Abstract
Vancomycin remains the mainstay treatment for methicillin-resistant Staphylococcus aureus (MRSA) infections, including pneumonia. There is concern regarding the emergence of vancomycin tolerance, caused by heterogeneous vancomycin-intermediate S. aureus (hVISA), and subsequent vancomycin treatment failure. Pneumonia is associated with high morbidity and mortality, especially with delays in appropriate therapy. This study evaluated the clinical outcomes of patients with hVISA pneumonia compared to those with vancomycin-susceptible S. aureus (VSSA) pneumonia. A retrospective cohort of patients with MRSA pneumonia from 2005 to 2014 was matched at a ratio of 2:1 VSSA to hVISA infections to compare patient characteristics, treatments, and outcomes. hVISA was determined by the 48-h population analysis profile area under the curve. Characteristics between VSSA and hVISA infections were compared by univariate analysis and multivariable logistic regression analysis to determine independent risk factors of inpatient mortality. Eighty-seven patients were included, representing 29 hVISA and 58 VSSA cases of pneumonia. There were no significant differences in demographics or baseline characteristics. Sequential organ failure assessment (SOFA) scores were a median of 7 (interquartile ratio [IQR], 5 to 8) in hVISA patients and 5 (IQR, 3 to 8) in VSSA (P = 0.092) patients. Inpatient mortality was significantly higher in hVISA patients (44.8% versus 24.1%; P = 0.049). Predictors of inpatient mortality upon multivariable regression were SOFA score (adjusted odds ratio [aOR], 1.36; 95% confidence interval [CI], 1.08 to 1.70), Panton-Valentine leukocidin (PVL) positivity (aOR, 6.63; 95% CI, 1.79 to 24.64), and hVISA phenotype (aOR, 3.95; 95% CI, 1.18 to 13.21). Patients with hVISA pneumonia experienced significantly higher inpatient mortality than those with VSSA pneumonia. There is a need to consider the presence of vancomycin heteroresistance in pneumonia caused by MRSA in order to potentially improve clinical outcomes.
INTRODUCTION
Lower respiratory tract infections (LRTIs), in particular, pneumonias, are a major cause of morbidity and mortality. The Centers for Disease Control and Prevention (CDC) report pneumonia to be the leading cause of infection-related death (1). The Extended Prevalence of Infection in Intensive Care (EPIC) II point prevalence study of 1,265 intensive care units (ICUs) across Europe found that respiratory infections accounted for more than 50% of all culture-positive patients, with the most common Gram-positive organism being Staphylococcus aureus (2). Additionally, the National Healthcare Safety Network at the CDC reported S. aureus to be the number one cause of ventilator-associated pneumonia (VAP) in a nationwide survey of health care-associated infections (3). Adding to the clinical dilemma, several publications have linked delays in appropriately targeted therapy to increased mortality, especially in critically ill patients with VAP (4–7).
Methicillin-resistant S. aureus (MRSA) is a common causative organism in pneumonia, particularly health care-associated pneumonia (HCAP) and hospital-acquired pneumonia (HAP) (8, 9). Vancomycin is the primary treatment for infections caused by MRSA, including pneumonia. Continued selective pressure, however, has led to tolerant and resistant strains, such as vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus, which has compromised the utility of this agent (10, 11). Of particular clinical concern is heterogeneous vancomycin-intermediate S. aureus (hVISA) as it often goes undetected by traditional clinical microbiology testing methods, such as automated MIC testing, and, at present, there is no suitable method for real-time identification in the clinical microbiology laboratory (12, 13). The prevalence of hVISA in many geographic regions remains unknown; however, reports of its occurrence range from 1.2% to 29.2% of MRSA isolates (14–18).
There have been numerous publications on the outcomes of S. aureus bloodstream infections (BSI) with hVISA versus vancomycin-susceptible S. aureus (VSSA) strains (19–24). Within these studies, however, pneumonia as a primary source is not prevalent; the most common sources of BSI were catheters, osteopathic hardware, or wound infections. Little evidence currently exists regarding S. aureus resistance or heteroresistance and associated outcomes specifically in the setting of pneumonia (25). This study aimed to present a comparative assessment between patients with hVISA pneumonia and those with VSSA pneumonia, with a focus on clinical outcomes.
MATERIALS AND METHODS
Study design and population.
This retrospective cohort study was conducted using data from adult patients (age of ≥18 years) treated at the Detroit Medical Center from 2005 to 2014 for clinically defined pneumonia (26, 27). Patients with pneumonia as diagnosed by treating physicians and with MRSA identified as the causative pathogen were included in the study, and available isolates from the blood and/or respiratory tract were used in the analysis. Patients were required to have new or progressive infiltrates on a chest radiograph as well as at least two of the following signs and symptoms: cough, purulent sputum, rales/crackles, dyspnea, hypoxemia (≤90% oxygen saturation [SpO2]), temperature alteration (>38°C or <35°C), altered mental status, or white blood cell count of >10,000 cells/mm3 or <4,500 cells/mm3. Patients were matched by age (grouped as 18 to 45 years, 45 to 75 years, >75 years) and infection/admission year (within 3 years) at a ratio of 2:1 hVISA to VSSA pneumonias based on the results of a 48-h modified population analysis profile area under the curve (PAP-AUC). Patients were excluded from the analysis if they were less than 18 years of age, if S. aureus was not identified as the causative pathogen, if the vancomycin MICs of their isolates were >2 mg/liter, or if mortality occurred before anti-MRSA therapy was received for at least 72 h. The Institutional Review Board at Wayne State University approved the study, and a wavier of informed consent was obtained.
Clinical data, outcome assessments, and definitions.
Patient data included the following: demographics, comorbidities, Charlson comorbidity score, location prior to admission, hospitalization history, receipt of prior antibiotic therapy in the previous 30 days, sequential organ failure assessment (SOFA) score within 24 h of admission, location at onset of infection (medical floor versus ICU), type of ICU (medical, surgical, or trauma/burn), concurrent sites of infection, pharmacokinetic variables (antibiotic dosing and vancomycin levels), presence of an infectious disease (ID) consult, and anti-MRSA therapies used in treatment. The type of pneumonia was determined using definitions from the Infectious Diseases Society of America (IDSA) guidelines for community, hospital-acquired, ventilator-associated, and health care-associated pneumonia (28, 29). Outcome parameters included duration of concurrent bacteremia as applicable, temperature and white blood cell count (WBC) for up to 14 days postdiagnosis of pneumonia, length of hospital stay (LOS), length of ICU stay, length of mechanical ventilation as applicable, vancomycin failure, and inpatient all-cause as well as MRSA-related mortality. Length of hospital stay was calculated as total number of calendar days of inpatient admission as well as postpneumonia length of stay (LOSPNA); similarly, ICU length of stay was defined as the number of calendar days the patient was admitted to a dedicated intensive care unit as well as postpneumonia ICU stay (ICU LOSPNA), defined as the LOS after the diagnosis of pneumonia. Therapy was defined as appropriate if the isolate demonstrated in vitro susceptibility and was dosed based on institutional guidelines according to renal function, weight, and therapeutic monitoring, as applicable. Vancomycin was not considered appropriate therapy in hVISA-positive patients as increased treatment failure and morbidity have been demonstrated.
Inpatient mortality was defined as expiration of the patient due to any cause while admitted. Mortality was defined as MRSA related if death occurred while the patient was bacteremic with MRSA, if death occurred before documented resolution of pneumonia, or if infection with MRSA was indicated as the cause of death within the electronic medical records (eMRs). Vancomycin treatment failure was defined as a composite of nonresolving signs and symptoms of infection for at least 7 days, a need to change to a different anti-MRSA agent according to the treating physician, recurrent MRSA respiratory infection while the patient was hospitalized, or MRSA-related mortality.
Microbiological and molecular assessment.
Microbiological testing was performed on the first available respiratory and/or blood isolate for each patient included in the analysis. Susceptibility to vancomycin was determined by broth microdilution (BMD) in duplicate, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines, at the Anti-Infective Research Laboratory (Detroit, MI) (30). All isolates also underwent additional testing to determine hVISA status by a modified 48-h PAP-AUC method as previously described by Wooten et al. (31). Isolates were inoculated at 1 × 108 CFU/ml on brain heart infusion (BHI) plates containing increasing concentrations of vancomycin (0, 0.5, 1, 1.5, 2, 3, 4, and 8 mg/liter) using automatic spiral dispensers (Whitley Automated Spiral Plater; Don Whitley Scientific, West Yorkshire, United Kingdom) and incubated at 35°C for 48 h. Subsequent colony counts (log10 CFU/ml) were determined using a laser colony counter (Scan 1200; Interscience, Saint Nom, France) and plotted against vancomycin concentration. The area under the population curve was computed using the trapezoidal method in SigmaPlot (version 10; Systat Software, Inc., San Jose, CA, USA) and compared to the AUC of the reference strain Mu3 (ATCC 700698). Isolates tested were considered to be hVISA if the PAP-AUC ratio of the test isolate to Mu3 was ≥0.9 (18, 31). The staphylococcal cassette chromosome mec (SCCmec), USA300 or USA400, and accessory gene regulator (agr) type as well as the presence of Panton-Valentine leukocidin (PVL) were determined through multiplex PCR as described previously (32, 33). agr dysfunction was determined phenotypically by δ-hemolysin assay (34).
Statistical analysis.
Descriptive analysis was performed on patient characteristics. Dichotomous variables were compared using a chi-square or Fisher's exact test, as appropriate, and continuous variables were compared using a Wilcoxon rank sum test or Student's t test, as appropriate. All variables with P values of ≤0.05 were considered significant. Assuming a 20% difference in inpatient mortality between hVISA and VSSA patients at a power of 80% and a two-sided significance level of 0.05 at a 2:1 allocation ratio, the minimum sample size was determined to be 29 hVISA patients and 58 VSSA patents. Multivariable conditional backward stepwise logistic regression was performed to determine variables independently associated with inpatient mortality. All variables associated with the outcome of interest upon univariate analysis with a P value of ≤0.1 or determined to be clinically relevant a priori were included in the regression model. Model fit was determined by a Hosmer-Lemeshow goodness-of-fit test with a P value of >0.05. Statistical analysis was performed using IBM SPSS, version 21.0 (IBM Corp., Armonk, NY).
RESULTS
A total of 87 patients, representing 29 (33.3%) hVISA and 58 (66.7%) VSSA cases of pneumonia, were included in the final analysis, meeting the minimum sample size determined a priori. There were no significant differences in terms of baseline characteristics between hVISA and VSSA patients with the noted exception of estimated glomerular filtration rate (eGFR), which was significantly lower in the hVISA cohort (Table 1). Patients in the hVISA cohort had a median baseline eGFR of 60.0 ml/min/1.73 m2 (interquartile ratio [IQR], 36.5 to 100.0 ml/min/1.73 m2) while VSSA patients had a median baseline eGFR of 86.0 ml/min/1.73 m2 (IQR, 60.0 to 115.5 ml/min/1.73 m2) (P = 0.022). Severity of illness as measured by SOFA scores was numerically higher in the hVISA cohort than in the VSSA cohort (7.0 [IQR, 5.0 to 8.0] versus 5 [IQR, 3.0 to 8.0]). S. aureus was isolated more commonly from induced sputum (n = 46; 52.9%) than from bronchoalveolar lavage (BAL) fluid (n = 13; 14.9%), and concurrent bacteremia was common (n = 79; 90.1%). There were no significant differences in concurrent sites of infection between hVISA and VSSA patients.
TABLE 1.
Baseline characteristics of hVISA and VSSA patients
| Parametera | Value for the cohort |
P value | |
|---|---|---|---|
| hVISA (n = 29) | VSSA (n = 58) | ||
| Median age (yr [IQR]) | 63.0 (56.0–76.0) | 59.00 (50.0–68.25) | 0.117 |
| Median wt (kg [IQR]) | 70.0 (63.5–87.5) | 69.9 (60.4–79.7) | 0.595 |
| Median Charlson score (IQR) | 5 (2.5–7.0) | 5 (2.0–7.0) | 0.995 |
| Median SOFA score (IQR)b | 7.0 (5.0–8.0) | 5 (3.0–8.0) | 0.092 |
| No. (%) of female patients | 10 (34.5) | 24 (41.4) | 0.534 |
| No. (%) of African American patients | 21 (72.4) | 40 (69.0) | 0.907 |
| Clinical factors (no. of patients [%]) | |||
| Diabetes | 10 (34.5) | 22 (37.9) | 0.753 |
| History of CVA | 7 (24.1) | 16 (27.6) | 0.731 |
| Any respiratory pathology | 13 (44.8) | 33 (56.9) | 0.241 |
| HIV/AIDS | 2 (6.9) | 3 (5.2) | 0.745 |
| Liver disease | 2 (6.9) | 7 (12.1) | 0.455 |
| Intravenous drug use | 8 (27.6) | 11 (19.0) | 0.359 |
| Hemodialysis | 3 (10.3) | 4 (6.9) | 0.577 |
| eGFR (median [IQR]) | |||
| Baseline | 60.0 (36.5–100.0) | 86.0 (60.0–115.5) | 0.022 |
| At start of antimicrobial treatment | 31.5 (17.3–68.0) | 62.0 (38.0–96.5) | 0.013 |
| Prior hospitalization (no. of patients [%]) | 15 (51.7) | 32 (55.2) | 0.761 |
| Antibiotic use in the last 30 days (no. of patients [%]) | 7 (24.1) | 7 (12.1) | 0.149 |
| Admission data (no. of patients [%]) | |||
| Home | 19 (65.5) | 35 (60.3) | 0.851 |
| SNF | 8 (27.6) | 15 (25.9) | 1.000 |
IQR, interquartile range; SOFA, sequential organ failure assessment; CVA, cerebrovascular accident; HIV, human immunodeficiency virus; eGFR, estimated glomerular filtration rate; SNF, skilled nursing facility.
First 24 h after hospital admission.
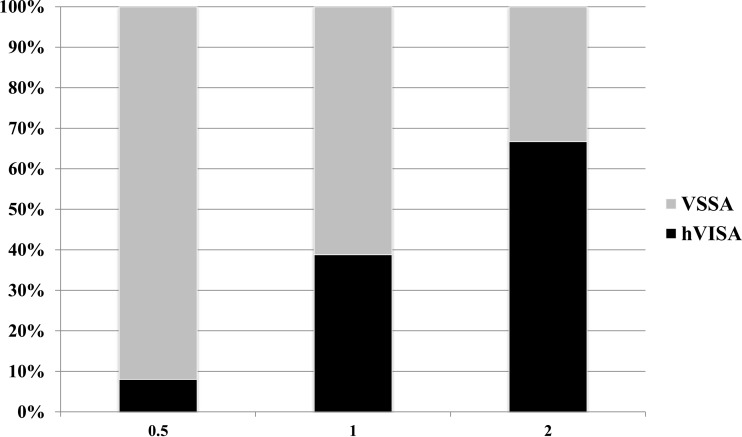
Vancomycin was the empirical MRSA agent for all patients included in the analysis. There was no difference in the loading doses between the two groups. Among the hVISA cohort, loading doses were given to 20 (69.0%) patients at a mean dose of 22.0 ± 4.7 mg/kg total body weight. A total of 41 (70.1%) patients with VSSA received a loading dose at a mean of 22.4 ± 4.5 mg/kg total body weight. Additionally, maintenance doses were not significantly different (14.8 ± 4.8 mg/kg for the hVISA cohort versus 15.2 ± 4.3 mg/kg for the VSSA cohort). Initial steady-state vancomycin levels often did not reach target trough serum vancomycin concentrations of at least 15 mg/liter (42.3% versus 59.6%, respectively; P = 0.148) (35). Patients were switched from vancomycin to another MRSA agent that was appropriate based on the above definition more commonly in the hVISA cohort (48.3% versus 34.5% for the VSSA cohort; P = 0.214) at a median time of 66.6 h (IQR, 32.3 to 109.2 h). Linezolid was the most common MRSA agent that patients were switched to (8/29, or 29.6%, for the hVISA cohort versus 12/58, or 20.7%, for the VSSA cohort; P = 0.416). Isolates available for vancomycin MIC testing were collected from blood (n = 69; 79.3%), the respiratory tract (n = 12; 13.8%), or both (n = 6; 6.9%) (Fig. 1). Although there was a 20% difference (95% confidence interval [CI], 13.8% to 31.1%) in vancomycin failure between the hVISA and VSSA cohorts (79.3% versus 58.6%, respectively; P = 0.056), this was not significant.
FIG 1.
Vancomycin MIC (mg/liter) frequency and distribution by broth microdilution.
Patients in both the hVISA and VSSA cohorts had a median length of stay of 17.5 days (censored for inpatient mortality). Patients in the hVISA cohort had a longer length of stay before diagnosis of pneumonia (6.5 days for the hVISA cohort versus 2 days for the VSSA cohort; P = 0.134). Length of stay postpneumonia diagnosis (LOSPNA) was shorter in the hVISA cohort (14.0 days versus 15.5 days for the VSSA cohort; P = 0.902) until censored for inpatient mortality (20.0 days versus 16.5 days, respectively; P = 0.631). ICU admissions occurred in 23 (79.3%) hVISA patients and in 40 (70.7%) VSSA patients (Table 2); there was no difference in distributions of the type of ICU (burn/trauma versus medical versus surgical). In addition there was no difference in the incidence of mechanical ventilation or ID consultations. More patients in the hVISA cohort presented with a body temperature of less than 36.0°C (24.1% versus 10.3% for the VSSA cohort; P = 0.089). More patients in the VSSA cohort achieved normalized WBC measurements within 14 days of pneumonia onset (51.7% for the VSSA cohort versus 37.9% for the hVISA cohort; P = 0.224). Additionally, there were no differences in molecular characteristics, including the presence of SCCmec type II (41.1% for the hVISA cohort versus 43.1% for the VSSA cohort), USA300 (20.7% for the hVISA cohort versus 27.6% for the VSSA cohort), PVL (20.7% for the hVISA cohort versus 29.3% for the VSSA cohort), or agr group II (55.2% for the hVISA cohort versus 43.1% for the VSSA cohort) (Table 2).
TABLE 2.
Clinical features and course of hVISA versus VSSA infection
| Characteristica | Value for the cohort |
P value | |
|---|---|---|---|
| hVISA (n = 29) | VSSA (n = 58) | ||
| Type of pneumonia (no. of patients [%]) | |||
| Community acquired | 6 (20.7) | 12 (20.7) | 1.000 |
| Health care associated | 12 (41.4) | 24 (41.4) | 1.000 |
| Hospital acquired | 5 (17.2) | 8 (13.7) | 0.753 |
| Ventilator associated | 6 (20.7) | 14 (24.1) | 0.792 |
| Antibiotic therapy (no. of patients [%]) | |||
| Vancomycin | 15 (51.7) | 38 (65.5) | 0.214 |
| Linezolid | 8 (29.6) | 12 (20.7) | 0.416 |
| Ceftaroline | 3 (10.3) | 3 (5.2) | 0.395 |
| TMP/SMX | 1 (3.4) | 2 (3.4) | 1.000 |
| Median time (h) to change in therapy (IQR) | 66.6 (32.3–109.2) | 72.8 (38.7–160.4) | 0.263 |
| Median (IQR) LOS (days)b | 17.5 (11.0–30.5) | 17.5 (11.0–35.8) | 0.554 |
| Median (IQR) LOSPNA (days)b | 13 (7–24) | 11 (6–18) | 0.797 |
| ID consult (no. of patients [%]) | 16 (55.2) | 34 (58.6) | 0.717 |
| Concurrent bacteremia (no. of patients [%]) | 27 (93.1) | 52 (89.7) | 0.742 |
| Median (IQR) duration of bacteremia (days) | 2 (1–5) | 2 (1–5) | 0.901 |
| Presence of concurrent sites of infection (no. of patients [%]) | |||
| Skin/soft tissue | 7 (24.1) | 8 (13.8) | 0.229 |
| Deep abscess | 1 (3.4) | 0 (0.0) | 0.155 |
| Bone/joint | 2 (6.9) | 1 (1.7) | 0.213 |
| Infective endocarditis | 2 (6.9) | 2 (3.4) | 0.469 |
| Unknown | 2 (6.9) | 10 (17.2) | 0.187 |
| ICU admission by type (no. of patients [%]) | 23 (79.3) | 40 (70.7) | 0.390 |
| Burn/trauma | 6 (26.1) | 5 (12.5) | 0.187 |
| Medical | 15 (65.2) | 31 (77.5) | 0.379 |
| Surgical | 2 (8.7) | 5 (12.5) | 1.000 |
| Median (IQR) ICU LOS (days)b | 12 (5.5–22.5) | 13 (5.0–25.0) | 0.725 |
| Mechanical ventilation (any) (no. of patients [%]) | 18 (62.1) | 37 (63.8) | 0.875 |
| Presence of S. aureus isolate type (no. of patients [%]) | |||
| PVL positive | 6 (20.7) | 17 (29.3) | 0.390 |
| USA300 | 6 (20.7) | 16 (27.6) | 0.485 |
| SCCmec type II | 12 (41.4) | 25 (43.1) | 0.878 |
| agr group II | 16 (55.2) | 25 (43.1) | 0.288 |
| agr dysfunction | 12 (41.4) | 19 (32.8) | 0.429 |
| Presence of other respiratory pathogens (no. of patients [%]) | |||
| Acinetobacter spp. | 5 (17.2) | 6 (10.3) | 0.642 |
| Pseudomonas spp. | 5 (17.2) | 5 (8.6) | 0.362 |
| Klebsiella spp. | 1 (3.4) | 7 (12.1) | 0.190 |
| Streptococcal spp. | 2 (6.9) | 4 (6.9) | 1.000 |
IQR, interquartile range; LOS, length of stay; LOSPNA, length of stay postdiagnosis of pneumonia; ICU, intensive care unit; PVL, Panton-Valentine leukocidin; TMP/SMX, trimethoprim-sulfamethoxazole.
Censored for inpatient mortality.
Inpatient mortality occurred in 27 (31.0%) patients. There were more cases of inpatient mortality among the hVISA cohort (44.8% versus 24.1% for the VSSA cohort; P = 0.049). In the subgroup of patients with respiratory cultures positive for MRSA (n = 60), inpatient mortality was numerically higher in the hVISA cohort (40.0% versus 22.5% for the VSSA cohort; P = 0.162). Variables significantly associated with inpatient mortality upon univariate analysis include the presence of the hVISA phenotype, presence of PVL, higher SOFA score, ICU stay, mechanical ventilation, higher Charlson comorbidity score, history of prior hospitalization, ID consult, and acute kidney injury at initiation of antibiotics (Table 3). Upon multivariable backward stepwise conditional logistic regression (Table 4), variables independently associated with inpatient mortality included SOFA score (adjusted odds ratio [aOR], 1.36; 95% CI, 1.08 to 1.70), ID consult (aOR, 0.35; 95% CI, 0.11 to 1.13), and hVISA phenotype (aOR, 3.95; 95% CI, 1.18 to 13.21). The variables of mechanical ventilation and ICU stay were not entered into the final model due to colinearity with the SOFA score. A Hosmer-Lemeshow goodness-of-fit test demonstrated good overall model fit, with a P value of 0.559.
TABLE 3.
Univariate analysis of clinical features associated with inpatient mortality
| Parametera | Value for the group |
P value | |
|---|---|---|---|
| Nonsurvivors (n = 27) | Survivors (n = 60) | ||
| Median (IQR) age (yr) | 65.0 (56.0–80.0) | 59.0 (56.0–80.0) | 0.132 |
| Median SOFA score (IQR) | 8 (6–9) | 5 (3–7) | 0.001 |
| Median Charlson score (IQR) | 6 (4–7) | 5 (2–7) | 0.115 |
| No. (%) of female patients | 8 (29.6) | 26 (43.3) | 0.226 |
| Clinical factors (no. of patients [%]) | |||
| Diabetes | 10 (37.0) | 22 (36.7) | 0.974 |
| History of CVA | 7 (25.9) | 16 (26.7) | 0.942 |
| Any respiratory pathology | 14 (51.9) | 26 (43.3) | 0.635 |
| HIV/AIDS | 2 (7.4) | 3 (5.0) | 0.655 |
| Liver disease | 3 (11.1) | 6 (10.0) | 0.875 |
| Intravenous drug use | 6 (22.2) | 13 (21.7) | 0.952 |
| Acute kidney injury | 8 (29.6) | 31 (51.7) | 0.152 |
| Hemodialysis | 1 (3.7) | 6 (10.0) | 0.318 |
| No. (%) of patients with prior hospitalization | 11 (40.7) | 36 (60.0) | 0.095 |
| Antibiotic use in the last 30 days (no. of patients [%]) | 2 (7.4) | 11 (18.3) | 0.396 |
| Previous vancomycin treatment (30 days) | 2 (7.4) | 10 (16.7) | 0.247 |
| Vancomycin therapy (no. of patients [%]) | |||
| Vancomycin trough of <15 mg/liter | 11 (45.8) | 31 (57.4) | 0.344 |
| No change in antibiotic therapy | 14 (51.9) | 39 (65.0) | 0.245 |
| ID consult (no. of patients [%]) | 11 (40.7) | 40 (66.7) | 0.023 |
| Concurrent bacteremia (no. of patients [%]) | 25 (92.6) | 54 (90.0) | 0.207 |
| Median (IQR) duration of bacteremia (days) | 3 (1–6) | 2 (1–5) | 0.717 |
| Presence of concurrent sites of infection (no. of patients [%]) | |||
| Skin/soft tissue | 4 (14.8) | 11 (18.3) | 0.688 |
| Deep abscess | 0 (0.0) | 1 (1.7) | 0.500 |
| Infective endocarditis | 2 (7.4) | 2 (3.3) | 0.401 |
| Bone/joint | 1 (3.7) | 2 (3.3) | 0.930 |
| Unknown | 4 (14.8) | 8 (13.3) | 0.853 |
| ICU admission (no. of patients [%]) | 23 (85.2) | 41 (63.8) | 0.099 |
| No. (%) of patients receiving mechanical ventilation (any) | 21 (77.8) | 34 (56.7) | 0.059 |
| Presence of other respiratory pathogens (no. of patients [%]) | |||
| Acinetobacter spp. | 4 (14.8) | 7 (11.7) | 0.683 |
| Pseudomonas spp. | 2 (7.4) | 8 (13.3) | 0.423 |
| Klebsiella spp. | 0 (0.0) | 8 (13.3) | 0.046 |
| Streptococcal spp. | 1 (3.7) | 8 (8.3) | 0.430 |
| Presence of MRSA isolate type (no. of patients [%]) | |||
| hVISA phenotype | 13 (48.1) | 14 (23.3) | 0.049 |
| VAN MIC of 2 mg/liter (BMD) | 4 (15.4) | 5 (8.8) | 0.501 |
| SCCmec type II | 13 (48.1) | 30 (50.0) | 0.873 |
| PVL positive | 13 (48.1) | 10 (16.7) | 0.002 |
| USA300 | 8 (29.6) | 14 (23.3) | 0.532 |
| agr type II | 14 (51.9) | 27 (45.0) | 0.554 |
| agr dysfunction | 8 (29.6) | 23 (38.3) | 0.433 |
IQR, interquartile range; SOFA, sequential organ failure assessment; CVA, cerebrovascular accident; HIV, human immunodeficiency virus; MRSA, methicillin-resistant S. aureus; hVISA, heterogeneous vancomycin-intermediate S. aureus.
TABLE 4.
Multivariable logistic regression for risk factors associated with inpatient mortality
| Characteristica | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| hVISA phenotype | 2.55 (0.99–6.59) | 3.95 (1.18–13.21) |
| PVL positive | 1.39 (1.45–11.08) | 6.63 (1.79–24.64) |
| ID consult | 0.37 (0.15–0.94) | 0.35 (0.11–1.13) |
| SOFA score | 1.36 (1.14–1.65) | 1.36 (1.08–1.70) |
hVISA, heterogeneous vancomycin-intermediate S. aureus; PVL, Panton-Valentine leukocidin; ID consult, infectious disease consultation; SOFA, sequential organ failure assessment.
DISCUSSION
In this single-center cohort study of outcomes of patients with hVISA pneumonia compared to those with VSSA pneumonia, the presence of the hVISA phenotype was found to be an independent predictor of inpatient mortality after adjustment for patient severity. This is unique from previously published studies that compared outcomes in patients with VSSA infection to those with hVISA infection in that it focuses on patients with pneumonia, a population known to have high rates of morbidity and mortality. There remains considerable debate regarding the pathogenicity of hVISA compared to that of VSSA, with reports of increased antibiotic failure and longer duration of bacteremia for hVISA strains but conflicting results regarding mortality (19, 21, 22, 24, 36, 37). Two observational studies have reported increased mortality among patients with hVISA infections; however, these studies were purely descriptive in nature, and neither confirmed the hVISA phenotype by PAP-AUC (36, 37). Subsequently, Maor et al. conducted a case-control study with 27 hVISA patients and 223 VSSA patients and found no difference in overall mortality rates (51% versus 46%, respectively; P = 0.6) or infection-attributable mortality rates (44% versus 36%, respectively; P = 0.4) (19). The source of bacteremia was not fully detailed, and the report mentioned only that the rates of pneumonia were similar in both groups. Additionally, our laboratory has previously published a study on the clinical outcomes of hVISA versus VSSA BSI and found a numerically higher rate of MRSA-related mortality in the hVISA cohort, but the difference was not significant (21% versus 10% for the VSSA cohort; P = 0.081). In this study, the most common primary diagnosis was infective endocarditis (39.9%), followed by catheter-related infections (24.6%), with pneumonia representing 8.2% of patients. In 2011, van Hal et al. reported that the presence of the hVISA phenotype was independently associated with decreased mortality among 409 MRSA BSI cases (11.5% hVISA infections) resembling the ST239 MRSA III clone (22). Overall mortality was reported to be 29.0%, with hVISA mortality significantly lower at 10.9%, a stark contrast to the 44.8% inpatient mortality in our study. The diagnosis of pneumonia was an independent predictor of mortality, and it is important to note that only 2 (4.3%) hVISA patients, in contrast to 50 (14.2%) VSSA patients, had a principal diagnosis of pneumonia.
The majority of the literature comparing hVISA and VSSA infections is retrospective in nature and focuses on S. aureus BSI from a variety of primary sources. It is clear from previous literature on MRSA BSI that the sources of hVISA infections tend to differ from those of VSSA infections. Isolates demonstrating the hVISA phenotype have more commonly been isolated from central intravenous line and wound infections (23, 38). MRSA is a frequent cause of pneumonia and has been consistently reported to be associated with significant morbidity and mortality. In 2012 Mendes et al. evaluated the clinical characteristics of MRSA isolates recovered from an international prospective phase IV pneumonia trial (39). Among the 434 MRSA isolates tested, 14.5% were determined to be hVISA by the macromethod Etest (bioMérieux, Marcy l'Etoile, France). In a retrospective observational study of 251 critically ill patients with MRSA-positive HCAP, HAP, or VAP, Haque et al. reported that hVISA accounted for 15.9% of infections (40). The 28-day all-cause mortality was 37.1%. hVISA was not significantly associated with mortality; however, this was not a primary outcome metric for the study. Our current study is unique with regard to its focus on clinical outcomes in patients with hVISA pneumonia compared to those in patients with VSSA pneumonia. Although baseline and clinical characteristics were balanced between the two groups, patients with hVISA pneumonia experienced a significantly higher rate of mortality. This was in spite of the fact that vancomycin treatment failure rates were not significantly different. The occurrence of vancomycin failure in the hVISA cohort was similar to that in our previous analysis (82% in the previous study versus 79% in the present study) but considerably higher in the VSSA cohort (33% in the previous study versus 59% in the present study) (20). This difference was largely driven by a change in antibiotic therapy in the VSSA cohort.
Delayed appropriate antibiotic therapy (greater than 24 to 48 h after initial diagnosis) may help to explain the current finding of increased mortality in the hVISA cohort. Previous publications have related delayed appropriate therapy in the setting of pneumonia to increased mortality (5, 41). In a recent publication by Inchai et al., late antibiotic therapy (>24 h) was associated with a hazard ratio of 2.23 (95% CI, 1.12 to 4.45; P = 0.022) among a single-center cohort of patients diagnosed with VAP (6). In contrast, DeRyke et al. reported no significant difference in inpatient mortality rates among VAP patients that received timely versus delayed appropriate antibiotic therapy (42). Evidence in community-acquired pneumonia (CAP) also fails to demonstrate consistent conclusions (41, 43, 44). Timely administration of appropriate antibiotics has been previously demonstrated in MRSA BSI (45). In patients with hospital-acquired S. aureus BSI, delays in appropriate antibiotic therapy of greater than 44.8 h resulted in a 3-fold-increased odds of infection-related mortality. In our current hVISA cohort, over 90% of patients had positive blood cultures, and the median time to change in antibiotic therapy was over 60 h, which is over the proposed breakpoint of 44.8 h established by Lodise et al. To our knowledge there is no available literature that directly addresses the timing of appropriate antibiotic therapy in hVISA versus VSSA infection; therefore, literature from MRSA BSI has been extrapolated to the current study. Additionally, our current sample size is not powered to adequately determine if delayed appropriate antibiotic therapy is an independent predictor of inpatient mortality; however, this cannot be ruled out and deserves further future investigation.
Several isolate-related characteristics have also been associated with the presence of the hVISA phenotype, increased mortality, or both. In the current study, patients with hVISA infection were more likely to have an elevated vancomycin MIC (2 mg/liter by BMD) than patients with VSSA infection. This has been demonstrated to be the case in previous hVISA studies (20, 46). Additionally, elevated vancomycin MICs have been linked to increased mortality in MRSA pneumonia (47). In the current study, there was a strong association between PVL positivity and increased mortality. The presence of PVL has been inconsistently associated with mortality (48, 49). In fact, in the study by Haque et al., PVL negativity was associated with increased mortality on univariate analysis but was not an independent predictor of mortality (25). Dysfunction of agr was not associated with the hVISA phenotype although this association has been demonstrated in the past (50). Also of note, agr dysfunction was not associated with worse outcomes or increased mortality, as has been previously demonstrated (34).
This study has several limitations, namely, those associated with retrospective single-center studies. Efforts were made to control for confounding variables through matching on relevant parameters. Additionally, physiological parameters were well balanced between the cohorts. We cannot exclude the possibility that confounding still occurred, however, as the small sample size precludes an extensive analysis. In patients who were bacteremic, every attempt was made to secure both respiratory and blood isolates. Due to the retrospective nature of the research, however, this was unachievable for some patients, and PAP-AUC and molecular analysis were carried out on the available isolate. Without pulse-field gel electrophoresis it is not possible to definitely determine if this study represents the impact of a predominant clonal strain of hVISA; however, through the molecular testing that was completed, there is evidence of a diversity of characteristics among the available strains. Although the rate of concurrent bacteremia is significantly higher in this study than that in a recent publication by Shorr et al. (51), this is due to the limitations listed above. Additionally, since the rates of bacteremia were not significantly different in the two groups studied, they likely represent a nondifferential bias. Lastly, the diagnosis of pneumonia was based on clinically defined criteria as described above; cases may have been missed in the eMRs.
In conclusion, in patients diagnosed with MRSA pneumonia, the presence of the hVISA phenotype was independently associated with increased inpatient mortality. Patients with hVISA pneumonia were more likely to have an elevated vancomycin MIC by BMD, which may contribute to this increased mortality. The current findings with respect to delayed appropriate therapy in hVISA versus VSSA infection are exploratory and deserve further, focused investigation.
ACKNOWLEDGMENTS
S.L.D. has received grant support from Cubist (now Merck) and Actavis and served as a consultant for Actavis, Premier, and Pfizer. M.J.R. has received grant support from and participated on speaker bureaus or consulted for Actavis, Bayer, Cempra, Cubist (now Merck), The Medicine Company, Sunovian, and Theravance.
Funding Statement
This research received no financial support.
REFERENCES
- 1.Eiland EH, Wargo KA, Hamm W, Hassoun AA. 2007. Analysis of adherence to national nosocomial pneumonia treatment guidelines. Ther Clin Risk Manag 3:983–988. [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 4.Frei CR, Attridge RT, Mortensen EM, Restrepo MI, Yu Y, Oramasionwu CU, Ruiz JL, Burgess DS. 2010. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther 32:293–299. doi: 10.1016/j.clinthera.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. 2002. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 6.Inchai J, Pothirat C, Liwsrisakun C, Deesomchok A, Kositsakulchai W, Chalermpanchai N. 2015. Ventilator-associated pneumonia: epidemiology and prognostic factors of 30-day mortality. Jpn J Infect Dis 68:181–186. doi: 10.7883/yoken.JJID.2014.282. [DOI] [PubMed] [Google Scholar]
- 7.Mathevon T, Souweine B, Traore O, Aublet B, Caillaud D. 2002. ICU-acquired nosocomial infection: impact of delay of adequate antibiotic treatment. Scand J Infect Dis 34:831–835. doi: 10.1080/0036554021000026934. [DOI] [PubMed] [Google Scholar]
- 8.Woods C, Colice G. 2014. Methicillin-resistant Staphylococcus aureus pneumonia in adults. Expert Rev Respir Med 8:641–651. doi: 10.1586/17476348.2014.940323. [DOI] [PubMed] [Google Scholar]
- 9.Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin SK, Hageman J, McDougal LK, Mohammed J, Jarvis WR, Perl TM, Tenover FC. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis 36:429–439. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Chambers HF. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 47:3040–3045. doi: 10.1128/AAC.47.10.3040-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG Jr, International Collaboration on Endocarditis-Microbiology Investigator. 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis 200:1355–1366. doi: 10.1086/606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhari CN, Tandel K, Grover N, Sen S, Bhatt P, Sahni AK, Praharaj AK. 2015. Heterogeneous vancomycin-intermediate among methicillin resistant Staphylococcus aureus. Med J Armed Forces India 71:15–18. doi: 10.1016/j.mjafi.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sancak B, Yagci S, Gur D, Gulay Z, Ogunc D, Soyletir G, Yalcin AN, Dundar DO, Topcu AW, Aksit F, Usluer G, Ozakin C, Akalin H, Hayran M, Korten V. 2013. Vancomycin and daptomycin minimum inhibitory concentration distribution and occurrence of heteroresistance among methicillin-resistant Staphylococcus aureus blood isolates in Turkey. BMC Infect Dis 13:583. doi: 10.1186/1471-2334-13-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitz AM, Yu F, Hermsen ED, Rupp ME, Fey PD, Olsen KM. 2011. Vancomycin susceptibility trends and prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus in clinical methicillin-resistant S. aureus isolates. J Clin Microbiol 49:269–274. doi: 10.1128/JCM.00914-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J Clin Microbiol 46:2950–2954. doi: 10.1128/JCM.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maor Y, Hagin M, Belausov N, Keller N, Ben-David D, Rahav G. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J Infect Dis 199:619–624. doi: 10.1086/596629. [DOI] [PubMed] [Google Scholar]
- 20.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother 57:4252–4259. doi: 10.1128/AAC.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KH, Kim ES, Kim HS, Park SJ, Bang KM, Park HJ, Park SY, Moon SM, Chong YP, Kim SH, Lee SO, Choi SH, Jeong JY, Kim MN, Woo JH, Kim YS. 2012. Comparison of the clinical features, bacterial genotypes and outcomes of patients with bacteraemia due to heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-susceptible S. aureus. J Antimicrob Chemother 67:1843–1849. doi: 10.1093/jac/dks131. [DOI] [PubMed] [Google Scholar]
- 22.van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6:e21217. doi: 10.1371/journal.pone.0021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 38:521–528. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 24.van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother 55:405–410. doi: 10.1128/AAC.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque NZ, Zuniga LC, Peyrani P, Reyes K, Lamerato L, Moore CL, Patel S, Allen M, Peterson E, Wiemken T, Cano E, Mangino JE, Kett DH, Ramirez JA, Zervos MJ. 2010. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest 138:1356–1362. doi: 10.1378/chest.09-2453. [DOI] [PubMed] [Google Scholar]
- 26.National Healthcare Safety Network. 2012. Ventilator-associated event (VAE) surveillance for adults special edition. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/pdfs/newsletters/vae-newsletter-september2012.pdf. [Google Scholar]
- 27.Centers for Disease Control and Prevention. 2013. CDC/NHSN surveillance definitions for specific types of infections. Centers for Disease Control and Prevention, Atlanta, GA: Accessed 18 September 2014. [Google Scholar]
- 28.American Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 29.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. Document M07-19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. 2008. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and Panton-Valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol 46:1118–1122. doi: 10.1128/JCM.01309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milheirico C, Oliveira DC, de Lencastre H. 2007. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: “SCCmec IV multiplex.”. J Antimicrob Chemother 60:42–48. [DOI] [PubMed] [Google Scholar]
- 34.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN. 2011. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother 55:1082–1087. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 36.Maor Y, Rahav G, Belausov N, Ben-David D, Smollan G, Keller N. 2007. Prevalence and characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a tertiary care center. J Clin Microbiol 45:1511–1514. doi: 10.1128/JCM.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariza J, Pujol M, Cabo J, Pena C, Fernandez N, Linares J, Ayats J, Gudiol F. 1999. Vancomycin in surgical infections due to methicillin-resistant Staphylococcus aureus with heterogeneous resistance to vancomycin. Lancet 353:1587–1588. doi: 10.1016/S0140-6736(99)01017-X. [DOI] [PubMed] [Google Scholar]
- 38.Horne KC, Howden BP, Grabsch EA, Graham M, Ward PB, Xie S, Mayall BC, Johnson PD, Grayson ML. 2009. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob Agents Chemother 53:3447–3452. doi: 10.1128/AAC.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendes RE, Deshpande LM, Smyth DS, Shopsin B, Farrell DJ, Jones RN. 2012. Characterization of methicillin-resistant Staphylococcus aureus strains recovered from a phase IV clinical trial for linezolid versus vancomycin for treatment of nosocomial pneumonia. J Clin Microbiol 50:3694–3702. doi: 10.1128/JCM.02024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haque NZ, Arshad S, Peyrani P, Ford KD, Perri MB, Jacobsen G, Reyes K, Scerpella EG, Ramirez JA, Zervos MJ. 2012. Analysis of pathogen and host factors related to clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus. J Clin Microbiol 50:1640–1644. doi: 10.1128/JCM.06701-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houck PM, Bratzler DW. 2005. Administration of first hospital antibiotics for community-acquired pneumonia: does timeliness affect outcomes? Curr Opin Infect Dis 18:151–156. doi: 10.1097/01.qco.0000160905.94684.91. [DOI] [PubMed] [Google Scholar]
- 42.DeRyke CA, Lodise TP Jr, Rybak MJ, McKinnon PS. 2005. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest 128:1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 43.Yu KT, Wyer PC. 2008. Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia. Ann Emerg Med 51:651–662. doi: 10.1016/j.annemergmed.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Cheng AC, Buising KL. 2009. Delayed administration of antibiotics and mortality in patients with community-acquired pneumonia. Ann Emerg Med 53:618–624. doi: 10.1016/j.annemergmed.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 36:1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 46.Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, Khatib R. 2009. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol 47:1640–1644. doi: 10.1128/JCM.02135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadros M, Williams V, Coleman BL, McGeer AJ, Haider S, Lee C, Iacovides H, Rubinstein E, John M, Johnston L, McNeil S, Katz K, Laffin N, Suh KN, Powis J, Smith S, Taylor G, Watt C, Simor AE. 2013. Epidemiology and outcome of pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) in Canadian hospitals. PLoS One 8:e75171. doi: 10.1371/journal.pone.0075171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyrani P, Allen M, Wiemken TL, Haque NZ, Zervos MJ, Ford KD, Scerpella EG, Mangino JE, Kett DH, Ramirez JA, IMPACT-HAP Study Group . 2011. Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis 53:766–771. doi: 10.1093/cid/cir541. [DOI] [PubMed] [Google Scholar]
- 49.Sharma-Kuinkel BK, Ahn SH, Rude TH, Zhang Y, Tong SY, Ruffin F, Genter FC, Braughton KR, Deleo FR, Barriere SL, Fowler VG Jr. 2012. Presence of genes encoding Panton-Valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J Clin Microbiol 50:848–856. doi: 10.1128/JCM.06219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butterfield JM, Tsuji BT, Brown J, Ashley ED, Hardy D, Brown K, Forrest A, Lodise TP. 2011. Predictors of agr dysfunction in methicillin-resistant Staphylococcus aureus (MRSA) isolates among patients with MRSA bloodstream infections. Antimicrob Agents Chemother 55:5433–5437. doi: 10.1128/AAC.00407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. 2015. Outcomes associated with bacteremia in the setting of methicillin-resistant Staphylococcus aureus pneumonia: a retrospective cohort study. Crit Care 19:312. doi: 10.1186/s13054-015-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]