Abstract
This study assessed the functional importance of residues located at the i−2 position of face 4 of the tandem repeat loops of the quinolone resistance protein QnrVC7 through mutagenesis studies. The i−2 position of face 4 on different coils required residues with different natures. Some substitutions reduced the protective activity of QnrVC7, while some of them increased it. These findings advanced our understanding on the detailed structural organization and functional requirements of Qnr proteins.
TEXT
Qnr proteins are pentapeptide repeat proteins (PRPs) that contribute to reduced susceptibility to quinolones in Gram-negative bacteria (1). Knowledge regarding the crystal structure of QnrB1, which was reported previously (1), indicates that the protein comprises 10 tandem-repeated loops, with each containing 4 faces and each face represented by the i, i+1, i+2, i−1, and i−2 positions. This class of proteins exhibits structural similarity to double-stranded DNA, with 5 amino acids forming a tandem-repeat unit, which in turn folds into a right-handed quadrilateral β-helix. The Qnr proteins confer reduced susceptibility to quinolones through competition with DNA in binding to DNA gyrase and topoisomerase IV, thereby preventing DNA breakage due to quinolone binding (2, 3). To date, a total of 6 classes of Qnr proteins, namely, QnrA, QnrB, QnrC, QnrD, QnrS, and QnrVC, have been discovered, among which QnrB is the most prevalent subtype and can be further subdivided into 80 variants (4–8). Mutational analyses of QnrA, QnrB, QnrC, and QnrS have identified several conserved residues that play an important role in the stabilization of the Qnr protein structure (1, 3, 9–11), most of which being located at the i and i−2 positions, as defined by Vetting et al. (1). In addition, the C terminus of Qnr proteins, which has been suggested to be responsible for the formation of dimers, was found to be critical for Qnr function (3). Two conservative extra loops of QnrB1 were also shown to be critical for Qnr protective activity, as the deletion of these loops dramatically affected its protective function (1). Recently, our group identified a novel chromosomally encoded Qnr variant, QnrVC7, which exhibited lower protection activity than that of other Qnr proteins, such as QnrVC5 and QnrVC6, and other Qnr proteins. Sequence comparison and mutational analysis showed that amino acid T152, located at the i−2 position on face 4, was responsible for the reduced protective activity of QnrVC7 (12). This study extended the characterization to all residues located at the i−2 position of face 4 to elucidate the structure-activity relationships of QnrVC7 and other Qnr proteins.
The entire coding region of QnrVC7 was amplified using chromosomal DNA from Vibrio cholerae V122 as the template, ligated to pCR2.1 vector, and then transformed into Escherichia coli strain TG1 to construct pCR2.1-qnrVC7 (12). Site-directed mutagenesis using the GeneArt site-directed mutagenesis system (Invitrogen) was performed on all residues located at the i−2 position of face 4, including A36, S62, A82, A102, A152, Q132, and C172 (Fig. 1A and B). E. coli TG1 isolates carrying different qnrVC7 mutations were assayed, upon induction by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), for their quinolone susceptibilities by the broth microdilution method, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (13), to determine the effect of amino acid substitutions of each residue on the protective effect of the qnrVC7 gene product. To better understand the mutational analysis data, the structure of QnrVC was modeled using QnrB1(2XTW) as the template, through the use of SWISS-MODEL, and the final structure was refined by PyMOL, as previously described (14). Structural analysis showed that coils 1 to 4 and 5 to 7 of QnrVC7 were arranged in the form of relatively tight and well-organized stacks, with similar space between the two stacks, whereas stacks between coils 4 and 5 and 7 and 8 were in a less-organized format, with wider spaces between the stacks (Fig. 1B). On the other hand, residues A36, S62, A82, and A102 were found to be located at the i−2 position of face 4 of coils 1 to 4 of QnrVC7, respectively.
FIG 1.
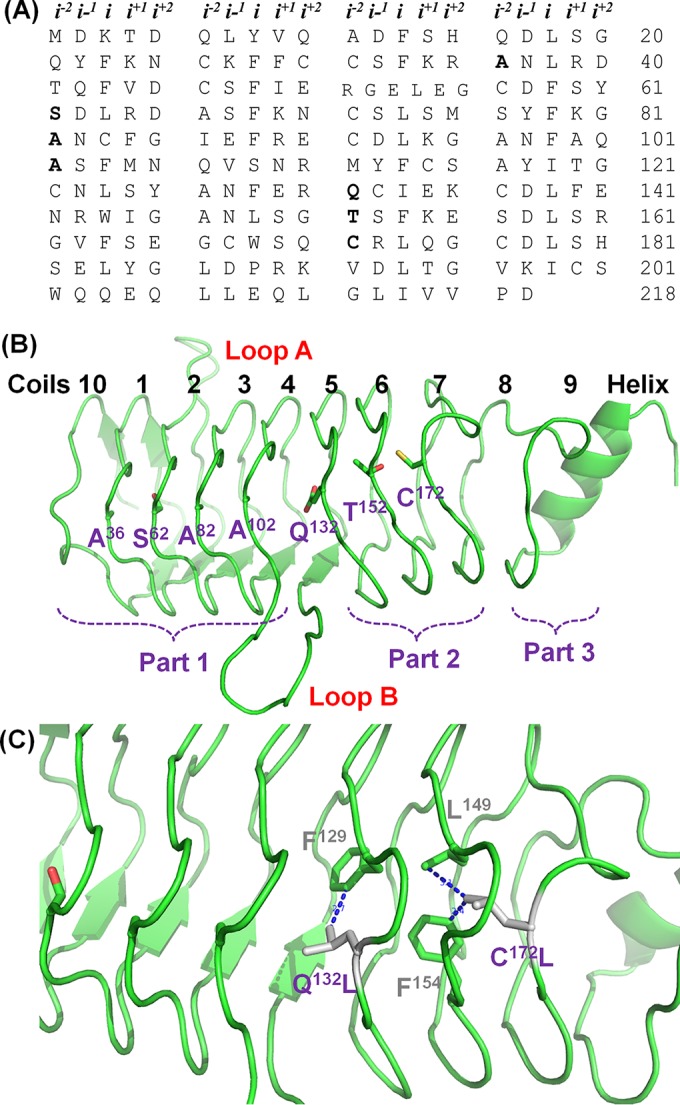
Amino acid sequence and structural representation of QnrVC7 and specific mutant proteins. (A) Tabular array of QnrV7 amino acids grouped by pentapeptide repeats, with coils along the vertical axis and faces along the horizontal axis; i−2 residues characterized in this study are in bold. (B) Overall structure of QnrVC7 and its organization. The sites at the i−2 position of face 4 are indicated in purple. (C) Amino acid substitutions that led to an increase in protective activity of QnrVC7. The potential hydrophobic interactions that may enhance the stability of QnrVC7 are indicated in gray and purple.
The amino acid substitutions A36T and A36G did not exhibit apparent effects on QnrVC7 protective activity, according to the ciprofloxacin MIC data, yet replacement with larger-chain residues, as exemplified by the A36L and A36D changes, completely abolished the protective function of QnrVC7 (Table 1). Similarly, the S62A substitution exhibited no effect, and the S62T amino acid change was found to display only minor effects on the protective activity of QnrVC7; however, other substitutions, such as S62G, S62L, and S62D, completely abolished the protective activity of QnrVC7. For residues A82 and A102, all substitutions, including those with Gly, completely abolished its protective activity, with an exception being A102S, which exhibited minor effects (Table 1). These data suggest that Ala, Ser, and, in some cases, Thr, were the best fitting residues at the i−2 position of face 4 on coils 1 to 4, and that the bulky side chain residues, such as Leu and Asp, would disrupt the well-organized tight stacks of these coils. The requirement for small side chain residues at the i−2 position was consistent with the finding in other Qnr proteins, such as QnrA and QnrC (11). However, substitution with Gly was not tolerated either, suggesting that the small side chain is required for forming the stable stack between coils. The best-fitting residues in these well-organized structure were also observable in the i−2 positions of other faces in QnrVC and other Qnr proteins, such as QnrB1 (3, 9, 12).
TABLE 1.
Ciprofloxacin MICs of Qnr-producing E. coli TG1 mutants
| Protein or mutation type, residue | Strain IDa | CIP MIC (μg/ml)b |
|---|---|---|
| Control | TG1(pCR2.1-qnrVC5) | 0.25 |
| TG1(pCR2.1-qnrVC7) | 0.06 | |
| TG1(pCR2.1) | 0.015 | |
| TG1 | 0.015 | |
| QnrVC7, A36 | TG1(pCR2.1-qnrVC7-A36T) | 0.06 |
| TG1(pCR2.1-qnrVC7-A36G) | 0.06 | |
| TG1(pCR2.1-qnrVC7-A36L) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A36D) | 0.015 | |
| QnrVC7, S62 | TG1(pCR2.1-qnrVC7-S62T) | 0.03 |
| TG1(pCR2.1-qnrVC7-S62G) | 0.015 | |
| TG1(pCR2.1-qnrVC7-S62L) | 0.015 | |
| TG1(pCR2.1-qnrVC7-S62D) | 0.015 | |
| TG1(pCR2.1-qnrVC7-S62A) | 0.06 | |
| QnrVC7, A82 | TG1(pCR2.1-qnrVC7-A82T) | 0.015 |
| TG1(pCR2.1-qnrVC7-A82G) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A82L) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A82D) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A82I) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A82S) | 0.03 | |
| QnrVC7, A102 | TG1(pCR2.1-qnrVC7-A102T) | 0.015 |
| TG1(pCR2.1-qnrVC7-A102G) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A102L) | 0.015 | |
| TG1(pCR2.1-qnrVC7-A102D) | 0.015 | |
| QnrVC7, Q132 | TG1(pCR2.1-qnrVC7-Q132T) | 0.06 |
| TG1(pCR2.1-qnrVC7-Q132G) | 0.06 | |
| TG1(pCR2.1-qnrVC7-Q132L) | 0.25 | |
| TG1(pCR2.1-qnrVC7-Q132D) | 0.015 | |
| TG1(pCR2.1-qnrVC7-Q132A) | 0.125 | |
| QnrVC7, T152 | TG1(pCR2.1-qnrVC7-T152G) | 0.015 |
| TG1(pCR2.1-qnrVC7-T152L) | 0.015 | |
| TG1(pCR2.1-qnrVC7-T152D) | 0.015 | |
| TG1(pCR2.1-qnrVC7-T152A) | 0.25 | |
| QnrVC7, C172 | TG1(pCR2.1-qnrVC7-C172T) | 0.06 |
| TG1(pCR2.1-qnrVC7-C172G) | 0.015 | |
| TG1(pCR2.1-qnrVC7-C172L) | 0.25 | |
| TG1(pCR2.1-qnrVC7-C172D) | 0.015 | |
| TG1(pCR2.1-qnrVC7-C172A) | 0.06 | |
| Double mutations | TG1(pCR2.1-qnrVC7-T152A/Q132L) | 0.25 |
| TG1(pCR2.1-qnrVC7-T152A/C172L) | 0.25 | |
| QnrVC5 | TG1(pCR2.1-qnrVC5-Q132L) | 0.25 |
| TG1(pCR2.1-qnrVC5-C172L) | 0.25 |
Strains TG1 and TG1(pCR2.1) denote the E. coli host strain and the corresponding strain harboring the pCR2.1 vector, respectively. The amino acid change of the respective protein is depicted in the strain identification (ID).
CIP, ciprofloxacin.
It was shown in our previous study that QnrVC7 differed from QnrVC5, QnrVC6, and other Qnr proteins by having a threonine at the 152 site (12). In this work, a mutation causing the T152A change was found to effectively convert QnrVC7 to other Qnr proteins through enhancement of its protective activity to the same level as that exhibited by the other Qnr proteins. However, other substitutions, including T152G, T152L, and T152D, completely abolished its protective activity. Structural analysis showed that the stack formed by coils 5 and 6 was a tight structure similar to that formed by coils 1 to 4; therefore, the rule for best-fitting residues also applies to this stack, in which the amino acid Ala or Ser was the best-fitting residue for maintenance of the stack structures of QnrVC7 and other Qnr proteins. Surprisingly, an i−2 residue of face 4 on coil 5, Q132, was found to exhibit different functional properties. The amino acid substitutions Q132T and Q132G had no effect on the protective function of the protein, whereas the Q132D change completely abolished its activity. On the other hand, the Q132A and Q132L changes increased QnrVC7-protective strength by 2- and 4-fold, respectively, almost reaching the full capacity exhibited by the other Qnr proteins. Similarly, the reduced protective strength of QnrVC7 due to the T152 substitution could also be complemented through the C172L change at the i−2 position of face 4 on coil 7, presumably through interaction with residue F154 of QnrVC7 to stabilize the stack formed by coils 6 and 7 and coils 7 and 8. However, the stack formed by coils 7 and 8 was similar to other stacks, in that the amino acid substitutions C172A and C172T had no effect on QnrVC7-protective activity, whereas the C172D and C172G changes completely abolished such activity. These findings suggest that the protective effects of the stacks formed by coils 6 and 7 and coils 7 and 8 could be increased by enhancing the hydrophobic nature of residue on i−2 of face 4; for example, the C172L and Q132L substitutions could complement the less-optimal protective effect due to residue T152 in QnrVC7.
To further characterize the effects of different substitutions of residues in the i−2 position of face 4, double-amino acid substitutions (T152A/Q132L and T152A/C172L) were introduced but were found to exhibit no further improvement in the QnrVC7 functions. In addition, the Q312L or C712L change created in QnrVC5 was found to exhibit a full protective effect but no further functional improvement. These data suggest that most Qnr proteins already exhibited full protective activity in their natural form, and that any reduction in protective activity due to amino acid substitutions in the i−2 position of coil 6, 7, or 8 could be readily reversed by the introduction of a hydrophobic residue, such as Leu.
Integrated analysis of the findings from this study and currently available data has advanced our understanding of Qnr proteins in the following aspects. First, face 2 of the Qnr protein was found to be in a well-organized status, whereas face 4 could be separated into three parts, in which part 1 was formed by coils 10, 1, 2, 3, and 4, part 2 by coils 5, to 7, and part 3 by coils 8 and 9 (Fig. 1B). Parts 1 and 2 were separated by loop B, whereas part 3 comprised a less-ordered coil and a C-terminal helix. Second, residues at the i position from all four faces were hydrophobic residues facing the inside of the Qnr protein and were found to play a very important role in stabilizing the overall structure of the protein, accommodating only small side chain residues at the i−2 position in all four faces. Previous mutational analysis data showed that some residues in the i position could tolerate substitutions by Ala, such as F25A, F30A, L35A, and F40A, yet some were sensitive to these substitutions, such as F56A, F66A, W166A, and L176A (3). Due to the space constraint of the i-position hydrophobic residues, only small side chain residues, such as Thr, Ala, Ser, or Cys, were allowed at the i−2 position, according to our data on the i−2 position of face 4 and data from previous studies (3, 9). Third, an exception to this rule is that the i−2 position of face 4 in coils 5 and 7 is located at the interface of three different parts. Due to the insertion of loop B into the region between parts 1 and 2, the stack formed by coils 4 and 5 was much wider than other well-organized stacks formed by other coils. It should be noted that the i−2 position on coil 5 tolerated the residue Gln, Thr, or Gly but not Asp. Interestingly, residues of a hydrophobic nature, such as Leu, helped stabilize parts 1 and 2 of the protein. In the case of QnrVC7, its protective effect was reduced by the substitution A152T. The substitution may cause a conformational change of QnrVC7, therefore affecting the correct docking of QnrVC7 into gyrase A. The substitution Q312L could complement such a reduction in protective activity by stabilizing coil 5. Similarly, due to the less-organized structure of coil 8, an amino acid substitution at the i−2 of coil 7, C172L, also mediated strong hydrophobic interactions with residues in the i position, such as L149 and F154, further stabilized the structure, and improved protective activity (Fig. 1C). The restoration of a higher protective activity of QnrVC7 by mutations Q132L and C172L, with predicted tighter stacking between coils 5 and 6 and 6 and 7, possibly functions by a better positioning of loop B, which is required for gyrase to disrupt quinolone action (5, 15). In summary, this study characterized a series of residues in the i−2 position of face 4 of QnrVC7 and demonstrated their role in stabilizing the structure of Qnr proteins for the maintenance of proper functions of such proteins, which advanced our understanding of the structure-activity relationship of Qnr proteins.
ACKNOWLEDGMENTS
This work was supported by the Chinese National Key Basic Research and Development (973) Program (grant 2013CB127200) and the Health and Medical Research Fund from the Food and Health Bureau, the Government of the Hong Kong SAR (grant HMRF:13121422 to S.C.).
We report no conflicts of interest.
REFERENCES
- 1.Vetting MW, Hegde SS, Wang M, Jacoby GA, Hooper DC, Blanchard JS. 2011. Structure of QnrB1, a plasmid-mediated fluoroquinolone resistance factor. J Biol Chem 286:25265–25273. doi: 10.1074/jbc.M111.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegde SS, Vetting MW, Roderick SL, Mitchenall LA, Maxwell A, Takiff HE, Blanchard JS. 2005. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 308:1480–1483. doi: 10.1126/science.1110699. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby GA, Corcoran MA, Mills DM, Griffin CM, Hooper DC. 2013. Mutational analysis of quinolone resistance protein QnrB1. Antimicrob Agents Chemother 57:5733–5736. doi: 10.1128/AAC.01533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahey Clinic. 2015. qnr numbering and sequence. Lahey Clinic, Burlington, MA: http://www.lahey.org/qnrstudies/. [Google Scholar]
- 5.Xiong X, Bromley EH, Oelschlaeger P, Woolfson DN, Spencer J. 2011. Structural insights into quinolone antibiotic resistance mediated by pentapeptide repeat proteins: conserved surface loops direct the activity of a Qnr protein from a Gram-negative bacterium. Nucleic Acids Res 39:3917–3927. doi: 10.1093/nar/gkq1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HB, Wang M, Ahmed S, Park CH, LaRocque RC, Faruque AS, Salam MA, Khan WA, Qadri F, Calderwood SB, Jacoby GA, Hooper DC. 2010. Transferable quinolone resistance in Vibrio cholerae. Antimicrob Agents Chemother 54:799–803. doi: 10.1128/AAC.01045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca ÉL, dos Santos Freitas F, Vieira VV, Vicente ACP. 2008. New qnr gene cassettes associated with superintegron repeats in Vibrio cholerae O1. Emerg Infect Dis 14:1129–1131. doi: 10.3201/eid1407.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegde SS, Vetting MW, Mitchenall LA, Maxwell A, Blanchard JS. 2011. Structural and biochemical analysis of the pentapeptide repeat protein EfsQnr, a potent DNA gyrase inhibitor. Antimicrob Agents Chemother 55:110–117. doi: 10.1128/AAC.01158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Martínez JM, Briales A, Velasco C, Conejo MC, Martínez-Martínez L, Pascual A. 2009. Mutational analysis of quinolone resistance in the plasmid-encoded pentapeptide repeat proteins QnrA, QnrB and QnrS. J Antimicrob Chemother 63:1128–1134. doi: 10.1093/jac/dkp111. [DOI] [PubMed] [Google Scholar]
- 10.Cattoir V, Poirel L, Nordmann P. 2007. In-vitro mutagenesis of qnrA and qnrS genes and quinolone resistance in Escherichia coli. Clin Microbiol Infect 13:940–943. doi: 10.1111/j.1469-0691.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 11.Guo Q, Weng J, Xu X, Wang M, Wang X, Ye X, Wang W, Wang M. 2010. A mutational analysis and molecular dynamics simulation of quinolone resistance proteins QnrA1 and QnrC from Proteus mirabilis. BMC Struct Biol 10:33. doi: 10.1186/1472-6807-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Po KH, Wong MH, Chen S. 2015. Identification and characterisation of a novel plasmid-mediated quinolone resistance gene, qnrVC7, in Vibrio cholerae of seafood origin. Int J Antimicrob Agents 45:667–668. doi: 10.1016/j.ijantimicag.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline, 2nd ed CLSI document M45-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Guo J, Chen S. 2013. Unique substrate recognition mechanism of the botulinum neurotoxin D light chain. J Biol Chem 288:27881–27887. doi: 10.1074/jbc.M113.491134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim ES, Chen C, Braun M, Kim HY, Okumura R, Wang Y, Jacoby GA, Hooper DC. 2015. Interactions between QnrB, QnrB mutants, and DNA gyrase. Antimicrob Agents Chemother 59:5413–5419. doi: 10.1128/AAC.00771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


