Abstract
The current recommendations for intravenous (i.v.) acyclovir dosing in obese patients suggest using ideal body weight (IBW) rather than total body weight (TBW). To our knowledge, no pharmacokinetic analysis has validated this recommendation. This single-dose pharmacokinetic study was conducted in an inpatient oncology population. Enrollment was conducted by 1:1 matching of obese patients (>190% of IBW) to normal-weight patients (80 to 120% of IBW). All patients received a single dose of i.v. acyclovir, 5 mg/kg, infused over 60 min. Consistent with current recommendations, IBW was used for obese patients and TBW for normal-weight patients. Serial plasma concentrations were obtained and compared. Seven obese and seven normal-weight patients were enrolled, with mean body mass indexes of 45.0 and 22.5 kg/m2, respectively. Systemic clearance was substantially higher in the obese than normal-weight patients (mean, 19.4 ± 5.3 versus 14.3 ± 5.4 liters/h; P = 0.047). Area under the concentration-time curve was lower in the obese patients (15.2 ± 2.9 versus 24.0 ± 9.4 mg · h/liter; P = 0.011), as was maximum concentration (5.8 ± 0.9 versus 8.2 ± 1.3 mg/liter; P = 0.031). Utilization of IBW for dose calculation of i.v. acyclovir in obese patients leads to lower systemic exposure than dosing by TBW in normal-weight patients. While not directly evaluated in this study, utilization of an adjusted body weight for dose determination appears to more closely approximate the exposure seen in normal-weight patients. (This study has been registered at ClinicalTrials.gov under registration no. NCT01714180.)
INTRODUCTION
Thirty-five percent of adults older than 20 years of age are classified as obese (body mass index [BMI], ≥30 kg/m2) in the United States and an additional 34% as overweight (BMI, <30 but ≥25 kg/m2). Class III obesity (BMI, ≥40 kg/m2) has risen from 1.3% in the 1980s to 6.6% currently (1). Body composition is a major factor influencing drug pharmacokinetics (PK) and can alter several parameters, including volume of distribution (V) and clearance (CL) (2). Unfortunately, published PK data specific to obese patients are limited. While biomarkers and surrogate endpoints can direct dosing of certain agents, such as antihypertensives and lipid-lowering drugs, no such biomarkers exist for antimicrobials; thus, clinicians must rely on published PK and pharmacodynamic (PD) data.
Acyclovir is a nucleoside analogue that possesses activity against the herpes family of viruses, including herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) and varicella-zoster virus (VZV) (3). The efficacy of acyclovir for treating HSV has been linked to both area under the plasma concentration-time curve (AUC) and the time that drug concentration remains above the 50% inhibitory concentration (T>IC50) (4–7). The T>IC50 target that has been proposed is 50% of the dosing interval (4–9). Controversy exists regarding which of these PD parameters is most important in predicting clinical success.
The manufacturer recommends calculation of intravenous (i.v.) acyclovir dose to be based on total body weight (TBW); however, in obese patients it recommends dosing by ideal body weight (IBW) (3). The only literature to support this recommendation is a single PK analysis comparing morbidly obese (MO) to normal-weight (NW) healthy females, presented as an abstract in 1991 by Davis and colleagues (10). All participants received doses based on TBW. Dosing by TBW in MO patients resulted in approximately 2-fold-higher maximum concentrations than in NW patients. The authors concluded that dosing by TBW in MO patients was inappropriate and recommended the use of IBW in this population (10). The conclusions of this study remain to be validated. The decision to not use TBW in dosing morbidly obese patients is supported by at least one case of an obese patient developing acyclovir-induced renal failure following i.v. acyclovir dosed by TBW (11). A prospective evaluation of IBW to dose acyclovir in obese patients has not been published. The objective of the present study was to evaluate the PK of i.v. acyclovir in MO patients utilizing dosing recommendations from the manufacturer's prescribing information.
(This study was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC, 2014.)
MATERIALS AND METHODS
Study design.
This was a prospective, matched-pair study in patients admitted to an inpatient oncology ward. The primary outcome was difference in acyclovir systemic clearance (CL) between MO and NW patients. Secondary outcomes included AUC0–∞, T>IC50 using a standardized IC50, and maximum concentration (Cmax). This study was approved by the Institutional Review Board of West Virginia University. Written informed consent was obtained before patient enrollment.
Inclusion/exclusion criteria.
Patients at least 18 years of age requiring acyclovir as part of routine clinical care were screened for inclusion. Patients were excluded if they had a serum creatinine (SCr) level of >1.4 mg/dl, exhibited clinical instability (defined as ICU admission or receipt of vasopressor support in the prior 24 h), were receiving medications known to interact with acyclovir, or had received acyclovir or valacyclovir in the previous 24 h. Enrollment was conducted by 1:1 matching of MO patients (TBW greater than or equal to 190% of IBW, calculated by the Devine equation [12]) to NW patients (TBW from 80 to 120% of IBW) by gender and by an age of ±10 years.
Acyclovir administration.
Patients received i.v. acyclovir sodium, 5 mg/kg, utilizing IBW for MO patients and TBW for NW patients, consistent with current recommendations. Acyclovir was prepared in 100 ml of 0.9% sodium chloride and infused over 60 min via an infusion pump.
Acyclovir concentration determination.
Blood samples were collected serially immediately prior to the first dose of i.v. acyclovir and at 30, 60, 75, 90, 120, 180, 300, 420, 540, and 720 min following initiation of the infusion. Blood samples were immediately placed on ice and subsequently centrifuged for isolation of plasma by the WVU Health Sciences Biospecimen Processing Core. Samples were stored at −20°C until analysis.
Samples were analyzed within 30 days of collection at NMS Labs (Willow Grove, PA) using liquid chromatography-tandem mass spectrometry that was internally validated. Briefly, labeled internal standard (acyclovir-d4) was added to diluted plasma samples, which were then deproteinized with trichloroacetic acid. The supernatant was analyzed by high-performance liquid chromatography separation using positive-ion electrospray tandem mass spectrometry (LC-MS/MS) for detection and quantitation. Calibration curves were constructed to quantify the concentration of acyclovir using a quadratic regression with 1/x weighting. The lower limit of quantitation for acyclovir was 0.020 μg/ml with additional calibration points at 0.050, 0.10, 0.50, 1.0, and 2.0 μg/ml. Within-run precision for the high, low, and lowest observable quantities assay were 2.6 to 5.2%, whereas between-run values were 3.7 to 6.8%. Similarly, assay accuracy for the high, low, and lowest observable quantities were 92 to 95% and 94 to 96% for within-run and between-run values, respectively. Plasma samples with initial concentrations greater than 2 μg/ml were diluted until the concentration was within the range of the assay. Two levels of quality control, 0.050 and 1.0 μg/ml, were prepared in bulk and frozen. These were utilized to evaluate each analytic run. Coefficients of variation for the assay were 7.69% and 10.20% for the high and low controls, respectively.
Acyclovir pharmacokinetics.
Pharmacokinetic parameter estimates for each patient's data set were generated by the standard two-stage approach using compartmental modeling (WinNonlin version 2.1; Pharsight Corporation, Mountain View, CA) by the WVU Health Sciences Clinical Pharmacology Core. Selection of the most appropriate model for each patient's data was primarily based on the Akaike information criterion. The absolute dose (nonnormalized) was utilized as the input variable for all analyses. Actual sample times obtained from initiation of the dose were calculated from the case report forms and used as primary input data. The optimal model was utilized to generate simulated data for estimation of the individual patients' IC50s at steady state with 12-h dosing intervals. Evaluations of T>IC50 were conducted using IC50s of 0.5625 mg/liter for HSV and 1.125 mg/liter for VZV, which had been reported previously and correspond to more resistant strains (8, 13).
Statistics.
A sample size of 7 patients per group was estimated to provide an 80% power to detect a 19% difference in CL using a two-sided paired t test at significance level of 0.05 when the correlation coefficient is 0.15. In the data analysis of comparison on the primary and secondary outcomes, Wilcoxon signed-rank test was used for continuous variables with paired data and Wilcoxon rank sum test was used for continuous variables between two groups, while Fisher's exact test was used in the data analysis between categorical variables. To explore optimal dosing strategies, CL was compared between MO and NW patients after normalizing by different measures of body size, including body surface area (BSA), TBW, IBW, lean body weight (LBW), and adjusted body weight (AjBW). AjBW was calculated as IBW plus 40% of TBW greater than IBW [AjBW = IBW + 0.4 × (TBW − IBW)] and LBW as previously described (14). Simple linear regression was performed to assess correlation of these body parameters with PK parameters. Analyses were considered statistically significant if P was <0.05. All statistical analyses were performed using Stata (Stata statistical software, release 13, 2014; StataCorp LP, College Station, TX) and R software (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
A total of 14 patients were enrolled and completed the study (7 patients per group). Baseline data were similar between groups with the exception of TBW, BMI, percentage of IBW, and BSA (Table 1). All patients in the MO group were classified as class III obesity (BMI, ≥40 kg/m2). All patients were receiving acyclovir for prophylaxis with chemotherapy regimens with an anticipated prolonged duration of neutropenia. None of the patients in the study were febrile or neutropenic or had any signs of viral infection at the time of sampling.
TABLE 1.
Baseline characteristics
| Characteristica | Value for patientsb |
Pc | |
|---|---|---|---|
| Morbidly obese (n = 7) | Normal wt (n = 7) | ||
| Age (yr) | 54.3 ± 9.6 | 53.0 ± 16.3 | 0.87 |
| Caucasian race (%) | 7 (100.0) | 7 (100.0) | 1.0 |
| Female (%) | 6 (85.7) | 6 (85.7) | 1.0 |
| Weight (kg) | 120.5 ± 15.7 | 61.2 ± 5.1 | 0.016 |
| IBW (kg) | 57.1 ± 8.8 | 58.5 ± 5.5 | 0.69 |
| % of IBW | 212.4 ± 15.4 | 105.2 ± 10.7 | 0.001 |
| BMI (kg/m2) | 45.0 ± 3.4 | 22.5 ± 2.2 | 0.016 |
| BSA (m2) | 2.3 ± 0.2 | 1.7 ± 0.1 | 0.016 |
| SCr (mg/dl) | 0.78 ± 0.26 | 0.76 ± 0.15 | 0.69 |
| GFR (ml/min/1.73 m2) (21) | 93.4 ± 24.9 | 93.7 ± 25.7 | 0.94 |
BMI, body mass index; BSA, body surface area; GFR, glomerular filtration rate; IBW, ideal body weight; SCr, serum creatinine.
Data are means ± standard deviations unless otherwise noted.
Determined by the Wilcoxon rank sum or Fisher exact test, as appropriate.
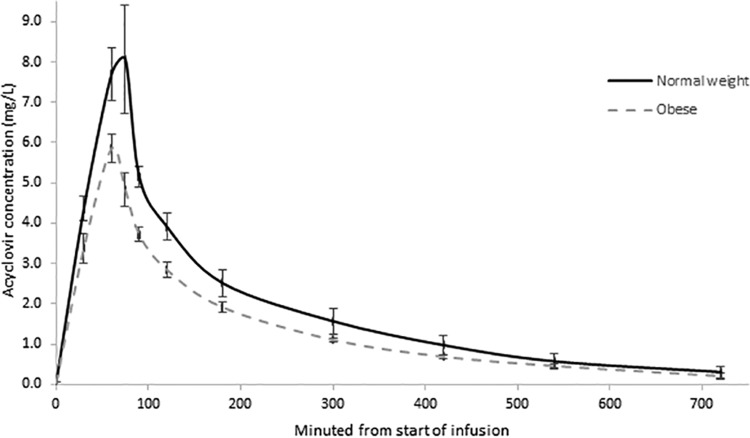
CL was significantly higher in MO than NW patients, while AUC0–∞ and Cmax were significantly lower in MO patients (Table 2; Fig. 1). This difference in AUC0–∞ represents a 37% (95% confidence interval [CI], 6.5% to 66.5%) relative decrease in total exposure for MO patients. The T>IC50 for HSV- and VZV-inhibitory concentrations were 23% (95% CI, −17% to 63%) and 29% (95% CI, −9% to 67%) lower in the MO than NW patients (Table 2).
TABLE 2.
Comparison of mean pharmacokinetic parameters
| Parametera | Value for patients |
Pb | |
|---|---|---|---|
| Morbidly obese (n = 7) | Normal wt (n = 7) | ||
| Dose (mg) | 285 ± 44 | 303 ± 26 | 0.55 |
| Cmax (mg/liter) | 5.8 ± 0.9 | 8.2 ± 1.3 | 0.031 |
| AUC0-∞ (mg·hr/liter) | 15.2 ± 2.9 | 24.0 ± 9.4 | 0.011 |
| Time > 0.5625 mg/liter (min) | 402.6 ± 204.2 | 524.3 ± 253.0 | 0.22 |
| Time > 1.125 mg/liter (min) | 264.9 ± 54.5 | 373.1 ± 181.6 | 0.08 |
| CL (liters/h) | 19.4 ± 5.3 | 14.3 ± 5.4 | 0.047 |
| V (liters) | 31.8 ± 9.9 | 25.9 ± 10.4 | 0.29 |
AUC0–∞, area under the curve from time zero to infinity; CL, systemic clearance; Cmax, maximum concentration; V, volume of distribution.
Determined by the Wilcoxon signed-rank test.
FIG 1.
Mean acyclovir concentration-time curve for normal-weight and morbidly obese patients.
Simple linear regression indicated that CL was more closely correlated to AjBW (r2 = 0.48, P = 0.006) and BSA (r2 = 0.45, P = 0.008) than IBW (r2 = 0.30, P = 0.052), LBW (r2 = 0.34, P = 0.34), or TBW (r2 = 0.40, P = 0.016). Using MO-patient-specific PK parameters, a dose determined by AjBW would result in an estimated AUC0–∞ similar to that observed in NW patients (22.2 ± 4.9 versus 24.0 ± 9.4 mg · h/liter; P = 0.49). While these data are based on this single-dose analysis, we expect that dosing over several days would provide similar results.
DISCUSSION
The objective of this study was to evaluate the currently recommended dosing strategy for i.v. acyclovir in MO patients. The PK parameters observed in the current study for NW patients are similar to those previously reported for healthy, nonobese patients. Average CL for NW patients in our study (14.6 liters/h/1.73 m2) was similar to that previously reported by Laskin and colleagues (16.1 liters/h/1.73 m2) and Blum and colleagues (19.6 liters/h/1.73 m2) (15, 16). Laskin and colleagues reported a similar AUC of 23.2 mg · h/liter in volunteer patients following a single dose of 5 mg/kg (15). In addition, steady-state volume (VSS) for NW patients in our study (40.5 liters/1.73 m2 or 0.63 liter/kg) was similar to that reported by Laskin and colleagues (43 liters/1.73 m2), Blum and colleagues (49.7 liters/1.73 m2), Spector and colleagues (0.80 liter/kg), and Acosta and colleagues (0.89 liter/kg) (15–17, 20).
The only previous study evaluating dosing in MO patients was presented in abstract form in 1991 by Davis and colleagues (10). The investigators enrolled 7 MO and 5 NW healthy females (mean of 203% and 96% of IBW, respectively). They administered a single infusion of i.v. acyclovir, 5 mg/kg, dosed by TBW in all patients. They identified a higher Cmax (14.9 versus 7.5 mg/liter; P = 0.003) in the MO patients but similar clearance (18.0 versus 16.9 liters/h; P reported as not significant) and volume of distribution (43.5 versus 42.5 liters; P reported as not significant). To guide dosing recommendations in MO patients, they normalized CL and VSS by IBW and TBW in both MO and NW groups. CL and VSS in MO patients more closely approximated those in NW patients when dosing wads normalized by IBW than TBW, leading to their recommendation to use IBW for dosing. Adjusted body weight was not evaluated in this study.
While previous data (10, 11) have shown that using TBW for dose determination leads to excessive acyclovir exposure in obese patients, our study found that dosing by IBW will provide substantially lower exposure than in nonobese controls. Using patient-specific PK parameters in the MO patients, utilizing an AjBW to dose acyclovir would result in similar exposure (AUC0–∞) compared to our NW patients. While we agree with Davis and colleagues (10) that TBW leads to excess exposure in MO patients, dosing by AjBW (correction factor of 0.4) may more closely approximate drug exposure in NW patients. Utilizing BSA to dose obese patients may also result in exposure similar to that in NW patients; however, dosing by BSA is uncommon with antimicrobials and may lead to additional difficulties, as the dosing recommendation for i.v. acyclovir in NW patients is based on weight.
While the difference did not reach statistical significance, we found MO patients to have lower T>IC50 than NW patients (Table 2). With dosing every 12 h, a similar number of MO and NW patients achieved the T>IC50 goal of 50% in plasma for both HSV (57.1% and 71.4% for MO and NW patients; P = 1.0) and VZV (14.3% and 42.9% for MO and NW patients; P = 0.56). Note that achievement of this target is based on samples collected from blood and is likely different from PD parameters in other body compartments.
Limited studies evaluating dosing of acyclovir and valacyclovir for treatment of genital herpes suggest that AUC and T>IC50 are both associated with efficacy (4–7). It should be noted that genital herpes is commonly treated with oral rather than i.v. acyclovir. Unfortunately, trials evaluating the PD of i.v. acyclovir in treating more invasive infections, such as HSV encephalitis, are lacking, and extrapolation of PD targets may not be appropriate.
Several important limitations exist in this study. First, our study was conducted with lower doses of i.v. acyclovir (5 mg/kg). While escalating doses result in linear increases in AUC and similar V (15, 17), caution should be exercised in extrapolating these data to other dosing regimens. Second, these patients were all receiving acyclovir for prophylaxis and were not actively infected or critically ill. Altered PK are often present in patients that are critically ill (18, 19). Third, our study evaluated the PK from blood samples only. Concentrations at different body sites, such as in cerebral spinal fluid, were not evaluated in this study and the PK parameters obtained in our study cannot be easily transposed to these body sites. Fourth, we evaluated only obese patients with BMIs of ≥40 kg/m2 (class III obesity) and did not include any patients with BMIs from 25.0 to 39.9 kg/m2. Finally, these were cancer patients receiving active chemotherapy treatments, but we have no reason to believe this would affect the PK characteristics with i.v. administration compared to nononcology patients.
Conclusions.
Our data suggest that MO (BMI, ≥40.0 kg/m2) patients treated with i.v. acyclovir dosed by IBW experience substantially decreased overall exposure compared to NW patients dosed by TBW. While not directly evaluated in this study, utilization of an AjBW [IBW + 0.4 × (TBW − IBW)] appears to more closely approximate the exposure seen in NW patients. Future research is needed to verify this finding and to explore appropriate dosing in this population and in other classes of obesity.
ACKNOWLEDGMENTS
S.W. and W.P. acknowledge support from the National Institute of General Medical Sciences grant U54GM104942. W.P. acknowledges support for the core labs of the West Virginia University Health Sciences Center, which are supported in part by NIH grants P30 RR 032138, P30 GM103488, and U54GM104942, as well as the Mylan Chair of Pharmacology at WVU.
Research reported in this publication was supported internally by the Center of Quality Outcomes at West Virginia University Healthcare.
We have no conflicts of interest to report.
Funding Statement
This project was funded by internal funding from the Center of Quality Outcomes at West Virginia University Healthcare. None of the above grant funding was for the submitted research project.
REFERENCES
- 1.Center for Disease Control and Prevention. 2014. Health, United States, 2013: with special feature on prescription drugs. Center for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 3.GlaxoSmithKline. 2010. Acyclovir (Zovirax) prescribing information. GlaxoSmithKline LLC, Research Triangle Park, NC. [Google Scholar]
- 4.Reitano M, Tyring S, Lang W, Thoming C, Worm AM, Borelli S, Chambers LO, Robinson JM, Corey L. 1998. Valaciclovir for the suppression of recurrent genital HSV infection: a large-scale dose range-finding study. J Infect Dis 178:603–610. doi: 10.1086/515385. [DOI] [PubMed] [Google Scholar]
- 5.Spruance SL, Tyring SK, De Gregoris B, Miller C, Beutner K. 1996. A large scale, placebo-controlled, dose-ranging trial of peroral valaciclovir for episodic treatment of recurrent herpes genitalis. Arch Intern Med 156:1729–1739. doi: 10.1001/archinte.156.15.1729. [DOI] [PubMed] [Google Scholar]
- 6.Saiag P, Praindhi D, Chastang C. 1999. A double-blind, randomized study assessing the equivalence of valacyclovir 1000 mg once daily versus 500 mg twice daily in the episodic treatment of recurrent genital herpes. J Antimicrob Chemother 44:525–531. doi: 10.1093/jac/44.4.525. [DOI] [PubMed] [Google Scholar]
- 7.Bye A. 1997. A mechanistic approach to understanding the response to antiinfectives in the population approach: measuring and managing variability in response, concentration and dose, p 105–113. European Commission, Brussels, Belgium. [Google Scholar]
- 8.Tod M, Lokiec F, Bidault R, De Bony F, Petitjean O, Aujard Y. 2001. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother 45:150–157. doi: 10.1128/AAC.45.1.150-157.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, Smiley ML. 1993. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther 54:595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]
- 10.Davis RL, Quenzer RW, Weller S, Blum R. 1991. Acyclovir pharmacokinetics in morbid obesity, abstr 765. In Abstr 31st Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 11.Hernandez JO, Norstrom J, Wysock G. 2009. Acyclovir-induced renal failure in an obese patient. Am J Health-Syst Pharm 66:1288–1291. doi: 10.2146/ajhp080307. [DOI] [PubMed] [Google Scholar]
- 12.Devine BJ. 1974. Gentamicin therapy. Drug Intell Clin Pharm 8:650–655. [Google Scholar]
- 13.Zeng L, Nath CE, Blair EYL, Shaw PJ, Stephen K, Earl JW, Coakley JC, MacLachlan AJ. 2009. Population pharmacokinetics of acyclovir in children and young people with malignancy after administration of intravenous acyclovir or oral valacyclovir. Antimicrob Agents Chemother 53:2918–2927. doi: 10.1128/AAC.01138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean body weight. Clin Pharmacokinet 44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 15.Laskin OL, Longstreth JA, Saral R, De Miranda P, Kenney R, Lietman PS. 1982. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother 21:393–398. doi: 10.1128/AAC.21.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum MR, Liao SHT, De Miranda P. 1982. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med 73:186–192. doi: 10.1016/0002-9343(82)90088-2. [DOI] [PubMed] [Google Scholar]
- 17.Spector SA, Connor JD, Hintz M, Quinn RP, Blum MR, Keeney RE. 1981. Single-dose pharmacokinetics of acyclovir. Antimicrob Agents Chemother 19:608–612. doi: 10.1128/AAC.19.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pea F. 2013. Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr Clin Pharmacol 8:5–12. doi: 10.2174/1574884711308010003. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello Rhodes JA, Starr T, Wallis SC, Lipman J. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 20.Acosta EP, Balfour HH Jr. 2001. Acyclovir for treatment of postherpetic neuralgia: efficacy and pharmacokinetics. Antimicro Agents Chemother 45:2771–2774. doi: 10.1128/AAC.45.10.2771-2774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. [DOI] [PubMed] [Google Scholar]