Abstract
Our recent report of dihydroartemisinin-piperaquine failure to treat Plasmodium falciparum infections in Cambodia adds new urgency to the search for alternative treatments. Despite dihydroartemisinin-piperaquine failure, and higher piperaquine 50% inhibitory concentrations (IC50s) following reanalysis than those previously reported, P. falciparum remained sensitive to atovaquone (ATQ) in vitro. There were no point mutations in the P. falciparum cytochrome b ATQ resistance gene. Mefloquine, artemisinin, chloroquine, and quinine IC50s remained comparable to those from other recent reports. Atovaquone-proguanil may be a useful stopgap but remains susceptible to developing resistance when used as blood-stage therapy.
TEXT
The Thai-Cambodian border has long been a focus of the growing public health crisis of Plasmodium falciparum multidrug resistance. In 2007 to 2008, the first treatment failures with artesunate monotherapy (7 days) were reported in western Cambodia (1). We recently reported unacceptably high failure levels in northern Cambodia with the current national first-line treatment, dihydroartemisinin-piperaquine (DP), with a drop in efficacy from 90% in 2010 (2) to only 46% in 2013, associated with a 3-gene mutation in kelch-13, MAL10, and MAL13 of the P. falciparum genome (3). Cambodian health officials are considering alternatives to replace it as the national first-line P. falciparum treatment regimen.
Malarone, a fixed-dose combination of atovaquone (ATQ) and proguanil (PG), has recently been used as part of a multidrug-resistant malarial containment program in western Cambodia (4). ATQ (a coenzyme Q analogue) specifically targets the cytochrome bc1 complex of the mitochondrial respiratory chain in the malarial parasite (5). Various single nucleotide polymorphisms (SNPs) in the quinine binding site of the Plasmodium species cytochrome b (cytb) gene have been implicated in conferring resistance to ATQ (6). While M133I and L271V have been implicated in murine malarial models (7, 8), and L144S, K272R, and V284F have been demonstrated in cultures exposed to high concentrations of ATQ (9), SNPs in position 268 (Y268C, Y268S, and Y268N) have been associated with clinical failure (10). ATQ resistance also appears to be associated with the delayed recrudescence of resistant parasites ≥3 weeks after initial clearance of parasitemia by ATQ-PG therapy (11). While likely of critical importance in the setting of rapidly worsening antimalarial resistance, little is known about the prevalence of P. falciparum cytb 268 mutations or ATQ susceptibility in Cambodia.
We isolated P. falciparum from blood samples collected prior to treatment from a total of 108 patients with uncomplicated P. falciparum, obtained during screening for a 2013 dihydroartemisinin (DHA)-piperaquine (PPQ) treatment efficacy study and diagnosed by microscopy and confirmatory Plasmodium species quantitative real-time PCR (12). All patients signed informed consent. All isolates were evaluated for susceptibility to a panel of standard antimalarials, including artesunate (AS), DHA, mefloquine (MQ), quinine (QN), chloroquine (CQ), and PPQ. ATQ had not been part of the initial panel but was added after the first 21 subjects were enrolled, due to high observed PPQ failure rates. ATQ was dissolved in dimethyl sulfoxide (DMSO) and diluted in 70% ethanol and then sterile water for a final concentration range of 0.14 to 100 ng/ml, while the conditions used for other drugs were as previously described (13). Susceptibility was measured by histidine-rich protein-2 (HRP-2) enzyme-linked immunosorbent assay (ELISA) testing on fresh isolates within 4 h of phlebotomy after being incubated for 72 h in 0.5% AlbuMAX with RPMI medium on drug-coated plates, according to previously published methods (13, 14).
Figure 1 shows the ex vivo susceptibility results for all isolates, and the values were comparable to our other recent ex vivo observations for all drugs except piperaquine, which was more resistant (15). The atovaquone-susceptible P. falciparum W2 clone was used as an established reference (16); the highly ATQ-resistant C2B clone was also used (17). All clinical isolates and the W2 clone were sensitive to atovaquone (geometric mean 50% inhibitory concentration [IC50], 6.0 nM), while the geometric mean IC50 for the C2B strain was 11,368 nM (range, 9,214 to 12,242 nM) (Fig. 1).
FIG 1.
Ex vivo drug susceptibility of P. falciparum isolates from Cambodia. IC50s (in nanomoles) of P. falciparum monoinfection are plotted for each drug, with their geometric mean indicated by a red bar, and indicated by the value below each cluster of data points. The dashed lines denote geometric mean IC50s against the P. falciparum W2 (green) and C2B (blue) reference clones. Red circles indicate isolates that were not originally cleared by 674 nM PPQ.
In retrospect, 23 of 108 (22%) isolates were found to have yielded inaccurate piperaquine IC50 curves following publication of the original report, as they had been capable of growing in the presence of the maximum piperaquine concentration tested (674 nM). To better determine the IC50s in these resistant isolates, we reinterpolated the IC50 dose-response curves by including the optical density (OD) values of the individual patient cultures in the presence of 2,000 ng/ml CQ, the value at which 100% HRP-2 inhibition occurred. Fitting these “zero-growth” OD values for individual isolates to the previously derived piperaquine curves yielded extrapolated a PPQ concentration of 53,905 nM at the point of 100% inhibition (see Fig. 2). Following this reanalysis, piperaquine resistance in some clones was higher than what we had previously reported for this study, with 22 of 92 (24%) evaluable isolate IC50s substantially >50 nM, the highest value seen from previous years (2009 to 2012 [15]). Further, isolates from subjects with P. falciparum recrudescence had a significantly increased median IC50 of 40.3 nM, compared to 28.6 nM for those without P. falciparum recrudescence (P < 0.05, Mann-Whitney U test).
FIG 2.

PPQ dose-response curves for 3 illustrative P. falciparum isolates that were not cleared at the maximum PPQ concentration used in the original assay. The colors denote different isolates. The solid lines are growth inhibition curves interpolated by HRP-2 OD, as measured by serial PPQ dilution with concentrations ranging from 0 to 674 nM. The dashed lines represent reanalyzed curves by including the control OD value for 100% HRP-2 inhibition (2,000 ng/ml CQ) and refitting the curve. This was equivalent to a concentration of 53,905 nM PPQ (denoted by closed circles); reinterpolated IC50s using these values are denoted by vertical colored lines for each isolate.
High-resolution melting (HRM)-PCR genotyping has been suggested as a fast and inexpensive tool for use in tracking parasite genetic polymorphisms (18). We applied HRM real-time PCR assay techniques to the samples to probe for single nucleotide polymorphisms (SNPs) associated with P. falciparum cytochrome b (cytb) variants at codon 268. Synthetic constructs were prepared to scan cytb and develop HRM curve profiles to distinguish ATQ-resistant mutations (Tyr268Asn, Tyr268Ser, and Tyr268Cys) from the wild-type haplotype (Tyr268Tyr) using P. falciparum reference DNA from C2B (ATQ-resistant) and 3D7 (ATQ-susceptible) clones. While we were unable to replicate prior melting point curves or temperature ranges reported by Gan and Loh (18) using our constructs, our assay was able to recognize the controls and revealed 2 potential mutants (data not shown). Secondary analysis of the 2 flagged isolates by fluorescent peak trace chromatography revealed a heterozygous 2-base mutation at position 271, a change not known to confer ATQ resistance (data not shown). Confirmatory Sanger sequencing (19) showed all 108 isolates to be wild type, including 3 marked as indeterminate by the HRM-PCR assay, and all were sensitive to ATQ in vitro (Fig. 3).
FIG 3.
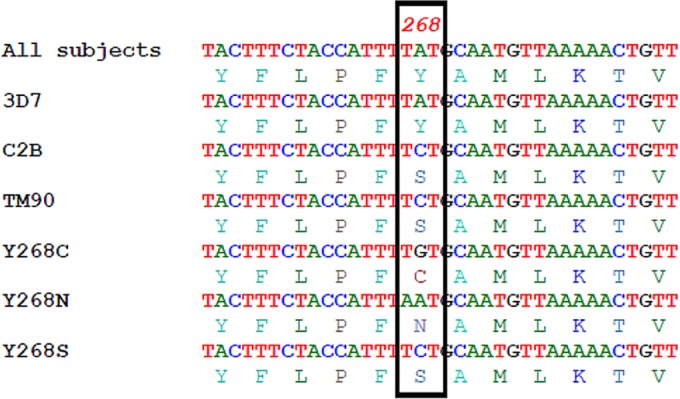
DNA sequencing analysis for cytb mutations revealed all of the 108 isolates to be wild-type, despite 2 P. falciparum isolates positive for mutations in the HRM-PCR. Y268C, N and S are previously described SNP associated with atovaquone resistance. While the 3D7 P. falciparum clone shares the wild-type sequence, the C2B and TM90 clones share the Y268N SNP.
While P. falciparum in vitro antimalarial resistance patterns to most currently available drugs in Cambodia were unchanged, we found worse in vitro resistance to piperaquine than we had previously reported in isolates from a study that found high-level clinical DHA-piperaquine failure. Despite this, and the recent use of atovaquone-proguanil as a part of public health containment activities in Cambodia, we found little evidence of ATQ resistance in P. falciparum clinical isolates from northern Cambodia. Although HRM-PCR was able to identify the 2 mutant isolates in our samples, disparities between our results and those of other published studies suggest that further evaluation of HRM-PCR is needed to determine if the method is viable as a reliable alternative to genetic sequencing in tracking resistance. Atovaquone-proguanil remains highly effective for both the causal (liver-stage) prophylaxis of malaria (20) and as a blood-stage therapy. However, rapid parasitic blood-stage resistance known to occur with atovaquone (21) may quickly obviate its use both as a treatment and as one of the last remaining effective prophylactic agents. At the time of this writing, we are currently conducting a study comparing ATQ-PG in combination with oral artesunate to ATQ-PG alone (ClinicalTrials.gov registration no. NCT02297477), using a low 15-mg dose of primaquine administered on day 1. If ATQ-PG is used to replace failing first-line malarial combination therapies, such as DHA-PPQ, meticulous clinical follow-up and molecular surveillance for emerging ATQ resistance are advised.
ACKNOWLEDGMENTS
We thank the patients of Oddar Meancheay who volunteered for the study, and we thank the combined staff of the Armed Forces Research Institute of Medical Sciences, the National Center for Parasitology, Entomology and Malaria Control, and the Royal Cambodian Armed Forces for their tremendous work and dedication toward making the study possible.
The views expressed in this paper are those of the authors and do not represent the views of the U.S. or Cambodian governments.
Funding Statement
Funding was provided by the Armed Forces Health Surveillance Center, Department of Defense. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 2.Lon C, Manning JE, Vanachayangkul P, So M, Sea D, Se Y, Gosi P, Lanteri C, Chaorattanakawee S, Sriwichai S, Chann S, Kuntawunginn W, Buathong N, Nou S, Walsh DS, Tyner SD, Juliano JJ, Lin J, Spring M, Bethell D, Kaewkungwal J, Tang D, Chuor CM, Satharath P, Saunders D. 2014. Efficacy of two versus three-day regimens of dihydroartemisinin-piperaquine for uncomplicated malaria in military personnel in northern Cambodia: an open-label randomized trial. PLoS One 9:e93138. doi: 10.1371/journal.pone.0093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders DL, Vanachayangkul P, Lon C, U.S. Army Military Malaria Research Program, National Center for Parasitology Entomology, and Malaria Control (CNM), Royal Cambodian Armed Forces. 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2012. National treatment guidelines for malaria in Cambodia. World Health Organization, Geneva, Switzerland: http://whothailand.healthrepository.org/bitstream/123456789/1442/1/NTG%20in%20English-Final.pdf. [Google Scholar]
- 5.Boggild AK, Parise ME, Lewis LS, Kain KC. 2007. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg 76:208–223. [PubMed] [Google Scholar]
- 6.Fry M, Pudney M. 1992. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem Pharmacol 43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 7.Siregar JE, Syafruddin D, Matsuoka H, Kita K, Marzuki S. 2008. Mutation underlying resistance of Plasmodium berghei to atovaquone in the quinone binding domain 2 (Qo(2)) of the cytochrome b gene. Parasitol Int 57:229–232. doi: 10.1016/j.parint.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Syafruddin D, Siregar JE, Marzuki S. 1999. Mutations in the cytochrome b gene of Plasmodium berghei conferring resistance to atovaquone. Mol Biochem Parasitol 104:185–194. doi: 10.1016/S0166-6851(99)00148-6. [DOI] [PubMed] [Google Scholar]
- 9.Siregar JE, Kurisu G, Kobayashi T, Matsuzaki M, Sakamoto K, Mi-ichi F, Watanabe Y, Hirai M, Matsuoka H, Syafruddin D, Marzuki S, Kita K. 2015. Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex. Parasitol Int 64:295–300. doi: 10.1016/j.parint.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Schwobel B, Alifrangis M, Salanti A, Jelinek T. 2003. Different mutation patterns of atovaquone resistance to Plasmodium falciparum in vitro and in vivo: rapid detection of codon 268 polymorphisms in the cytochrome b as potential in vivo resistance marker. Malar J 2:5. doi: 10.1186/1475-2875-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland CJ, Laundy M, Price N, Burke M, Fivelman QL, Pasvol G, Klein JL, Chiodini PL. 2008. Mutations in the Plasmodium falciparum cytochrome b gene are associated with delayed parasite recrudescence in malaria patients treated with atovaquone-proguanil. Malar J 7:240. doi: 10.1186/1475-2875-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. 2013. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One 8:e71539. doi: 10.1371/journal.pone.0071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaorattanakawee S, Tyner SD, Lon C, Yingyuen K, Ruttvisutinunt W, Sundrakes S, Sai-gnam P, Johnson JD, Walsh DS, Saunders DL, Lanteri CA. 2013. Direct comparison of the histidine-rich protein-2 enzyme-linked immunosorbent assay (HRP-2 ELISA) and malaria SYBR green I fluorescence (MSF) drug sensitivity tests in Plasmodium falciparum reference clones and fresh ex vivo field isolates from Cambodia. Malar J 12:239. doi: 10.1186/1475-2875-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyner SD, Lon C, Se Y, Bethell D, Socheat D, Noedl H, Sea D, Satimai W, Schaecher K, Rutvisuttinunt W, Fukuda MM, Chaorattanakawee S, Yingyuen K, Sundrakes S, Chaichana P, Saingam P, Buathong N, Sriwichai S, Chann S, Timmermans A, Saunders DL, Walsh DS. 2012. Ex vivo drug sensitivity profiles of Plasmodium falciparum field isolates from Cambodia and Thailand, 2005 to 2010, determined by a histidine-rich protein-2 assay. Malar J 11:198. doi: 10.1186/1475-2875-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaorattanakawee S, Saunders DL, Sea D, Chanarat N, Yingyuen K, Sundrakes S, Saingam P, Buathong N, Sriwichai S, Chann S, Se Y, Yom Y, Heng TK, Kong N, Kuntawunginn W, Tangthongchaiwiriya K, Jacob C, Takala-Harrison S, Plowe C, Lin JT, Chuor CM, Prom S, Tyner SD, Gosi P, Teja-Isavadharm P, Lon C, Lanteri CA. 2015. Ex vivo drug susceptibility testing and molecular profiling of clinical Plasmodium falciparum isolates from Cambodia from 2008 to 2013 suggest emerging piperaquine resistance. Antimicrob Agents Chemother 59:4631–4643. doi: 10.1128/AAC.00366-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutvisuttinunt W, Chaorattanakawee S, Tyner SD, Teja-Isavadharm P, Se Y, Yingyuen K, Chaichana P, Bethell D, Walsh DS, Lon C, Fukuda M, Socheat D, Noedl H, Schaecher K, Saunders DL. 2012. Optimizing the HRP-2 in vitro malaria drug susceptibility assay using a reference clone to improve comparisons of Plasmodium falciparum field isolates. Malar J 11:325. doi: 10.1186/1475-2875-11-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musset L, Pradines B, Parzy D, Durand R, Bigot P, Le Bras J. 2006. Apparent absence of atovaquone/proguanil resistance in 477 Plasmodium falciparum isolates from untreated French travellers. J Antimicrob Chemother 57:110–115. [DOI] [PubMed] [Google Scholar]
- 18.Gan LS, Loh JP. 2010. Rapid identification of chloroquine and atovaquone drug resistance in Plasmodium falciparum using high-resolution melt polymerase chain reaction. Malar J 9:134. doi: 10.1186/1475-2875-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakato H, Vivancos R, Hunter PR. 2007. A systematic review and meta-analysis of the effectiveness and safety of atovaquone proguanil (Malarone) for chemoprophylaxis against malaria. J Antimicrob Chemother 60:929–936. doi: 10.1093/jac/dkm337. [DOI] [PubMed] [Google Scholar]
- 21.Maude RJ, Nguon C, Dondorp AM, White LJ, White NJ. 2014. The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J 13:380. doi: 10.1186/1475-2875-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]



