16 | 1 | 128 | 64 |
| EA117 | 512 | 1,024 | 128 | >1,024 | 32 | 128 | 128 | 1,024 | 1,024 | 16 | 16 | 2 | 128 | 8 | 1 | 0.5 | 0.125 | 64 | 2 | 0.125 | >128 | 2 |
| EADFJ 85 | 1,024 | 1,024 | 64 | >1,024 | 32 | 64 | 16 | 1,024 | 512 | 8 | 8 | 2 | 64 | 16 | 1 | 1 | ND | 16 | 1 | 0.02 | >128 | 0.25 |
| EADFJ 46 | 1,024 | 1,024 | 256 | >1,024 | 256 | 128 | 128 | 1,024 | 1,024 | 32 | 8 | 8 | 128 | 64 | 1 | 1 | ND | 64 | 8 | 1 | >128 | 16 |
CHL, chloramphenicol; PAβN, phenylalanine-arginine β-naphthylamide; NOR, norfloxacin; NAL, nalidixic acid; CIP, ciprofloxacin; PIP, piperacillin; AVI, avibactam (1, 2, or 4 mg/liter); PMBN, polymyxin B nonapeptide (used at MIC/10 for combination); CAZ, ceftazidime; CPT, ceftaroline; IPM, imipenem; MEM, meropenem; ATM, aztreonam; ERT, ertapenem; ND, not determined because of intrinsic susceptibility preventing the reading of a reliable endpoint.
TABLE 4.
Antibiotic susceptibilities of K. pneumoniae strains, combination CAZ-AVI, and effect of PMBN
| Strain | MIC (mg/liter)a |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHL | PAβN | NOR | NAL | CIP | PIP | AVI | PMBN | CAZ | CAZ + AVI 1 | CAZ + AVI 2 | CAZ + AVI 4 | CAZ + PMBN | AVI + PMBN | CAZ + PMBN + AVI 1 | CAZ + PMBN +AVI 2 | CAZ + PMBN + AVI 4 | CPT | IPM | MEM | ATM | ERT | |
| ATCC 11296 | 4 | 4 | 0.25 | 16 | 0.06 | 16 | >128 | 256 | 0.25 | 0.5 | 0.5 | 0.125 | 0.25 | 128 | 0.5 | 0.25 | ND | 1 | 0.25 | 0.03 | 0.125 | 0.25 |
| KP45 | 16 | 1,024 | 0.25 | 4 | 0.06 | 8 | 128 | 1,024 | 0.5 | 0.125 | 0.06 | 0.5 | 0.125 | 16 | 0.06 | 0.06 | 0.06 | 0.25 | 0.125 | 0.03 | 0.06 | 0.01 |
| KP55 | 16 | 1,024 | 16 | 1,024 | 2 | 128 | 128 | >1,024 | 128 | 4 | 4 | 1 | 4 | 8 | 0.25 | 0.125 | 0.06 | 8 | 0.25 | 0.06 | >128 | 1 |
| KP63 | 512 | 1,024 | 1 | 32 | 0.125 | 64 | 16 | 512 | 512 | 8 | 8 | 2 | 8 | 8 | 0.5 | 1 | 1 | 8 | 0.25 | 0.06 | >128 | 1 |
| KP74 | 8 | 512 | 0.25 | 4 | 0.06 | >1,024 | 64 | 512 | 2,048 | 64 | 16 | 8 | 64 | 16 | 0.5 | 0.25 | 0.25 | 128 | 0.25 | 0.06 | 64 | 1 |
| KP80 | 128 | 1,024 | 16 | >1,024 | 8 | >1,024 | 16 | 1,024 | 8 | 4 | 2 | 0.25 | 0.125 | 8 | 0.125 | 0.125 | 0.125 | 8 | 0.06 | 0.03 | 1 | 0.5 |
| KP89 | 128 | 1,024 | 16 | >1,024 | 8 | 128 | 32 | 1,024 | 2 | 0.5 | 0.5 | 0.25 | 0.125 | 8 | 0.125 | 0.125 | 0.06 | 1 | 0.125 | 0.03 | 0.25 | 0.06 |
CHL, chloramphenicol; PAβN, phenylalanine-arginine β-naphthylamide; NOR, norfloxacin; NAL, nalidixic acid; CIP, ciprofloxacin; PIP, piperacillin; AVI, avibactam (1, 2, or 4 mg/liter); PMBN, polymyxin B nonapeptide (used at MIC/10 for combination); CAZ, ceftazidime; CPT, ceftaroline; IPM, imipenem; MEM, meropenem; ATM, aztreonam; ERT, ertapenem; ND, not determined because of intrinsic susceptibility preventing the reading of a reliable endpoint.
Effect of outer membrane permeabilization by PMBN on MICs of avibactam.
Avibactam displayed low antibacterial activity, which has been reported previously for Enterobacteriaceae (19, 43, 44). The mechanism of this growth inhibition is likely through weak inhibition of one or more penicillin-binding proteins (PBPs) (45), consistent with the original design concept of the diazabicyclooctane class of compounds of which avibactam is an example (15).
The intrinsic susceptibility to avibactam of the eight E. coli isolates when permeabilized was 8 mg/liter (±1 dilution in 2 cases) (Table 2). The higher MICs of 32 to 128 mg/liter observed in the absence of the permeabilizer in 3 of the isolates are interpreted as the presence of an outer membrane permeability barrier to avibactam. However, a clear inference could not be made that either OmpF or OmpC formed the main diffusion pathway for avibactam, because one of the 3 isolates with the permeability barrier (ARS237) expressed both of these pore proteins (Table 1). Moreover, isolate ARS273 was susceptible to avibactam in the absence of the permeabilizer despite lacking OmpF, consistent with avibactam diffusion through pores other than those formed by OmpF. From these results, we suggest that in E. coli the outer membrane presents a permeability barrier to avibactam that reduces the intrinsic susceptibility to the compound by 4- to 16-fold in the absence of a diffusion pathway(s) not mainly accounted for by OmpC- or OmpF-mediated pores.
Unlike the permeabilization results for E. coli, those for E. aerogenes were consistent with Omp35 and/or Omp36 forming the main pores able to conduct avibactam across the outer membrane. The avibactam MICs with or without PMBN revealed the presence of a permeability barrier to avibactam in strains EA3, EA5, EA27, and EA117 (Fig. 1; Table 3). Three of these four strains did not express active Omp35 or Omp36, and the other one only expressed Omp36 weakly. Also, permeabilization did not alter the avibactam MICs against isolates ATCC 15038, EA2, and EADFJ85, which produced both Omp35 and Omp36.
As was observed with E. coli and E. aerogenes, the use of PMBN revealed a permeability barrier to avibactam in K. pneumoniae strains KP45, KP55, KP74, and KP89 (Table 4). Notably, strain KP45 expressed both OmpK35 and OmpK36, excluding pores formed by them as major routes of diffusion of avibactam into the periplasm of K. pneumoniae (Table 1). In agreement with this, the avibactam MICs with or without PMBN showed the absence of a permeability barrier to avibactam in KP63 despite lacking OmpK35 and OmpK36.
Effect of outer membrane permeabilization by PMBN on the MICs of ceftazidime.
The permeability barrier to ceftazidime was clearly displayed in all isolates of E. coli, even in the susceptible strains AG100 and ARS100, as judged from the reductions in the MICs by 8- to 128-fold upon the addition of the permeabilizer PMBN (Table 2). The permeability barrier was also evident in OmpF/OmpC-sufficient cells, consistent with diffusion through those pores being sterically and electrostatically constrained (46). Interestingly, the extents of the reductions in the ceftazidime MICs against E. coli strains AG100 and ARS100 were similar (16-fold and 8-fold, respectively, Table 2), implying similar changes in permeability, despite the fact that AG100 expressed both OmpC and OmpF proteins, whereas ARS100 expressed neither. This implies that, similarly to avibactam, there are pathways for the diffusion of ceftazidime across the outer membrane other than those formed by OmpF and OmpC.
The ceftazidime-resistant strains of E. aerogenes tested displayed the removal of a significant permeability barrier when the ceftazidime MICs were measured in the presence of PMBN with decreases in the MICs of 8- to 32-fold compared with the MICs of ceftazidime alone (Table 3). These reductions in the MICs did not depend on selective production of Omp35 or Omp36, because the fold decreases were similar regardless of the production of those two proteins (Tables 1 and 3). For example, the ceftazidime MICs against strains EA2 and EA5 decreased 8-fold with the addition of PMBN even though strain EA2 produced both Omp35 and Omp36, whereas EA5 produced neither.
As was found with E. coli and E. aerogenes, the outer membrane permeability barrier was demonstrable in the ceftazidime-resistant isolates of K. pneumoniae by the 4- to 64-fold reductions in the MICs of ceftazidime observed on addition of PMBN (Table 4). Addition of PMBN did not cause any change in the ceftazidime MIC of 0.25 mg/liter against the susceptible strain ATCC 11296, which produced both porins OmpK35 and OmpK36 (Table 4). The MIC shift observed with resistant strain K. pneumoniae KP45, which also produced both OmpK35 and OmpK36, was 4-fold, whereas the MIC shifts observed with strains KP55 and KP63 lacking both proteins were greater, being 32- and 64-fold, respectively. High MIC shifts of 16- to 64-fold were obtained with strains KP74, KP80, and KP89 (Table 4), which only lacked one of the two proteins assessed (OmpK35 but not OmpK36) (Table 1). We conclude that OmpK35 forms a pore through which ceftazidime diffuses across the outer membrane of K. pneumoniae. However, in strain KP45 which produced OmpK35 (but not in strain ATCC 11296) ceftazidime diffusion was still restricted somewhat, because the permeability barrier was still measurable by the 4-fold decrease in the ceftazidime MIC upon the addition of PMBN (Table 4).
Effect of outer membrane permeabilization by PMBN on MICs of combined ceftazidime-avibactam.
Against many of the strains tested, an MIC of ceftazidime with avibactam at 4 mg/liter could not be reliably measured in the presence of PMBN owing to bacterial growth being weak or completely inhibited at all ceftazidime concentrations under that condition (Tables 2, 3, and 4). This was likely caused by simultaneous enhancement of the antibacterial activities of avibactam and ceftazidime, resulting from the removal of the outer membrane permeability barrier to both compounds. It was thus necessary in the combination experiments to test avibactam at concentrations lower than the standard 4 mg/liter (CLSI 2015) (44).
The addition of PMBN to ceftazidime plus avibactam (1 mg/liter) caused 8- to 1,024-fold decreases in the MICs for all 8 strains of E. coli (Table 2), demonstrating the removal of the common outer membrane permeability barrier to both compounds. The magnitudes of the decreases in the MICs could not clearly be attributed to the presence/absence of OmpC and/or OmpF. For example, the MICs decreased 8- and 32-fold, respectively, against strains ARS100 and ARS108 that lacked OmpC and OmpF, but decreased similarly by 16-, 16-, and 64-fold, respectively, against strains ARS144, ARS150, and ARS237 that produced both porins (Tables 1 and 3).
Against intact cells of ceftazidime-resistant isolates of E. aerogenes, the addition of avibactam at 1 mg/liter resulted in a decrease in all ceftazidime MICs (Table 3). Thus, avibactam penetrated to the periplasm and inhibited ceftazidime hydrolysis there, even in isolates EA5, EA27, and DFJ46 lacking both Omp35 and Omp36 (Table 3), revealing additional pathways of diffusion. This agreed with the effect of PMBN on the MICs of avibactam tested singly against E. aerogenes analyzed above. The further decreases in the MICs of ceftazidime plus avibactam (1 mg/liter) by the addition of PMBN against all 7 of the ceftazidime-resistant isolates of E. aerogenes demonstrated that a permeability barrier was still present before permeabilization. This likely resulted from simultaneous permeabilization of the cells to both compounds. Analysis of the different mutants did not identify specific diffusion pathways.
Identical conclusions were also made for K. pneumoniae. Avibactam at 1 mg/liter decreased the MICs of ceftazidime by 32- or 64-fold against intact cells of strains KP55 and KP63 (Table 4), both of which lacked porins OmpK35 and OmpK36 (Table 1), demonstrating the existence of diffusion pathways for avibactam other than the channels formed by those two proteins. The further addition of PMBN elicited additional reductions in the MICs of 16- to 128-fold against the ceftazidime-resistant isolates, KP55, KP63, KP74, and KP80, demonstrating the permeability barrier to the combination. However, again, it was not possible to conclude that either OmpK35 or OmpK36 formed the major diffusion pathways determining the MICs of ceftazidime-avibactam against intact K. pneumoniae.
Effect of inhibition of efflux by PAβN.
In order to investigate the role of efflux pumps in the activities of ceftazidime, avibactam, and ceftazidime-avibactam, the susceptibilities of the strains selected were determined in the presence and absence of the efflux inhibitor PAβN, as previously reported (7, 41). As a control, all of the isolates studied displayed chloramphenicol efflux revealed by decreases in the MIC by 4-fold or more on testing in the presence of PAβN (Table 5).
TABLE 5.
Antibiotic susceptibility of E. coli, E. aerogenes, and K. pneumoniae strains and combination with the efflux blocker (PAβN 20 mg/liter)
| Strain | MIC (mg/liter)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CHL | CHL + PAβN | PAβN | AVI | CAZ | CAZ + PAβN | AVI + PAβN | CAZ + AVI 1 + PAβN | CAZ + AVI 2 + PAβN | |
| E. coli | |||||||||
| AG100 | 8 | 2 | 512 | 16 | 0.5 | 1 | 32 | 0.5 | ND |
| ARS100 | 256 | 64 | 512 | 128 | 1 | 0.5 | 32 | 0.5 | ND |
| ARS108 | 512 | 32 | 256 | 32 | 32 | 32 | 32 | 0.5 | ND |
| ARS144 | 32 | 2 | 256 | 8 | 1,024 | 128 | 16 | 8 | ND |
| ARS150 | 8 | 4 | 256 | 8 | 512 | 128 | 16 | 1 | 1 |
| ARS237 | 64 | 8 | 256 | 32 | 128 | 128 | 32 | 2 | ND |
| ARS273 | 1,024 | 64 | 512 | 8 | 128 | 128 | 16 | 2 | 8 |
| ARS301 | 16 | 1 | 512 | 16 | 2,048 | 256 | 16 | 16 | ND |
| E. aerogenes | |||||||||
| ATCC 15038 | 4 | 1 | 1,024 | 8 | 0.06 | 0.125 | 16 | 0.125 | 0.125 |
| EA2 | 512 | 128 | 1,024 | 4 | 256 | 128 | 8 | 4 | 2 |
| EA3 | 512 | 128 | 1,024 | 64 | 1,024 | 1,024 | 128 | 16 | ND |
| EA5 | 256 | 128 | 1,024 | 64 | 1,024 | 1,024 | 64 | 64 | ND |
| EA27 | 512 | 128 | 1,024 | 256 | 1,024 | 1,024 | 512 | 128 | 64 |
| EA117 | 512 | 256 | 1,024 | 128 | 1,024 | 1,024 | 64 | 32 | 16 |
| EADFJ 85 | 1,024 | 64 | 1,024 | 16 | 512 | 1,024 | 64 | 16 | ND |
| EADFJ 46 | 1,024 | 64 | 1,024 | 128 | 1,024 | 1,024 | >1,024 | >128 | ND |
| K. pneumoniae | |||||||||
| ATCC 11296 | 4 | 1 | 4 | 256 | 0.25 | 0.5 | 128 | 0.5 | 0.5 |
| KP45 | 16 | 2 | 1,024 | 128 | 0.5 | 0.5 | 64 | 0.5 | ND |
| KP55 | 16 | 2 | 1,024 | 128 | 128 | 128 | 256 | 32 | 32 |
| KP63 | 512 | 128 | 1,024 | 16 | 512 | 512 | 32 | 32 | ND |
| KP74 | 8 | 1 | 512 | 64 | 2,048 | 1,024 | 64 | 64 | 64 |
| KP80 | 128 | 8 | 1,024 | 16 | 8 | 16 | 32 | 4 | 4 |
| KP89 | 128 | 32 | 1,024 | 8 | 2 | 1 | 16 | 1 | ND |
CHL, chloramphenicol; PAβN, phenylalanine-arginine β-naphthylamide; AVI, avibactam (1 or 2 mg/liter); CAZ, ceftazidime; ND, not determined.
There was a moderate level of efflux of avibactam in E. coli ARS100 based on a 4-fold decrease of the MIC of avibactam in the presence of the efflux inhibitor (Table 5). However, there was no evidence of avibactam efflux in any of the other E. coli strains (Table 5), implying that it is not a general property of the species. There was no evidence of any PAβN-inhibitable efflux of avibactam in any of the 8 isolates of E. aerogenes or the 7 isolates of K. pneumoniae studied (the addition of PAβN did not decrease avibactam MICs by more than 2-fold) (Table 5).
Three E. coli strains, ARS144, ARS150, and ARS301, showed some effect of inhibition of the efflux of ceftazidime as judged by 4- or 8-fold decreases in the MICs in the presence of PAβN (Table 5). However, there was no evidence of ceftazidime efflux in any of the isolates of the other two species (Table 5).
Comparison of the MICs of ceftazidime plus avibactam (1 mg/liter) in the absence (Tables 2, 3, and 4) or presence of PAβN (Table 5) yielded no evidence of any effect of efflux on the antibacterial activity of the combination against isolates of any of the 3 species, with the exception of one strain of E. coli (ARS273), against which the MIC decreased from 32 to 2 mg/liter on the addition of the efflux inhibitor. The reason for the enhancement of the activity of the combination by apparently inhibiting efflux in this strain was unclear, because neither the MIC of ceftazidime nor that of avibactam was affected by PAβN when each was tested singly (128 mg/liter or 32 mg/liter, respectively), whether PAβN was present or not (Table 5).
Antibiotic susceptibility, porin expression, and osmotic variation.
The osmotic strength of the growth medium regulates the OmpC/OmpF balance in E. coli, E. aerogenes, K. pneumoniae, and other Enterobacteriaceae (6, 11). We investigated the role of the two families, OmpC (OmpK36, Omp36) versus OmpF (OmpK35, Omp35), by using different test media. The MICs of avibactam alone against porin-sufficient isolates of all 3 species were generally 2- to 4-fold higher in the test media of low (NB, 46 mosmol/kg; high OmpF/OmpC ratio) than high (NBS, 1,290 mosmol/kg; low OmpF/OmpC ratio) osmolality (data not shown). However, the same result occurred against porin-deficient cells of E. coli or K. pneumoniae, indicating that the effect was likely not mediated through differential expression of OmpF/OmpC. In contrast, the MIC of avibactam against the porin-deficient E. aerogenes was higher in the higher-osmolality NBS medium than in the NB medium, but because OmpC and OmpF were not expressed, the difference between the results in the two media could not be ascribed to osmolality-dependent porin expression. Ultimately, the effects on the MICs of the combination of ceftazidime plus avibactam could not be unequivocally interpreted owing to the complexity of the system of two different compounds, coupled with the potential variation of the sensitivity of the peptidoglycan synthetic pathway to PBP inhibitors with test medium osmolality.
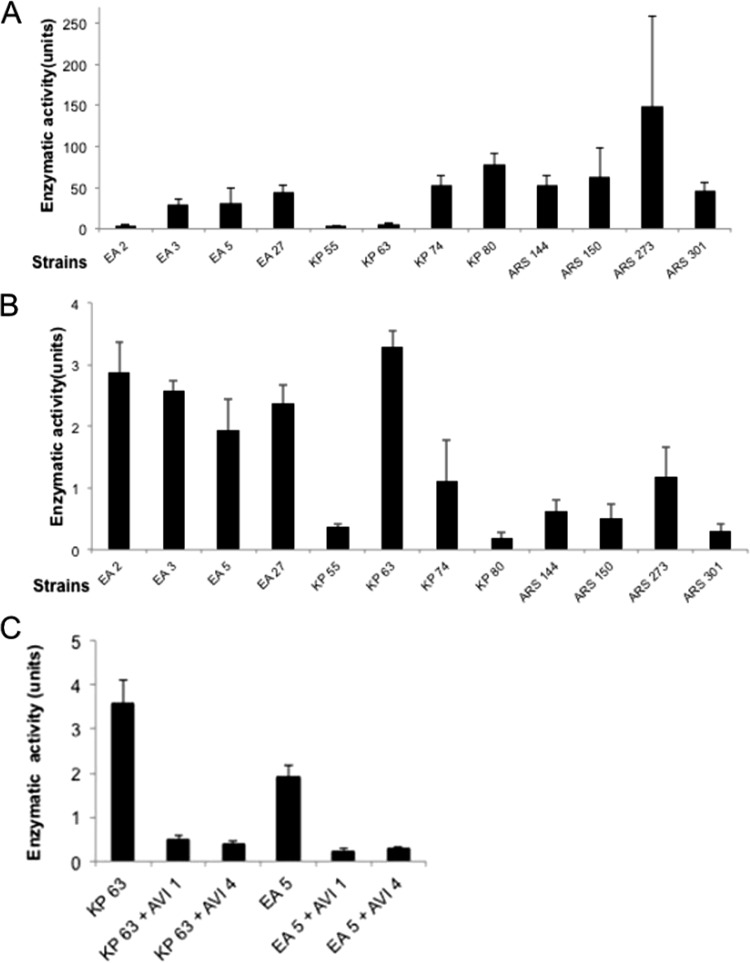
Antibiotic susceptibility and enzymatic activity, ceftazidimase versus nitrocefinase.
Determinations of ceftazidimase and nitrocefinase activity were performed in order to verify the activity of β-lactamases and the effect of inhibitors. The results indicated a large heterogeneity regarding the expression of the two activities analyzed. Figures 2A and B present the nitrocefinase and ceftazidimase activities measured in the various strains. As expected, the addition of avibactam to bacterial lysates containing the enzyme strongly decreased ceftazidime hydrolysis, and the residual activities were similar with avibactam at 1 or 4 mg/liter (Fig. 2C). The results confirmed that the outer membrane constituted a permeation barrier for the avibactam, as follows. The addition of avibactam at 1 mg/liter reduced the ceftazidime MIC against K. pneumoniae KP63 by 64-fold, and increasing the concentration to 4 mg/liter reduced the MIC by a further 4-fold. In contrast, the same two avibactam concentrations reduced the enzyme activity in disrupted cell extracts by about the same amount (25% in each case) (Fig. 2C). An identical observation was made with E. aerogenes EA5. The MIC change between testing ceftazidime plus avibactam at 1 mg/liter and at 4 mg/liter against E. aerogenes EA5 was 8-fold (64 and 8 mg/liter) (Table 3), whereas the difference in the ceftazidime hydrolysis activity of disrupted cells in the presence of avibactam at 1 or 4 mg/liter was negligible (Fig. 2C).
FIG 2.
Enzymatic assay in selected strain lysates. (A) β-Lactamase activity for the bacterial lysates obtained from the various bacterial suspensions was measured. β-Lactamase activity was determined using the chromogenic substrate nitrocefin. Units are micromoles of nitrocefin hydrolyzed per minute per milligram of protein. (B) Ceftazidimase activity on the different bacterial lysates was measured. (C) Inhibition of ceftazidimase activity by avibactam. Ceftazidimase activity on the different bacterial lysates in the presence of avibactam at 1 or 4 mg/liter was determined. Units are micromoles of ceftazidime hydrolyzed per minute per milligram of protein. The standard deviations (error bars) were obtained from three independent experiments.
DISCUSSION
Hydrophilic compounds do not readily cross the hydrophobic lipid bilayer of the Gram-negative outer membrane (11, 30). Thus, based on general principles, the outer membrane of members of the Enterobacteriaceae is predicted to form a permeability barrier to avibactam. The work reported here confirmed that hypothesis experimentally. Significant increases in the antibacterial activities of ceftazidime alone, avibactam alone, and ceftazidime-avibactam in combination were elicited by the addition of subinhibitory concentrations (1/10 MIC) of PMBN, an outer membrane permeabilizer, as has been previously reported for other antibacterial drugs (14, 38). In the case of ceftazidime-avibactam, addition of PMBN enhanced the activity of the pair of compounds against bacterial isolates that exhibited a permeability barrier and enzymatic resistance mechanisms simultaneously.
We also propose that the permeability barrier to ceftazidime-avibactam described above is a passive barrier, with little contribution from active efflux, based on an absence of any consistent strain- and species-wide effect on the MICs of the efflux inhibitor PAβN.
Although the Gram-negative bacterial outer membrane presents a permeability barrier to hydrophilic compounds, such compounds do cross the barrier via transmembrane channels that connect the external and periplasmic aqueous compartments (6, 11, 47). For example, ceftazidime diffuses through pores formed by OmpC and/or OmpF in E. coli (5-fold faster through OmpF pores) at rates consistent with those observed for other β-lactam and non-β-lactam compounds (48, 49). However, diffusion also occurs via other pathways, because the MIC against an OmpF/OmpC double mutant remained low at 0.5 mg/liter (48). From the MICs observed against the ceftazidime-resistant β-lactamase-producing isolates of Enterobacteriaceae, avibactam reaches the periplasm at high enough concentrations and rapidly enough to restore the activity of ceftazidime against ceftazidime-resistant, β-lactamase-producing clinical isolates and engineered strains (19, 26–29). We were able to exclude pores formed by OmpF and OmpC of E. coli and OmpK35 and OmpK36 of K. pneumoniae as the major channels by which avibactam penetrates to the periplasm in those species (see Results). Avibactam might still diffuse through those channels, but other channels were clearly accessible because avibactam inhibited β-lactamases and displayed its moderate antibacterial activity against isolates lacking those proteins. In contrast, Omp35 and Omp36 were not similarly excluded as forming the major channels for the influx of avibactam in E. aerogenes, but neither was it possible to exclude other channels.
The present work has also addressed the important question of whether the β-lactamase inhibitory activity of avibactam (8) and hence the antibacterial activity of the combination agent ceftazidime-avibactam (9) would be lost against clinical isolates exhibiting reduced permeability. Against the E. coli, E. aerogenes, and K. pneumoniae clinical isolates devoid of porins of both the OmpC and OmpF types (ARS100, ARS108, EA3, EA5, EA27, EA117, EADFJ46, KP55, and KP63), the MICs of ceftazidime-avibactam were still lower than or equal to the pharmacokinetic/pharmacodynamic cutoff of ≤8 mg/liter (50), which was recently adopted by the U.S. Food and Drug Administration as the interpretive criterion of susceptibility for Enterobacteriaceae and Pseudomonas aeruginosa (51). This is important because the loss of porins combined with the production of extended-spectrum β-lactamases (ESBLs) or class C β-lactamases can cause a lack of susceptibility to carbapenems in isolates of the Enterobacteriaceae without a carbapenemase (52–55). Of the 9 isolates listed above, an imipenem- or meropenem-nonsusceptible phenotype was observed in strains EA5, EA27, and EADFJ46, demonstrating a molecular mechanism-based difference between the activities of ceftazidime-avibactam and carbapenems, even in the absence of carbapenemases. This is consistent with studies of the activity of ceftazidime-avibactam against Enterobacteriaceae resistant to ertapenem by mechanisms of ESBL or AmpC production combined with loss of one or more outer membrane pore-forming proteins (22).
Using growth conditions in which the osmolality was varied (nutrient broth with or without sorbitol) (39), we modulated the balance of OmpF/OmpC (OmpK35/OmpK36, Omp35/Omp36) to evaluate the effect of the porin balance on the activity of ceftazidime-avibactam. The results obtained against the clinical isolates suggested a relationship between the moderate antibacterial activity of avibactam and osmolarity. However, a precise diagnosis of specific diffusion channels was not possible owing to the complexity of the results.
This is the first study that has investigated the role of membrane permeability in the activity of ceftazidime-avibactam against Enterobacteriaceae. It is clear that permeation is a significant factor governing the efficacy of this combination. It will be interesting in the future to assess the specific route and the penetration rate of avibactam though the pores formed by porins (OmpF or OmpC) or other hydrophilic outer membrane channels by using electrophysiological approaches (49, 56) or by determining the killing rate in isogenic strains expressing diverse outer membrane channels (57). Understanding the kinetics and mechanisms of influx of the individual components of β-lactam-β-lactamase inhibitor combinations such as ceftazidime-avibactam may inform the design of the next generation of such combinations as well as identify potential points of future resistance.
ACKNOWLEDGMENTS
We thank Anne Davin-Regli and Jacqueline Chevalier for their fruitful discussions.
Wright W. Nichols and Thomas A. Keating are former employees of AstraZeneca.
Funding Statement
This work was supported by Aix-Marseille University and IRBA and by a research grant “Ceftazidime-Avibactam” from AstraZeneca.
REFERENCES
- 1.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So DA, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta AZ, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. 2013. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 2.Wright GD, Poinar H. 2012. Antibiotic resistance is ancient: implications for drug discovery. Trends Microbiol 20:157–159. doi: 10.1016/j.tim.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Grundmann H, Klugman KP, Walsh T, Ramon-Pardo P, Sigauque B, Khan W, Laxminarayan R, Heddini A, Stelling J. 2011. A framework for global surveillance of antibiotic resistance. Drug Resist Updat 14:79–87. doi: 10.1016/j.drup.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Valade E, Davin-Regli A, Bolla JM, Pagès JM. 2013. Bacterial membrane, a key for controlling influx and efflux, p 217−240. In Gualerzi CO, Brandi L, Fabbretti A, Pon CL (ed), Antibiotics, targets, mechanisms and resistance. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 5.Davin-Regli A, Bolla JM, James C, Lavigne J-P, Chevalier J, Garnotel E, Molitor A, Pagès JM. 2008. Membrane permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr Drug Targets 9:750–759. [DOI] [PubMed] [Google Scholar]
- 6.Pagès JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 7.Nikaido H, Pagès JM. 2012. Broad specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drawz SM, Papp-Wallace KM, Bonomo RA. 2013. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother 58:1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PRS, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/ β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 10.Farmer TH, Degnan BA, Payne DJ. 1999. Penetration of β-lactamase inhibitors into the periplasm of Gram-negative bacteria. FEMS Microbiol Lett 176:11–15. [DOI] [PubMed] [Google Scholar]
- 11.Nikaido H. 2003. Molecular basis of outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikaido H, Rosenberg EY, Foulds J. 1983. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol 153:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolla JM, Alibert-Franco S, Handzlik J, Chevalier J, Mahamoud A, Boyer G, Kieć-Kononowicz K, Pagès JM. 2011. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett 585:1682–1690. doi: 10.1016/j.febslet.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Coleman K. 2011. Diazabicyclooctanes (DBOs): a potent new class of non-β-lactam β-lactamase inhibitors. Curr Opin Microbiol 14:550–555. doi: 10.1016/j.mib.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Ehmann DE, Jahić H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Réville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with β-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum β-lactamases and class C β-lactamases. Antimicrob Agents Chemother 55:2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiskirchen DE, Crandon JL, Furtado GH, Williams G, Nicolau DP. 2011. In vivo efficacy of a human-simulated regimen of ceftaroline combined with NXL104 against extended-spectrum-β-lactamase (ESBL)-producing and non-ESBL-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:3220–3205. doi: 10.1128/AAC.00024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of β-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of β-lactamases. Antimicrob Agents Chemother 56:258–270. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livermore DM, Mushtaq S, Barker K, Hope R, Warner M, Woodford N. 2012. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J Antimicrob Chemother 67:1354–1358. doi: 10.1093/jac/dks079. [DOI] [PubMed] [Google Scholar]
- 24.Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. 2013. Antimicrobial activity of ceftaroline-avibactam tested against clinical isolates collected from U.S. medical centers in 2010-2011. Antimicrob Agents Chemother 57:1982–1988. doi: 10.1128/AAC.02436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine United States census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flamm RK, Stone GG, Sader HS, Jones RN, Nichols WW. 2013. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J Chemother 26:333–338. doi: 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa R, Bush K. 2015. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, Foster AN, Winkler ML, Gatta JA, Nichols WW, Testa R, Bonomo R, Jacobs MR. 2015. Activities of ceftazidime, ceftaroline and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases. Diagn Microbiol Infect Dis 82:65–69. doi: 10.1016/j.diagmicrobio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizumi A, Ishii Y, Aoki K, Testa R, Nichols WW, Tateda K. 2015. In vitro susceptibility of characterized β-lactamase-producing Gram-negative bacteria isolated in Japan to ceftazidime-, ceftaroline-, and aztreonam-avibactam combinations. J Infect Chemother 21:148–151. doi: 10.1016/j.jiac.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Nichols WW. 2012. Permeability of bacteria to antibacterial agents, 849–879. In Dougherty TJ, Pucci MJ (ed), Antibiotic drug discovery and development, vol II. Springer Publishing Company, New York, NY. [Google Scholar]
- 31.Pagès J-M, Peslier S, Nichols WW, Lavigne JP, Keating T. 2014. Porins and the ceftazidime-avibactam (CAZ-AVI) susceptibility of β-lactamase-producing Enterobacteriaceae, abstr F-947 Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC, 5 to 9 September 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pantel A, Boutet-Dubois A, Jean-Pierre H, Marchandin H, Sotto A, Lavigne JP, CARB-LR Group . 2014. French regional surveillance program of carbapenemase-producing Gram-negative bacilli: results from a 2-year period. Eur J Clin Microbiol Infect Dis 33:2285–2292. doi: 10.1007/s10096-014-2189-5. [DOI] [PubMed] [Google Scholar]
- 33.Malléa M, Chevalier J, Bornet C, Eyraud A, Davin-Regli A, Bollet C, Pagès JM. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003–3009. [DOI] [PubMed] [Google Scholar]
- 34.Gayet S, Chollet R, Molle G, Pagès JM, Chevalier J. 2003. Modification of outer membrane protein profile and evidence suggesting an active drug pump in Enterobacter aerogenes clinical strains. Antimicrob Agents Chemother 47:1555–1559. doi: 10.1128/AAC.47.5.1555-1559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevalier J, Pagès JM, Eyraud A, Malléa M. 2000. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem Biophys Res Commun 274:496–499. [DOI] [PubMed] [Google Scholar]
- 36.Hasdemir UO, Chevalier J, Nordmann P, Pagès JM. 2004. Detection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from Turkey. J Clin Microbiol 42:2701–2706. doi: 10.1128/JCM.42.6.2701-2706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuete V, Alibert-Franco S, Eyong KO, Ngameni B, Folefoc GN, Nguemeving JR, Tangmouo JG, Fotso GW, Komguem J, Ouahouo BM, Bolla JM, Chevalier J, Ngadjui BT, Nkengfack AE, Pagès JM. 2011. Antibacterial activity of some natural products against bacteria expressing a multidrug-resistant phenotype. Int J Antimicrob Agents 37:156–161. doi: 10.1016/j.ijantimicag.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Mamelli L, Petit S, Chevalier J, Giglione C, Lieutaud A, Meinnel T, Artaud I, Pagès JM. 2009. New antibiotic molecules: bypassing the membrane barrier of Gram negative bacteria increases the activity of peptide deformylase inhibitors. PLoS One 4:e6443. doi: 10.1371/journal.pone.0006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bornet C, Saint N, Fetnaci L, Dupont M, Davin-Régli A, Bollet C, Pagès JM. 2004. Omp35, a new Enterobacter aerogenes porin involved in selective susceptibility to cephalosporins. Antimicrob Agents Chemother 48:2153–2158. doi: 10.1128/AAC.48.6.2153-2158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, Pagès JM, Amaral L. 2007. Antibiotic stress, genetic response and altered permeability of E. coli. PLoS One 2:e365. doi: 10.1371/journal.pone.0000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagès JM, Lavigne JP, Leflon-Guibout V, Marcon E, Bert F, Noussair L, Nicolas-Chanoine MH. 2009. Efflux pump, the masked side of β-lactam resistance in Klebsiella pneumoniae clinical isolates. PLoS One 4:e4817. doi: 10.1371/journal.pone.0004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antunes NT, Frase H, Toth M, Vakulenko SB. 2012. The class A β-lactamase FTU-1 is native to Francisella tularensis. Antimicrob Agents Chemother 56:666–671. doi: 10.1128/AAC.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkhout J, Melchers MJ, van Mil AC, Nichols WW, Mouton JW. 2015. In vitro activity of ceftazidime-avibactam combination in in vitro checkerboard assays. Antimicrob Agents Chemother 59:1138–1144. doi: 10.1128/AAC.04146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huband MD, Nichols WW, Stone GG, Otterson LG, Bradford PA. 2015. Ceftazidime-avibactam: use of a predictor panel to evaluate and optimize avibactam concentrations for in vitro susceptibility testing, abstr P1290 Abstr 25th Eur Congr Clin Microbiol Infect Dis, Copenhagen, Denmark, 25 to 28 April 2015. [Google Scholar]
- 45.Asli A, Brouillette E, Krause KM, Nichols WW, Malouin F. 16 November 2015. Distinctive binding of avibactam to penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob Agents Chemother. doi: 10.1128/AAC.02102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidal S, Bredin J, Pagès JM, Barbe J. 2005. β-Lactam screening by specific residues of the OmpF eyelet. J Med Chem 48:1395–1400. [DOI] [PubMed] [Google Scholar]
- 47.Schulz GE. 2002. The structure of bacterial outer membrane proteins. Biochim Biophys Acta 1565:308–317. [DOI] [PubMed] [Google Scholar]
- 48.Beceiro A, Maharjan S, Gaulton T, Doumith M, Soares NC, Dhanji H, Warner M, Doyle M, Hickey M, Downie G, Bou G, Livermore DM, Woodford N. 2011. False extended-spectrum β-lactamase phenotype in clinical isolates of Escherichia coli associated with increased expression of OXA-1 or TEM-1 penicillinases and loss of porins. J Antimicrob Chemother 66:2006–2010. doi: 10.1093/jac/dkr265. [DOI] [PubMed] [Google Scholar]
- 49.Mahendran KR, Kreir M, Weingart H, Fertig N, Winterhalter M. 2010. Permeation of antibiotics through Escherichia coli OmpF and OmpC porins: screening for influx on a single-molecule level. J Biomol Screen 15:302–307. doi: 10.1177/1087057109357791. [DOI] [PubMed] [Google Scholar]
- 50.Cerexa. 2014. Ceftazidime-avibactam briefing package. NDA 206494 Division of Anti-Infective Products, Office of Antimicrobial Products, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425458.pdf Accessed 21 May 2015. [Google Scholar]
- 51.Forest Pharmaceuticals. Avycaz package insert. Forest Pharmaceuticals, Cincinnati, OH: http://www.avycaz.com Accessed 21 May 2015. [Google Scholar]
- 52.Yang D, Guo Y, Zhang Z. 2009. Combined porin loss and extended spectrum β-lactamase production is associated with an increasing imipenem minimal inhibitory concentration in clinical Klebsiella pneumoniae strains. Curr Microbiol 58:366–370. [DOI] [PubMed] [Google Scholar]
- 53.Webster DP, Gaulton T, Woodford N, Pike R, Turton J, Perry C, Bowler IC. 2010. Emergence of carbapenem resistance due to porin loss in an extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae strain during meropenem therapy. Int J Antimicrob Agents 36:575–576. doi: 10.1016/j.ijantimicag.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y, Choi H, Yum JH, Kang G, Bae IK, Jeong SH, Lee K. 2012. Molecular mechanisms of carbapenem resistance in Enterobacter cloacae clinical isolates from Korea and clinical outcome. Ann Clin Lab Sci 42:281–286. [PubMed] [Google Scholar]
- 55.López-Camacho E, Gómez-Gil R, Tobes R, Manrique M, Lorenzo M, Galván B, Salvarelli E, Moatassim Y, Salanueva IJ, Pareja E, Codoñer FM, Alvarez-Tejado M, Garcillán-Barcia MP, De la Cruz F, Mingorance J. 2014. Genomic analysis of the emergence and evolution of multidrug resistance during a Klebsiella pneumoniae outbreak including carbapenem and colistin resistance. J Antimicrob Chemother 69:632–636. doi: 10.1093/jac/dkt419. [DOI] [PubMed] [Google Scholar]
- 56.Mahendran KR, Chimerel C, Mach T, Winterhalter M. 2009. Antibiotic translocation through membrane channels: temperature-dependent ion current fluctuation for catching the fast events. Eur Biophys J 38:1141–1145. doi: 10.1007/s00249-009-0495-0. [DOI] [PubMed] [Google Scholar]
- 57.James CE, Mahendran KR, Molitor A, Bolla JM, Bessonov AN, Winterhalter M, Pagès JM. 2009. How β-lactam antibiotics enter bacteria: a dialogue with the porins. PLoS One 4:e5453. doi: 10.1371/journal.pone.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young K. 2013. New broad-spectrum diazacyclo-octane inhibitors of β-lactamases, abstr F-873 Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO, 12 September 2013. [Google Scholar]