Abstract
Catheter-associated infections are difficult to treat with available antimicrobial agents because of their biofilm etiology. We examined the effect of low-amperage direct electrical current (DC) exposure on established bacterial and fungal biofilms in a novel experimental in vitro catheter model. Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida parapsilosis biofilms were grown on the inside surfaces of polyvinyl chloride (PVC) catheters, after which 0, 100, 200, or 500 μA of DC was delivered via intraluminally placed platinum electrodes. Catheter biofilms and intraluminal fluid were quantitatively cultured after 24 h and 4 days of DC exposure. Time- and dose-dependent biofilm killing was observed with all amperages and durations of DC administration. Twenty-four hours of 500 μA of DC sterilized the intraluminal fluid for all bacterial species studied; no viable bacteria were detected after treatment of S. epidermidis and S. aureus biofilms with 500 μA of DC for 4 days.
INTRODUCTION
Catheter-associated infections, including catheter-associated urinary tract infection (CAUTI) and catheter-related bloodstream infection (CRBSI), are associated with morbidity, mortality, and expense, often requiring catheter removal. The pathogenesis of these infections relates to the presence of biofilms on the surface of the catheters.
Compared with planktonic (i.e., free-floating) forms, microorganisms in biofilms exhibit increased resistance to host immunity and antimicrobial therapy (1). Proposed mechanisms underlying biofilm-associated antimicrobial resistance include limited penetration through or neutralization of antimicrobials within biofilms (2, 3); subpopulations of resistant phenotypes, referred to as “persister” cells (4, 5); and dormant stationary-phase zones within biofilms (4, 6, 7). As a result, most conventional systemically administered antimicrobial agents have little ability to cure catheter-associated infections. Catheter removal is necessary in the majority of cases, typically in conjunction with systemic antimicrobial treatment. Strategies to control biofilms, such as coating catheters with silver ions, chlorhexidine or minocycline plus rifampin, have been proposed (8–12), and catheter lock solutions, using conventional antimicrobial agents or antiseptics, have shown activity against catheter-associated biofilms (13–19). However, none of these strategies has solved the clinical challenge of catheter-associated infections, underscoring the need for new approaches.
We previously described an antibiofilm strategy that we termed the electricidal effect. Biofilms of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa on Teflon discs were exposed to 20, 200, or 2,000 μA direct current (DC) for up to 7 days, which resulted in time- and dose-dependent antibiofilm effects, as measured by decreases in numbers of viable cells (20). Subsequent studies confirmed the microbicidal activity of continuously and intermittently applied electrical current against established biofilms of several bacterial and fungal species in vitro and in animal models (21–25).
A potential avenue to deliver electrical current is to administer it to the lumen of catheters. This location of biofilm formation in CRBSI and CAUTI provides a site targetable by electrical current. Based on our prior work, we hypothesized that DC delivered via intraluminally placed electrodes would provide an antibiofilm strategy targeting intraluminal biofilms. This approach could limit the use of antibiotics as well as the replacement of infected catheters. In this study, we examined the effect of different amperages and delivery durations of DC on established intraluminal biofilms of four bacterial and one fungal species in a novel in vitro catheter model.
MATERIALS AND METHODS
Microorganisms.
Staphylococcus epidermidis Xen43, Staphylococcus aureus Xen30, Pseudomonas aeruginosa Xen5, Escherichia coli IDRL-7029, and Candida parapsilosis IDRL-7250 were studied. The Xen strains were generous gifts of PerkinElmer Caliper Life Sciences (formerly Xenogen Corporation), Waltham, MA, and the IDRL isolates were clinical isolates collected at Mayo Clinic, Rochester, MN.
Catheters.
Six-millimeter-inner-diameter 28 French (Fr) polyvinyl chloride (PVC) thoracic catheters (Atrium Medical Corporation, Hudson, NH) were cut to a length of 45 mm and sterilized by using ethylene oxide. Polyoxymethylene plastic caps were used to seal the catheter bottoms and tops; platinum electrodes (50 mm in length and 1.6 mm in diameter) were inserted through and held in place by the latter (Fig. 1).
FIG 1.
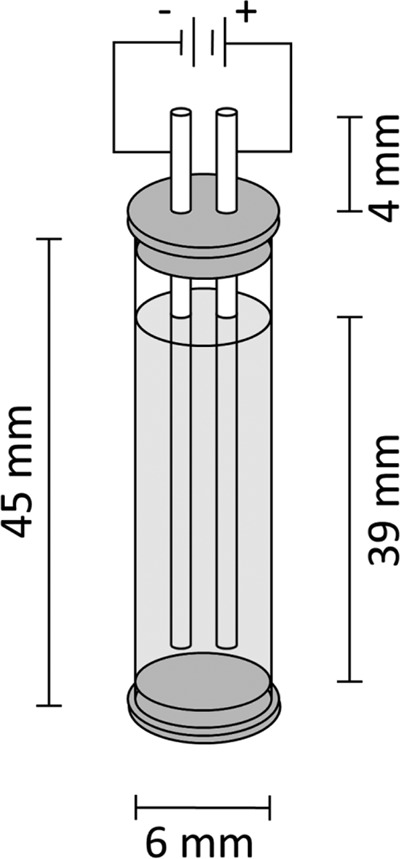
Catheter biofilm model. PVC catheters (inner diameter of 6 mm) were cut to a length of 45 mm, and polyoxymethylene plastic caps were used to seal the bottom of the catheters and were placed into catheter tops to hold platinum electrodes (50 mm in length and 1.6 mm in diameter) in place.
Biofilms.
Microorganisms were subcultured from frozen aliquots onto BBL Trypticase soy agar with 5% sheep blood plates (TSA II; Becton Dickinson, Franklin Lakes, NJ) and incubated at 37°C overnight. One to three colonies were added to 2.5 ml of Trypticase soy broth (TSB) and grown for 1 to 3 h at 37°C on an orbital shaker to reach a 0.5 McFarland standard. One hundred microliters of the 0.5 McFarland standard solutions was added to 0.9 ml TSB in sterile PVC catheters capped at one end, placed into a sterile glass box, and incubated on an orbital shaker at 37°C for 24 h. TSB was then removed by using a pipette and replaced with 1.1 ml phosphate buffer.
Phosphate buffer.
Phosphate buffer (1×) was prepared with 426 mg Na2HPO4, 205 mg KH2PO4, 640 mg glucose, and 1 liter distilled water; filter sterilized; and stored at 4°C. The stock phosphate buffer was diluted to 3% in sterile water for each experiment.
Electrical treatment.
Electrical current was applied by using an 8-channel computer-controlled current generator designed by the Mayo Clinic Division of Engineering (Rochester, MN) via anode and cathode electrical hooks connected to the electrodes. Catheters were treated with 0, 100, 200, or 500 μA DC current for either 24 h or 4 days, with testing being performed in triplicate.
Biofilm and planktonic cell densities.
Biofilm and planktonic cell densities were determined by quantitative culture. Intraluminal phosphate buffer was quantitatively cultured to obtain planktonic cell densities. To obtain biofilm densities, caps were aseptically removed from the catheters, which were gently rinsed in sterile saline and placed into test tubes containing 5 ml of sterile saline. Biofilms were removed by vortexing for 30 s, sonication for 5 min, and vortexing again for 30 s; the resultant fluid was quantitatively cultured. CFU were counted after 24 to 48 h. Biofilm reduction was expressed by subtracting the mean log10 CFU per square centimeter of exposed catheters from that of nonexposed catheters.
Statistical methods.
Comparison among 4 current levels (0, 100, 200, and 500 μA) was first performed by using the Kruskal-Wallis test. If the results were significant, further comparisons were performed in a pairwise manner (0 versus 100 μA, 0 versus 200 μA, 0 versus 500 μA, 100 versus 200 μA, 100 versus 500 μA, and 200 versus 500 μA) by using the Wilcoxon rank sum test. No adjustment was performed for multiple comparisons due to small sample sizes. All tests were two sided; P values of <0.05 were considered statistically significant. Analyses were performed by using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
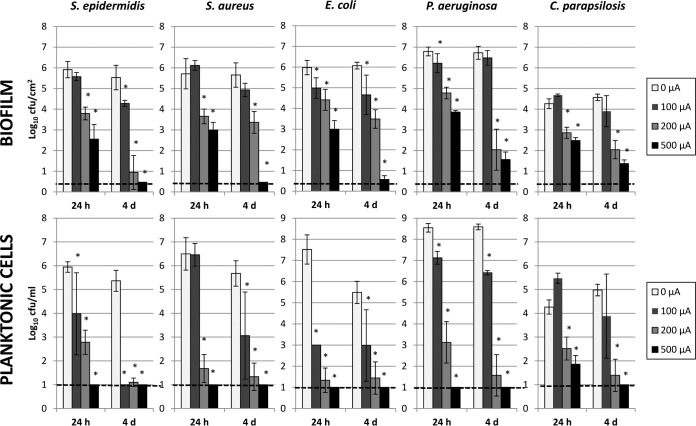
Results are shown in Fig. 2. We detected statistically significant differences between no electrical current exposure and electrical current exposure of 200 μA and higher for all microorganisms studied (P ≤ 0.02). Higher amperage yielded greater reductions of biofilm viability at all times studied. Time-dependent reductions in numbers of viable biofilm cells were observed, with lower viable-cell counts when electrical current was applied for longer periods of time. No viable cells were detected when S. aureus or S. epidermidis biofilms were exposed to 500 μA DC for 4 days. Reductions of 5.2 to 5.5 log10 CFU/cm2 were observed when P. aeruginosa and E. coli biofilms were exposed to 500 μA DC for 4 days (P < 0.02). C. parapsilosis biofilms were more resistant, with a maximum reduction of 3.2 log10 CFU/cm2 being achieved after 4 days of treatment with 500 μA DC (P value of 0.01). Significant biofilm reductions were also observed for treatment with 200 μA for 24 h, ranging from 1.4 to 2.1 log10 CFU/cm2 (P < 0.02). Application of 100 μA DC for 24 h (and 4 days) reduced E. coli biofilm densities and the number of planktonic cells (P ≤ 0.02); 100 μA applied for 4 days reduced the S. epidermidis biofilm density (P value of 0.01). A greater biofilm effect was measured with 500 μA than with 200 μA DC (P < 0.05) for all organisms and durations, except for C. parapsilosis after 24 h and S. epidermidis and P. aeruginosa after 4 days, possibly due to the small sample size. Likewise, 500 μA showed greater reductions than did 100 μA DC for biofilms and planktonic cells with 24 h and 4 days of application (P ≤ 0.05), except for S. aureus and S. epidermidis planktonic cells with 4 days of exposure. Comparison between treatments with 100 and 200 μA DC showed less marked differential reductions of biofilm densities and numbers of planktonic cells; however, a significant difference in effect was measured for 200 μA compared to 100 μA DC for both planktonic cells and biofilms at 24 h and 4 days for P. aeruginosa (P ≤ 0.05).
FIG 2.
Results for quantitative cultures of Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida parapsilosis biofilms on PVC catheters and planktonic cells in the study buffer after exposure to 0, 100, 200, and 500 μA DC for 24 h and 4 days. The x axis represents hours (h) and days (d) of DC exposure. The y axis shows results for quantitative cultures as log10 CFU per square centimeter for biofilm and log10 CFU per milliliter for planktonic cultures. Error bars indicate standard deviations. The dashed line indicates the limit of detection. *, statistical significance (P < 0.05) compared to 0 μA.
Generally, greater reductions were observed for planktonic cells than for biofilms. No viable planktonic cells were observed after exposure to 500 μA DC at any time point for all bacterial species studied. However, viable planktonic C. parapsilosis cells were found after 24 h of exposure to 500 μA DC (reduction of 2.4 log10 CFU/ml; P value of 0.01). Exposure to 200 μA achieved reductions ranging from 1.7 to 6.2 log10 CFU/ml (P < 0.02) after 24 h and from 3.6 to 7.0 log10 CFU/ml (P < 0.02) after 4 days for all bacterial species. Reductions in numbers of planktonic cells ranging from 1.1 to 4.4 log10 CFU/ml were measured after 4 days of exposure to 100 μA for the bacterial species. Again, C. parapsilosis was more resistant, with a reduction of just 0.7 log10 CFU/ml after 4 days of exposure to 100 μA DC (P = 0.16).
DISCUSSION
In this study, we demonstrated that DC reduces staphylococcal, E. coli, P. aeruginosa, and C. parapsilosis biofilms on the intraluminal surface of catheters in a time- and dose-dependent manner. The most dramatic effects (i.e., no detectable viable cells) were observed when S. epidermidis and S. aureus biofilms were exposed to 500 μA DC for 4 days, although large reductions (≥5.0 log10 CFU/cm2) were also observed when P. aeruginosa and E. coli biofilms were exposed to 500 μA DC for 4 days. Reductions of >1.0 log10 CFU/cm2 were observed with C. parapsilosis exposed to 200 or 500 μA DC; however, the degree of reduction was lower than that observed with the other organisms studied. Exposure to 100 μA DC yielded biofilm reductions for S. epidermidis (after 4 days) as well as for E. coli and P. aeruginosa (after 24 h). Overall, these results show that electrical current, applied via intraluminal electrodes, has a marked effect on microbial biofilms on catheter surfaces.
The underlying mechanism of the effect observed is not fully understood. Oxidative stress (26–30), damage to the cell walls (31, 32), changes in pH (33, 34), and the formation of hypochlorous acid by electrolysis (33) have been proposed. The results generated here support an electrochemical mechanism. Detachment promoted by enhanced repulsive forces between microorganisms and surface materials may also play a role (31, 35–38).
Although our results are consistent with previously reported data showing a bactericidal effect of DC on sessile and planktonic cells, previous studies either used different electrode positioning, focusing on biofilms grown on discs placed between two electrodes (22, 25, 39), or investigated the effects of custom-fabricated, electrically conductive catheters on bacterial colonization in agar plates (40). In this study, electrodes were simply placed into the lumen of commercially available catheters on which biofilms had been grown. Theoretically, this could be adapted to clinical settings by introducing electrodes into infected catheters, without the need for expensive changes to the design of available catheters.
Limitations of this study relate to the methodology employed and the ability to extrapolate our findings. The phosphate buffer did not resemble physiological body fluids such as blood or urine. Chlorine, which is abundantly present in body fluids and which might enhance the effect by formation of hypochlorous acid (33), was not added to the study buffer (but might have been present in small quantities as a result of having grown biofilms in TSB). Catheter materials like silicone or latex, which are widely used in urinary or venous catheters, might behave differently with regard to biofilm growth and/or reduction achieved by electrical current than the PVC catheters used in this study. It also has to be considered that in this model, electrodes were placed intraluminally, not affecting the outer catheter surface. In vivo biofilms may grow on the outer and inner surfaces of infected catheters (41, 42). Interestingly, studies using electrified catheters with comparable electrode positioning showed reduced encrustation by Proteus mirabilis biofilms at the catheter eyelet region in vitro (43) and reduced microbial populations associated with catheter-associated urinary tract infections in vivo (44, 45). Finally, safety issues need to be addressed to use this strategy in a clinical setting. The use of low-dose electrical current within the urinary tract appears to be safe; a study of electrified catheters in sheep reported no physical or chemical changes of urine or the tissues of the urinary tract with an amperage of 400 μA (44). Similarly, an electrified urinary catheter trial in humans did not show adverse effects or evidence of catheter damage (45). Possible adverse reactions to electrical current delivered into intravascular catheters, including cardiac arrhythmias, hemolysis, and thrombus formation, require further investigation.
In conclusion, our results demonstrate that biofilms in catheters can be reduced by using low-dose DC. Although further in vitro and in vivo studies are needed, this strategy might be useful to combat clinically challenging catheter-associated infections.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01 AR056647 and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01 AI091594.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Michelle E. M. Campeau for performing the preliminary work that inspired the present study.
We report no conflicts of interest in this work.
REFERENCES
- 1.Anwar H, Dasgupta M, Costerton J. 1990. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother 34:2043–2046. doi: 10.1128/AAC.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdi-Ali A, Mohammadi-Mehr M, Alaei YA. 2006. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents 27:196–200. doi: 10.1016/j.ijantimicag.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh R, Ray P, Das A, Sharma M. 2010. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 4.Spoering AL, Lewis K. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suci PA, Tyler BJ. 2003. A method for discrimination of subpopulations of Candida albicans biofilm cells that exhibit relative levels of phenotypic resistance to chlorhexidine. J Microbiol Methods 53:313–325. doi: 10.1016/S0167-7012(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 6.Anderl JN, Zahller J, Roe F, Stewart PS. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 47:1251–1256. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darouiche RO. 1999. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis 29:1371–1377. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 9.Saint S, Lipsky BA. 1999. Preventing catheter-related bacteriuria: should we? Can we? How? Arch Intern Med 159:800–808. [DOI] [PubMed] [Google Scholar]
- 10.Schierholz JM, Beuth J, Pulverer G, Konig D. 1999. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection. Am J Med 107:534–535. doi: 10.1016/S0002-9343(99)00205-3. [DOI] [PubMed] [Google Scholar]
- 11.Rupp ME, Fitzgerald T, Marion N, Helget V, Puumala S, Anderson JR, Fey PD. 2004. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am J Infect Control 32:445–450. doi: 10.1016/j.ajic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. 1999. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 281:261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 13.Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M. 2008. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis 47:83–93. doi: 10.1086/588667. [DOI] [PubMed] [Google Scholar]
- 14.Henrickson KJ, Axtell RA, Hoover SM, Kuhn SM, Pritchett J, Kehl SC, Klein JP. 2000. Prevention of central venous catheter-related infections and thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double-blind trial. J Clin Oncol 18:1269–1278. [DOI] [PubMed] [Google Scholar]
- 15.Sanders J, Pithie A, Ganly P, Surgenor L, Wilson R, Merriman E, Loudon G, Judkins R, Chambers S. 2008. A prospective double-blind randomized trial comparing intraluminal ethanol with heparinized saline for the prevention of catheter-associated bloodstream infection in immunosuppressed haematology patients. J Antimicrob Chemother 62:809–815. doi: 10.1093/jac/dkn284. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Hidalgo N, Almirante B. 2014. Antibiotic-lock therapy: a clinical viewpoint. Expert Rev Anti Infect Ther 12:117–129. doi: 10.1586/14787210.2014.863148. [DOI] [PubMed] [Google Scholar]
- 17.Vandenhende M-A, Buret J, Camou F, Morlat P, Bonnet F. 2014. Successful daptomycin lock therapy for implantable intra-arterial catheter infection in a patient with liver metastases of colon cancer. Diagn Microbiol Infect Dis 78:497–498. doi: 10.1016/j.diagmicrobio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Tan M, Lau J, Guglielmo BJ. 2014. Ethanol locks in the prevention and treatment of catheter-related bloodstream infections. Ann Pharmacother 48:607–615. doi: 10.1177/1060028014524049. [DOI] [PubMed] [Google Scholar]
- 19.Madsen M, Rosthøj S. 2013. Impact of hydrochloric acid instillation on salvage of infected central venous catheters in children with acute lymphoblastic leukaemia. Scand J Infect Dis 45:38–44. doi: 10.3109/00365548.2012.708941. [DOI] [PubMed] [Google Scholar]
- 20.Del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. 2009. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother 53:41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Pozo JL, Rouse MS, Euba G, Kang CI, Mandrekar JN, Steckelberg JM, Patel R. 2009. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother 53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Pozo JL, Rouse MS, Euba G, Greenwood-Quaintance KE, Mandrekar JN, Steckelberg JM, Patel R. 2014. Prevention of Staphylococcus epidermidis biofilm formation using electrical current. J Appl Biomater Funct Mater 12:81–83. doi: 10.5301/jabfm.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Borden AJ, Van Der Werf H, Van Der Mei HC, Busscher HJ. 2004. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl Environ Microbiol 70:6871–6874. doi: 10.1128/AEM.70.11.6871-6874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Borden AJ, Maathuis PG, Engels E, Rakhorst G, Van Der Mei HC, Busscher HJ, Sharma PK. 2007. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials 28:2122–2126. doi: 10.1016/j.biomaterials.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Malan SM, Karau MJ, Cede J, Greenwood-Quaintance KE, Brinkman CL, Mandrekar JN, Patel R. 2015. Anti-biofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrob Agents Chemother 59:4610–4615. doi: 10.1128/AAC.00483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boles BR, Singh PK. 2008. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A 105:12503–12508. doi: 10.1073/pnas.0801499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boor KJ. 2006. Bacterial stress responses: what doesn't kill them can make them stronger. PLoS Biol 4:e23. doi: 10.1371/journal.pbio.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabiscol E, Tamarit J, Ros J. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8. [PubMed] [Google Scholar]
- 29.Marles-Wright J, Lewis RJ. 2007. Stress responses of bacteria. Curr Opin Struct Biol 17:755–760. doi: 10.1016/j.sbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Miller RA, Britigan BE. 1997. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pareilleux A, Sicard N. 1970. Lethal effects of electric current on Escherichia coli. Appl Microbiol 19:421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sale A, Hamilton W. 1967. Effects of high electric fields on microorganisms. I. Killing of bacteria and yeasts. Biochim Biophys Acta 148:781–788. doi: 10.1016/0304-4165(67)90052-9. [DOI] [Google Scholar]
- 33.Sandvik EL, McLeod BR, Parker AE, Stewart PS. 2013. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS One 8:e55118. doi: 10.1371/journal.pone.0055118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson M, Baylor K, Stecker MM, Netherton BL. 2010. Electrical stimulation and electrode properties. Part 2: pure metal electrodes. Am J Electroneurodiagnostic Technol 50:263–296. [PubMed] [Google Scholar]
- 35.Jucker BA, Harms H, Zehnder A. 1996. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J Bacteriol 178:5472–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W-K, Brown M, Elliott T. 1997. Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 39:687–695. doi: 10.1093/jac/39.6.687. [DOI] [PubMed] [Google Scholar]
- 37.Ueshima M, Tanaka S, Nakamura S, Yamashita K. 2002. Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res 60:578–584. doi: 10.1002/jbm.10113. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Borden A, Van Der Mei H, Busscher H. 2005. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials 26:6731–6735. doi: 10.1016/j.biomaterials.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 39.Del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. 2009. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 53:35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amalou H, Negussie AH, Ranjan A, Chow L, Xu S, Kroeger C, Neeman Z, O'Grady NP, Wood BJ. 2014. Electrically conductive catheter inhibits bacterial colonization. J Vasc Interv Radiol 25:797–802. doi: 10.1016/j.jvir.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramanathan V, Riosa S, Al-Sharif AH, Mansouri MD, Tranchina A, Kayyal T, Abreo AP, Aslam S, Nassar G, Darouiche RO. 2012. Characteristics of biofilm on tunneled cuffed hemodialysis catheters in the presence and absence of clinical infection. Am J Kidney Dis 60:976–982. doi: 10.1053/j.ajkd.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarti A, Gangodawila S, Long MJ, Morris NS, Blacklock ARE, Stickler DJ. 2005. An electrified catheter to resist encrustation by Proteus mirabilis biofilm. J Urol 174:1129–1132. doi: 10.1097/01.ju.0000168618.79096.cb. [DOI] [PubMed] [Google Scholar]
- 44.Davis C, Shirtliff M, Scimeca J, Hoskins S, Warren M. 1995. In vivo reduction of bacterial populations in the urinary tract of catheterized sheep by iontophoresis. J Urol 154:1948–1953. doi: 10.1016/S0022-5347(01)66832-0. [DOI] [PubMed] [Google Scholar]
- 45.Shafik A. 1993. The electrified catheter. World J Urol 11:183–185. [DOI] [PubMed] [Google Scholar]