Abstract
Proteus mirabilis forms dense crystalline biofilms on catheter surfaces that occlude urine flow, leading to serious clinical complications in long-term catheterized patients, but there are presently no truly effective approaches to control catheter blockage by this organism. This study evaluated the potential for bacteriophage therapy to control P. mirabilis infection and prevent catheter blockage. Representative in vitro models of the catheterized urinary tract, simulating a complete closed drainage system as used in clinical practice, were employed to evaluate the performance of phage therapy in preventing blockage. Models mimicking either an established infection or early colonization of the catheterized urinary tract were treated with a single dose of a 3-phage cocktail, and the impact on time taken for catheters to block, as well as levels of crystalline biofilm formation, was measured. In models of established infection, phage treatment significantly increased time taken for catheters to block (∼3-fold) compared to untreated controls. However, in models simulating early-stage infection, phage treatment eradicated P. mirabilis and prevented blockage entirely. Analysis of catheters from models of established infection 10 h after phage application demonstrated that phage significantly reduced crystalline biofilm formation but did not significantly reduce the level of planktonic cells in the residual bladder urine. Taken together, these results show that bacteriophage constitute a promising strategy for the prevention of catheter blockage but that methods to deliver phage in sufficient numbers and within a key therapeutic window (early infection) will also be important to the successful application of phage to this problem.
INTRODUCTION
A frequent complication associated with long-term urethral catheterization is the encrustation and blockage of catheters due to infection with Proteus mirabilis, which can be isolated from around 45% of catheter-associated urinary tract infections (CAUTI) (1, 2). Blockage stems from the ability of P. mirabilis to form dense biofilms on catheter surfaces and the production of a potent urease enzyme which generates ammonia through hydrolysis of urea (1, 3, 4). Ammonia production elevates urinary pH, causing the precipitation of calcium and magnesium phosphates and the subsequent formation of crystals which become trapped within developing biofilms (1, 5). Once embedded in the biofilm, crystal growth is stabilized and enhanced by the biofilm matrix (6, 7). As this process continues, the biofilm gradually becomes mineralized, leading to development of extensive crystalline biofilm structures which ultimately block catheters (1, 5). If blockage is unnoticed, it can lead to reflux of infected urine to the upper urinary tract and the onset of serious clinical complications, including pyelonephritis, septicemia, and shock (1, 8).
Although catheters containing antimicrobial coatings are currently available, their efficacy in preventing infection during even short-term use remains questionable, and all available catheter types remain susceptible to P. mirabilis encrustation and blockage (9, 10). P. mirabilis is also extremely difficult to eliminate once established in the catheterized urinary tract and often responds poorly to conventional antibiotic therapy. It can persist despite multiple catheter changes or periods without catheterization and causes chronic infection and blockage in many patients (8, 9, 11). There are presently no truly effective strategies for the control of P. mirabilis CAUTI and associated blockage, and the development of new approaches is urgently required. The aim of this study was to determine if bacteriophage (phage) therapy may constitute a viable approach to the prevention of catheter encrustation and blockage.
MATERIALS AND METHODS
Bacterial strains, media, and routine culture.
Clinical isolates of P. mirabilis (designated RS1 and RS3) used in this study were obtained from the Royal Sussex County Hospital, and all were derived from urinary tract infections. All chemicals, reagents, and growth media were obtained from Fisher Scientific (United Kingdom), Oxoid (United Kingdom), or Sigma (United Kingdom), unless otherwise stated. Bacteria were routinely cultured in lysogeny broth-derivative broth (LBDB) medium (5 g/liter yeast extract, 10 g/liter vegetable peptone, 10 g/liter sodium chloride) at 37°C with shaking or on LBDB solidified by the addition of 15 g/liter technical agar (i.e., LBDA). Soft agar overlays, used for phage enrichments, purification, and enumeration, were derived from LBDA (i.e., S-LBDA), contained 5 g/liter yeast extract, 10 g/liter vegetable peptone, and 5.75 g/liter technical agar, and were kept molten at 45°C for use in agar overlays. The artificial urine (AU) medium previously described by Stickler et al. (12), was initially prepared as a 5× concentrated stock solution containing sodium disulfate (11.5 g/liter), magnesium chloride (hexahydrate) (3.25 g/liter), sodium chloride (23 g/liter), trisodium citrate (3.25 g/liter), sodium oxalate (0.1 g/liter), potassium dihydrogen orthophosphate (14 g/liter), potassium chloride (8 g/liter), ammonium chloride (5 g/liter), calcium chloride dihydrate (3.25 g/liter), urea (125 g/liter), gelatin (25 g/liter), and tryptone soya broth (5 g/liter). Stock solutions of urea and calcium chloride dihydrate were sterilized separately by membrane filtration (0.45 μm; Sartorius, United Kingdom), while other components were sterilized by autoclaving. For use in bladder models, all components were combined and diluted to 1× strength using sterile deionized water, with the final pH adjusted to 6.1.
Phage isolation and purification.
Phage were isolated from sewage collected from wastewater treatment plants in the United Kingdom (Anglian Water, Luton area). For initial enrichments of P. mirabilis phage, 387.5 ml of LBDB was mixed with 100 ml of sewage and inoculated with 2.5 ml of host growing cultures of P. mirabilis. Enrichments were incubated statically overnight at 37°C; the following day, 10-ml aliquots were recovered and centrifuged (3,000 × g for 30 min), and supernatants were filtered into fresh sterile tubes using 0.22-μm pore syringe filters (Sartorius, United Kingdom). Then, 100 μl of filtered enrichment was mixed with 100 μl of a P. mirabilis exponential-phase growing culture to be used as phage host (clinical isolate RS1 or RS3), combined with 3 ml of molten S-LBDA, swirled gently, and immediately poured over the surface of an LBDA plate. Plates were incubated at 37°C for 18 to 20 h. Phage replication was identified by zones of lysis (plaques) in the confluent bacterial growth within S-LBDA overlays. To isolate and purify distinct phage, individual plaques were picked off using Pasteur pipettes and resuspended in 300 μl of SM buffer (100 mM NaCl, 10 mM MgSO4·7H2O, 50 mM Tris-HCl [pH 7.5], 0.01% gelatin). The resulting phage suspensions were serially diluted in SM buffer (10−3 to 10−6), and dilutions were used to repeat agar overlays with host strains of P. mirabilis used in initial isolation. To ensure clonality of phage types, this process was repeated a further 5 times until bacterial lawns showed homogeneity of plaque morphology. Finally, an individual plaque was picked off and resuspended in SM buffer for use in subsequent experiments. These final clonal phage suspensions were stored at 4°C until required.
Preparation of high-titer phage stocks.
Phage were propagated on a P. mirabilis RS1 host, and high-titer stocks were obtained. Briefly, 100 μl of phage suspension and 100 μl of host growing culture were mixed, combined with 3 ml of molten S-LBDA, swirled gently, and poured onto agar plates. After a static overnight incubation at 37°C, plates displaying confluent lysis were selected, and 3 ml of SM buffer supplemented with 2% (vol/vol) chloroform (to lyse remaining bacterial cells and maximize yield) was added before incubation at 37°C for 4 h. High-titer phage solution was removed from the plates, centrifuged (8,000 × g for 10 min) to remove cell debris, and then filter sterilized (pore size, 0.22 μm) and stored at 4°C.
In vitro bladder models.
In vitro bladder models, originally described by Stickler et al. (12), were set up and operated, as described previously (13). The key features of models are illustrated in Fig. 1; the model consisted of a double-walled glass chamber (the bladder) maintained at 37°C by a water jacket supplied from a circulating water bath. Size-14 French all-silicone Foley catheters (Bard, United Kingdom) were used in all experiments and were inserted into the bladder via an outlet in the base of the glass chamber before retention balloons were inflated with 10 ml of sterile water. Catheters were subsequently attached to a drainage bag to form a sterile closed drainage system, and AU medium was supplied at a constant flow rate of 0.75 ml/min. P. mirabilis RS1 cell suspensions were inoculated directly into the residual bladder urine at either 1010 CFU or 103 CFU, representing late-sage or early stage-infection, respectively, and flow was suspended for 1 h to permit cells to establish within the system. At 45 min after bacterial inoculation, test models were treated with a single dose of 3 × 1010 PFU of a 3-phage cocktail (1:1:1; 1010 PFU of each phage) in a volume of 1 ml, and flow was restored 15 min later. The numbers of viable cells present in the residual bladder medium were enumerated at the start and end of experiments, and pH was also measured at the start and end of experiments by sampling the medium in the bladder.
FIG 1.
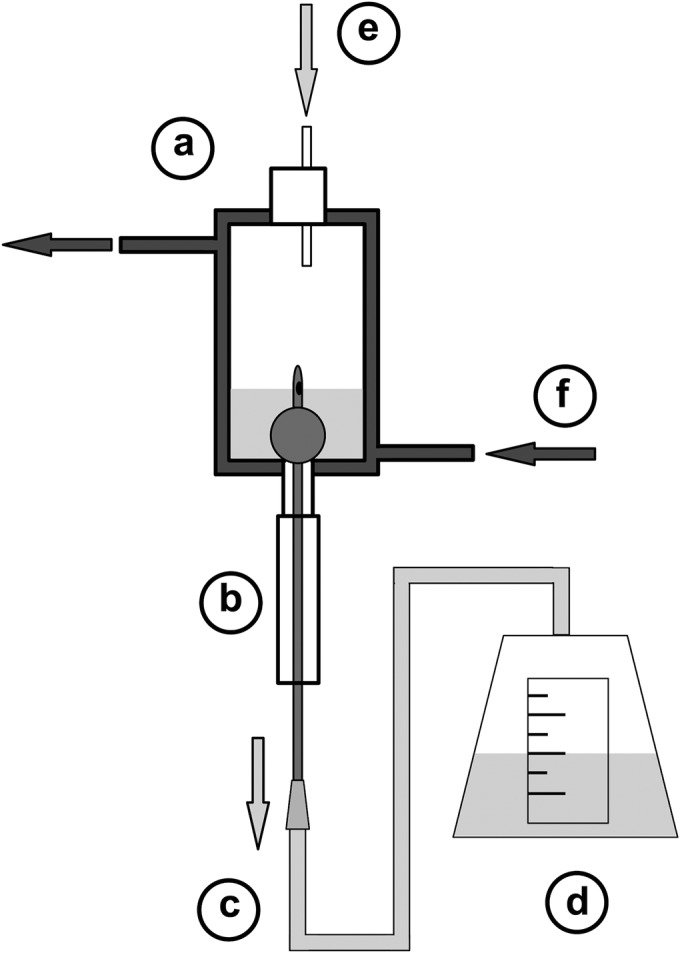
Illustration of in vitro bladder model system. Models were set up according to specifications originally described by Stickler et al. in 1999 (12). (a) Double-walled glass vessel representing the bladder. (b) Foley catheter inserted into the model and connected to drainage bag to form sterile closed drainage system. (c) Drainage tubing. (d) Drainage bag collects urine outflow. (e) Sterile urine/artificial urine supplied to bladder via peristaltic pump at a constant flow rate. (f) Water at 37°C circulated through outer bladder model chamber to maintain constant temperature. Diagram is adapted from Holling et al. (2014) (13).
Quantification of crystalline biofilm formation on catheter sections.
To measure the levels of crystalline biofilm formation and catheter encrustation in control and phage-treated models, the amount of calcium present on catheter sections removed from bladder models run for a set time (10 h) was quantified by flame photometry, as described previously (13). Briefly, 1-cm catheter sections were submerged in 2 ml of an ammonium oxalate and oxalic acid solution (95% and 5% [vol/vol], respectively, from 0.1 M stock solutions), subjected to vigorous mixing for 3 min, and then incubated at room temperature for 30 min. Catheter sections were then removed, the remaining mixture was centrifuged (3,000 × g for 10 min), and the supernatant was discarded. Pellets were resuspended in 5 ml of perchloric acid (0.05 M), samples were mixed thoroughly and centrifuged (3,000 × g for 2 min), and supernatants were recovered. Levels of calcium dissolved in supernatants were determined using a flame photometer (Flame Photometer 410; Corning) that was calibrated using calcium standards at 100, 75, 50, and 25 ppm.
SEM of catheter cross sections.
The thickness of biofilms and extent of encrustation on catheters recovered from timed models were visualized by scanning electron microscopy (SEM). Catheters were sectioned and mounted directly onto aluminum stubs using Leit adhesive carbon tabs (Agar Scientific, Stansted, United Kingdom). Mounted sections were stored overnight in a desiccator at room temperature, sputter coated with platinum using a Quorum Q150T ES system (Quorum Technologies, United Kingdom), and viewed using a Zeiss Evo LS15 microscope under high vacuum at an accelerating voltage of 5 kV and using a 5 quadrant backscatter detector (5Q-BSD).
Transmission electron microscopy of bacteriophage.
Purified phage particles (109 PFU/ml) were immobilized on a 200-mesh Formvar/carbon copper electron microscope grid (Agar Scientific, United Kingdom) and negatively stained with 2% phosphotungstic acid (pH 7.4) (Sigma, United Kingdom). Phage particles were imaged by field emission gun-scanning transmission electron microscopy (FEG-STEM) using a Zeiss Sigma FEG-STEM microscope at 20 kV of accelerating voltage, a 20-μm aperture, and a 2.7-mm working distance.
Analysis of data.
All statistical analysis was performed using Prism 6.0c for Mac OS X (GraphPad Software, Inc., USA; http://www.graphpad.com). Data were analyzed using either Student's t test or analysis of variance with the Bonferroni multiple-comparison test.
RESULTS
Bacteriophage isolation and characterization.
Three lytic phage, designated ΦRS1-PmA, ΦRS1-PmB, and ΦRS3-PmA, were isolated from wastewater through enrichments against clinical isolates of P. mirabilis. These phage showed distinct but overlapping host ranges (against a panel of 51 clinical isolates; data not shown) and differences in plaque morphology (Fig. 2). All were observed to generate halos around plaques, indicative of polysaccharide depolymerization activity, and were classified as members of the Podoviridae based on transmission electron microscopy observations of capsid morphology (Fig. 2). All 3 phage were included in a cocktail in equal proportions (1:1:1) for the evaluation of phage therapy in representative models of the catheterized urinary tract.
FIG 2.
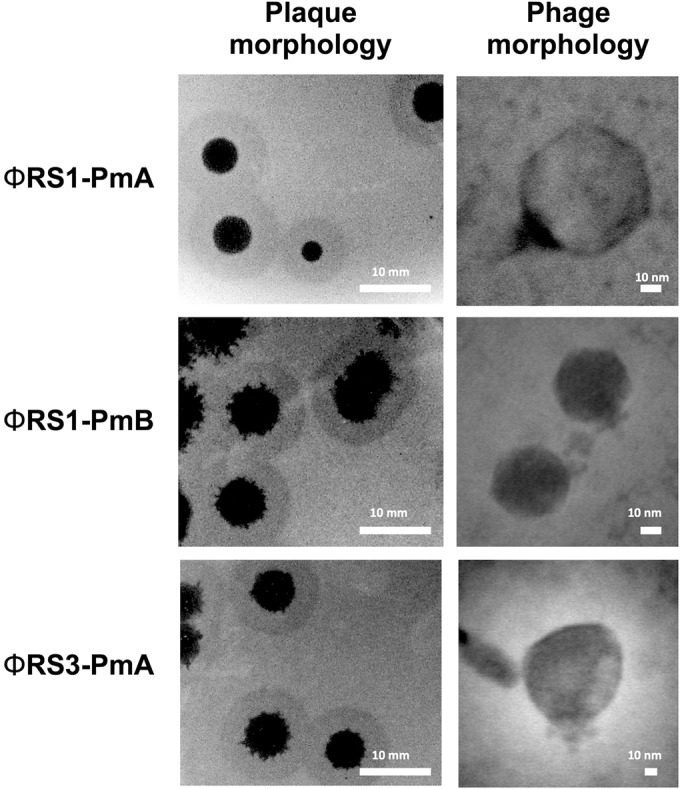
Example of plaque morphology and capsid morphology in P. mirabilis bacteriophage evaluated in bladder models. Images show plaque morphology for ΦRS1-PmA, ΦRS1-PmB and ΦRS3-PmA generated on lawns of host strains used for isolation (strain RS1 for ΦRS1-PmA and ΦRS1-PmB, and strain RS3 for ΦRS3-PmA). Associated transmission electron micrographs show structure of phage capsids, with morphology in all cases congruent with members of the Podoviridae family.
Effect of phage therapy on catheter blockage.
Initial experiments replicated a worst-case scenario, in which phage were used to treat an established infection (1010 CFU P. mirabilis in bladder models). Under these conditions, a single dose of the phage cocktail (1010 PFU; multiplicity of infection [MOI], 1:1 phage to bacteria) significantly extended the time taken for catheters to block (∼3-fold) (Fig. 3). Because interventions affecting blockage under these highly challenging conditions are likely to have greater impact when applied earlier in the infection process, we next evaluated the impact of the same phage dose in experiments replicating the early stages of infection (103 CFU P. mirabilis; MOI 1:10−7 phage to bacteria). Under these conditions, the phage cocktail completely prevented catheter blockage and eradicated infection, with models draining freely for >8 days until medium reserves were exhausted (Fig. 3). In contrast, catheters in corresponding control models developed substantial encrustation and became blocked after ∼2 days (Fig. 3).
FIG 3.
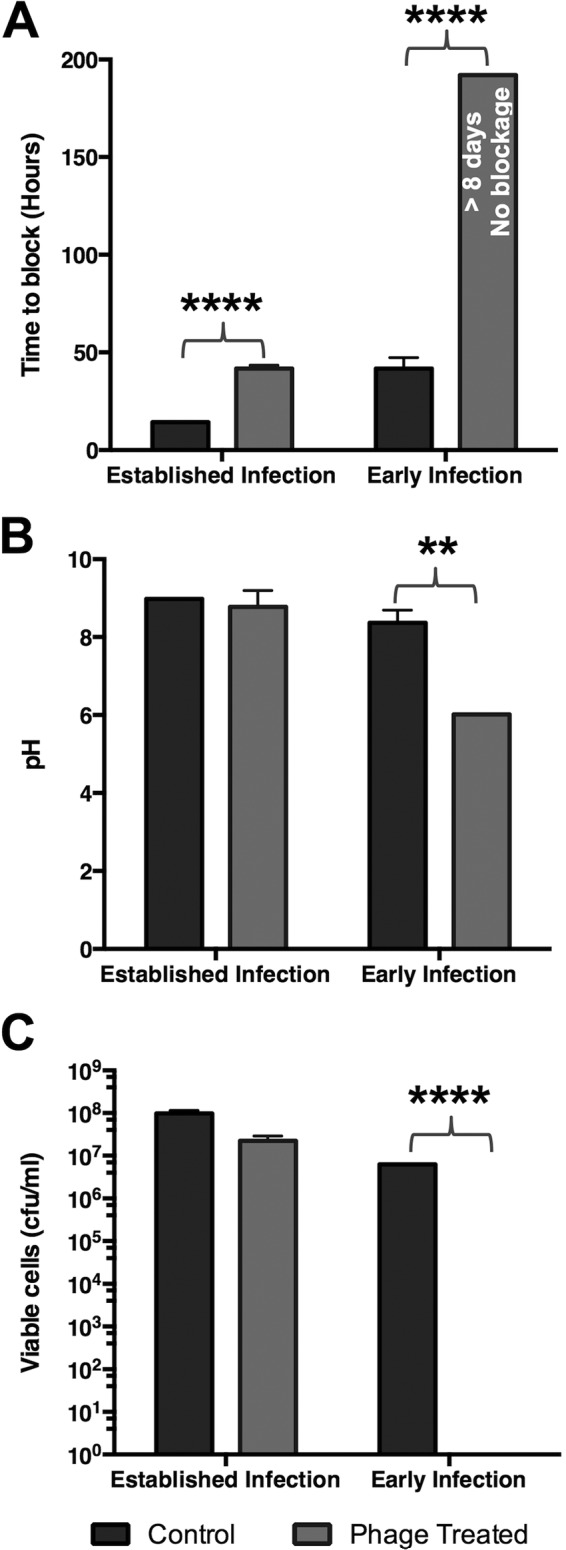
Impact of bacteriophage treatment on catheter blockage. In vitro models of the catheterized urinary tract replicating either a late-stage established infection (1010 CFU P. mirabilis), or early-stage colonization of the catheterized urinary tract (103 CFU P. mirabilis) were used to evaluate the impact of a single phage therapy treatment on blockage and encrustation. For established infection test models were treated with phage at an MOI of 1:1 phage to bacteria. Test models replicating early-stage infection were treated with the same phage dose (MOI, 1:10−7 phage to bacteria). Phage treatments were applied 45 min after models were inoculated with P. mirabilis. Models were run until catheters became blocked and urine ceased to accumulate in drainage bags, or when media were exhausted. (A) Time taken for catheters in control and phage-treated models to become blocked or for media to be exhausted. (B) pH of urine in residual bladder model media at end of experiments. (C) Enumeration of viable cells in residual urine in bladder models at the end of experiments. All data represent the means from 3 independent replicates. Error bars show the standard error of the mean. **, P < 0.01; ****, P < 0.0001 for treated versus control in each model set-up. In models representing early infection and treated with phage, no evidence of catheter blockage was observed, and models were deactivated after 8 days when media were exhausted.
Effect of phage treatment on crystalline biofilm formation.
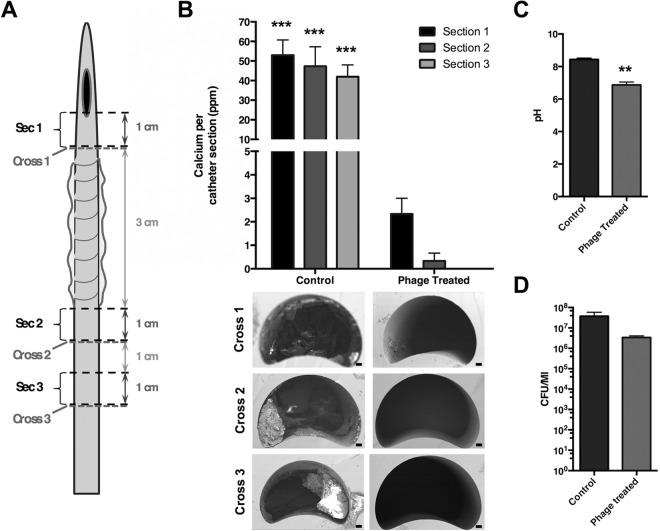
To specifically evaluate the impact of phage treatment on crystalline biofilm formation, models of late-stage infection were deactivated after 10 h, and levels of calcium on catheter sections were quantified. This demonstrated that phage treatment significantly reduced levels of encrustation (Fig. 4A). These data were supported by direct SEM visualization of catheter sections, which showed sections from models treated with phage to be devoid of visible crystalline deposits. This was in stark contrast to catheter sections from untreated models, which exhibited prominent encrustations (Fig. 4B). While these observations corresponded with a significant reduction in pH in treated models, the number of viable planktonic cells in residual urine from test or control models was not found to be significantly different (Fig. 4C and D).
FIG 4.
Impact of phage treatment on crystalline biofilm formation. Models replicating an established infection (1010 CFU P. mirabilis) were used to evaluate the impact of phage treatment on crystalline biofilm formation. Test models were treated with phage at an MOI of 1:1 phage to bacteria (1010 PFU to 1010 CFU) at 45 min after inoculation with P. mirabilis. Both test and control models were deactivated after 10 h, and levels of crystalline biofilm formation were measured on descending sections. (A) Schematic of urethral catheter showing sections subject to analysis in part B. (B) Quantification of crystalline biofilm formation and encrustation on catheter sections (total calcium present on each catheter section examined). Images below the chart provide examples of SEM visualization of catheter cross sections, distal to sections 1 to 3, and levels of encrustation. Bars on SEM images represent 200 μm. (C) pH of urine in residual bladder model media at end of experiments. (D) Enumeration of viable cells in residual urine in bladder models at the end of experiments (no significant differences). All data represent the means from 3 replicate experiments, and error bars show the standard error of the mean. **, P < 0.01; ***, P < 0.001 for control section versus phage-treated model.
DISCUSSION
Here, we demonstrate the potential for bacteriophage to constitute an effective countermeasure for one of the most common and serious complications of long-term urethral catheterization: encrustation and blockage. Our findings are congruent with previous studies examining the potential to control biofilm formation on urinary catheters using phage, where a reduction in biofilm formation by P. mirabilis, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus epidermidis has been reported when catheter sections were pretreated with phage suspensions (14–16). More recently, Lehman and Donlan (17) have described phage pretreatment for control of mixed-species biofilm formation (P. aeruginosa and P. mirabilis) and also have evaluated encrustation of catheter sections. However, these previous studies were able to show a reduction, rather than complete prevention, of biofilm formation by uropathogens tested; when P. mirabilis was used, phage did not fully prevent encrustation (14, 16, 17).
In contrast, our data highlight the potential for a more dramatic impact of phage therapy in preventing blockage and resolution of P. mirabilis infection. Although specific attributes of the phage used in this study may be important to the outcome of bladder model experiments reported here, the differences in phage performance noted between this and other studies most likely relate to the high titers of phage achieved in bladder models and to delivery directly to residual bladder urine. In contrast, previous studies targeting P. mirabilis or other uropathogens have focused on the pretreatment of catheter sections with phage suspensions prior to use in models of biofilm formation (14–17). As a result, the final titers of phage tested in these systems (and the resulting MOIs established) were unclear but were likely to be substantially lower than those obtained in our models.
In addition, previous evaluations of phage therapy for CAUTI have mainly been designed to evaluate the ability of phage to reduce biofilm formation in general, rather than prevent catheter blockage specifically. In this context, the focus of our study on blockage as a specific therapeutic endpoint, and the evaluation of phage using a full closed drainage system in the bladder model system, is also a key difference. This model provides an excellent representation of the catheterized urinary tract and assessment of phage therapy in this setting.
In contrast, previous studies have used simple static models of biofilm formation on catheter sections (14) or have deployed models that do not use whole intact catheters or do not fully replicate the closed drainage system (15–17). While such models of infection clearly provide useful and valid insights into the potential of phage therapy for CAUTI and the control of bacterial biofilms in this setting, the encrustation and blockage of catheters are also governed by physical characteristics of distinct regions of catheters and the physicochemical forces that develop in the closed drainage system (1).
Most notably, blockage typically occurs around the catheter eye-hole and the first few centimeters of the catheter, which provide more irregular surface topologies (arising from the manufacturing process) that are particularly supportive of bacterial colonization and are continually exposed to the sump of infected residual urine that accumulates in the bladder (1, 12, 13). Therefore, the bladder model system provides a particularly robust evaluation of interventions aimed at prevention of blockage and encrustation, and the use of this system strengthens the observations reported here about the potential of phage to prevent catheter blockage.
Nevertheless, it is notable that phage were only able to fully prevent blockage when used in models of early-stage infection. The simplest explanation for failure to prevent blockage in simulations of established infection is that the dose of phage used was insufficient to deal with the dense P. mirabilis population and, under MOIs, established P. mirabilis growth and crystalline biofilm formation simply outstripped the capacity of phage to eliminate infection. This may have been compounded by factors such as washout of phage from model systems during the course of experiments as well as by the rapid elevation of urinary pH in models of late-stage infection, which may reach a pH of 8 ∼2 to 3 h after model activation.
Conversely, the recovery of phage from models of early infection 8 days after model activation (albeit at low levels, ∼20 PFU/ml) despite an apparent absence of host bacteria for the majority of this time, and the far longer duration of these experiments compared with models of established infection, argues against washout as a significant factor. Under conditions of high pH, it is possible that phage may be inactivated or their ability to infect host cells may be reduced, leading to eventual therapeutic failure. Previous evaluations of P. mirabilis phage have indicated that these remain active even under conditions of high pH (17). Our own evaluation of specific phage used here confirms these remain capable of infection after exposure to high pH (data not shown), but the possibility that alterations to cell surface properties protect against infection with these specific phage at high pH cannot be excluded.
Alternatively, the failure of phage to prevent blockage in late-stage infection may be explained by the development of resistance to the phage used, and this has been observed in other studies of phage therapy for CAUTI over a similar time frame (16). Although the use of a 3-phage cocktail should guard against resistance, the phage used here have similar host range profiles, are all members of the Podoviridae family, and are yet to be characterized genetically. It is therefore possible that they constitute closely related phage types with comparable mechanisms of attachment and infection. This could allow the same mutation(s) in host bacteria to afford resistance to all 3 phage. In this context, it is also notable that many key surface structures of P. mirabilis that may be receptors for phage attachment are subject to phase-variable gene expression (18), and it is therefore not unlikely that a small proportion of a given P. mirabilis population may be naturally immune to particular phage types and selected for during phage treatment.
Despite this, there is clear potential to address the issue of resistance by ensuring selection of phage binding distinct cell surface structures and by generating a greater understanding of the mechanisms underpinning the phage-host interaction in P. mirabilis, particularly under conditions encountered in the catheterized urinary tract. Furthermore, the high MOIs achieved in models of early infection also raise the potential for the induction of lysis from without (LO), which could also explain the differences in efficacy of phage treatment in the two infection scenarios modeled. The induction of LO could be highly advantageous in control of P. mirabilis CAUTI, and subsequent studies should explore if P. mirabilis phage used here can induce LO and the applications of this to control of CAUTI.
It was also clear from timed bladder model experiments that phage treatment significantly reduced levels of crystalline biofilm formation in models of established infection. Intriguingly, this work also suggests that the impact of phage treatment on P. mirabilis crystalline biofilm formation may not be solely attributable to a reduction in the number of planktonic cells available to participate in biofilm formation, since no statistically significant differences were observed in the number of viable planktonic cells in residual urine from test or control models at the 10-h time point.
The putative polysaccharide depolymerase (PD) activity exhibited by the phage used here (based on halo production around plaques; Fig. 2) (19) may be important in this regard. These enzymes, expressed on the surface of phage capsids or produced by host cells during phage replication, are believed to facilitate phage attack on biofilm communities by enabling phage penetration of the exopolymeric matrix (19, 20). The bioengineering of phage T7 has already demonstrated the potential utility of PD-expressing phage in biofilm dispersal (20), and it is possible that any PD activity of phage used in this study may contribute to their ability to reduce crystalline biofilm formation and encrustation independent of cell lysis.
This highlights an additional feature of P. mirabilis phage that may be investigated further from the perspective of developing more broadly applicable anti-biofilm strategies. In the context of CAUTI, greater insights into the ability of phage to access biofilm-associated cells could improve activity not only against mature biofilms but, perhaps more importantly, against multispecies biofilms. Challenges to the efficacy of phage therapy posed by multispecies biofilms would stem not only from the relatively narrow spectrum of activity of most phage, but also the possibility that mechanisms used by phage to access host bacterial cells within biofilms (such as PD enzymes) may be undermined by the chimeric exopolysaccharide generated by multispecies biofilms (19).
Although recent work does indicate the potential to tackle multispecies biofilms with phage therapy (17), it would seem that the more detailed study of phage-biofilm interactions and elucidation of associated mechanisms, coupled with the powerful approach of phage genome engineering, holds much potential for enhancing the efficacy of phage therapy in this regard. Alternatively, the prophylactic administration of phage active against key pathogens, such as P. mirabilis, should also serve to offset issues associated with access to target cells in multispecies or even single-species biofilms once these become established. Furthermore, there is also considerable scope to combine phage therapy with other approaches to control infection, such as antibiotics or other antimicrobial agents, to enhance efficacy further.
In summary, the current study supports the potential efficacy of phage therapy in control of CAUTI and, in particular, of blockage caused by P. mirabilis. Although there is a clear need for further fundamental research into phage-host interactions and the ability of phage to control CAUTI to progress this approach, our work also suggests a major factor in the successful use of phage therapy in this setting will be the parallel development of strategies to deliver sufficient numbers of phage within the most effective therapeutic window (e.g., early-stage infection for P. mirabilis CAUTI).
ACKNOWLEDGMENTS
We thank Joseph Hawthorn, Rowena Berterelli, and Heather Catty for excellent technical support and Caroline Jones for constructive comments and criticism.
B.V.J. and C.G.M.G. conceived and designed the study. J.N., A.H., D.R.A., B.M., C.D., and J.S. conducted the experiments. B.V.J., A.T.A.J., B.F.G., and C.G.M.G. directed the research. All authors contributed to analysis and interpretation of data. B.V.J. wrote the manuscript, and all authors edited the manuscript.
J.C. is an employee of Novolytics Ltd., which develops commercial bacteriophage products. J.C. provided expert advice and scientific support, but Novolytics Ltd. provided no funding for the study and had no role in study design, interpretation of data, manuscript preparation, or decision to publish. The study funders also had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was primarily funded by the Queen Victoria Hospital NHS Foundation Trust and the Queen Victoria Hospital Charitable Trust. D.R.A. is supported by the Queen Victoria Hospital NHS Foundation Trust, the Blond McIndoe Research Foundation, and the University of Brighton. J.N. is supported by the Dunhill Medical Trust (via R394/1114, awarded to B.V.J.).
REFERENCES
- 1.Stickler DJ. 2008. Bacterial biofilms in patients with indwelling urinary catheters. Nat Rev Urol 5:598–608. doi: 10.1038/mcpuro1231. [DOI] [PubMed] [Google Scholar]
- 2.Mobley HLT. 1996. Virulence of Proteus mirabilis, p 245–265. In Mobley HLT, Warren JW (ed), Urinary tract infections, molecular pathogenesis and clinical management. ASM Press, Washington, DC. [Google Scholar]
- 3.Griffith DP, Musher DM, Itin C. 1976. Urease: the primary cause of infection-induced urinary stones. Invest Urol 13:346–350. [PubMed] [Google Scholar]
- 4.Jones BD, Mobley HL. 1987. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun 55:2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stickler D, Ganderton L, King J, Nettleton J, Winters C. 1993. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res 21:407–411. doi: 10.1007/BF00300077. [DOI] [PubMed] [Google Scholar]
- 6.Clapham L, McLean RJC, Nickel JC, Downey J, Costerton JW. 1990. The influence of bacteria on struvite crystal habit and its importance in urinary stone formation. J Cryst Growth 104:475–484. doi: 10.1016/0022-0248(90)90150-J. [DOI] [Google Scholar]
- 7.Dumanski AJ, Hedelin H, Edin-Liljegren A, Beauchemin D, McLean RJ. 1994. Unique ability of the Proteus mirabilis capsule to enhance mineral growth in infectious urinary calculi. Infect Immun 62:2998–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris NS, Stickler DJ, Winters C. 1997. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol 80:58–63. doi: 10.1046/j.1464-410X.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 10.Morgan S, Rigby D, Stickler DJ. 2009. A study of the structure of the crystalline biofilms that encrust and block silver Foley catheters. Urol Res 37:88–93. doi: 10.1007/s00240-009-0176-6. [DOI] [PubMed] [Google Scholar]
- 11.Sabbuba NA, Mahenthiralingam E, Stickler DJ. 2003. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol 41:4961–4965. doi: 10.1128/JCM.41.11.4961-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stickler DJ, Morris NS, Winters C. 1999. Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol 310:494–501. doi: 10.1016/S0076-6879(99)10037-5. [DOI] [PubMed] [Google Scholar]
- 13.Holling N, Lednor D, Tsang S, Bissell A, Campbell L, Nzakizwanayo J, Dedi C, Hawthorne JA, Hanlon G, Ogilvie LA, Salvage JP, Patel BA, Barnes LM, Jones BV. 2014. Elucidating the genetic basis of crystalline biofilm formation in Proteus mirabilis. Infect Immun 82:1616–1626. doi: 10.1128/IAI.01652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson L, Gorman SP, Gilmore BF. 2010. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol 59:447–455. doi: 10.1111/j.1574-695X.2010.00696.x. [DOI] [PubMed] [Google Scholar]
- 15.Curtin JJ, Donlan RM. 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob Agents Chemother 50:1268–1275. doi: 10.1128/AAC.50.4.1268-1275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. 2010. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman SM, Donlan RM. 2015. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother 59:1127–1137. doi: 10.1128/AAC.03786-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armbruster CE, Mobley HLT. 2012. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol 10:743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland IW, Hughes KA, Skillman LC, Tait K. 2004. The interaction of phage and biofilms. FEMS Microbiol Lett 232:1–6. doi: 10.1016/S0378-1097(04)00041-2. [DOI] [PubMed] [Google Scholar]
- 20.Lu TK, Collins JJ. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A 104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]