Abstract
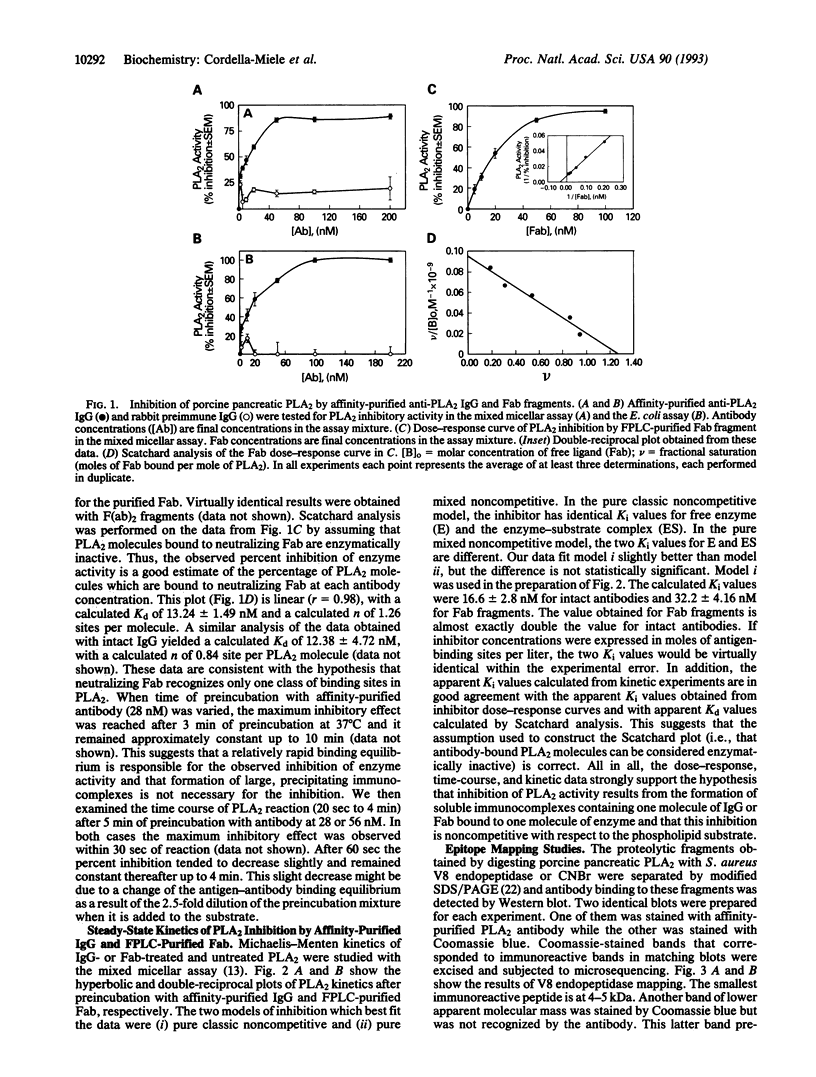
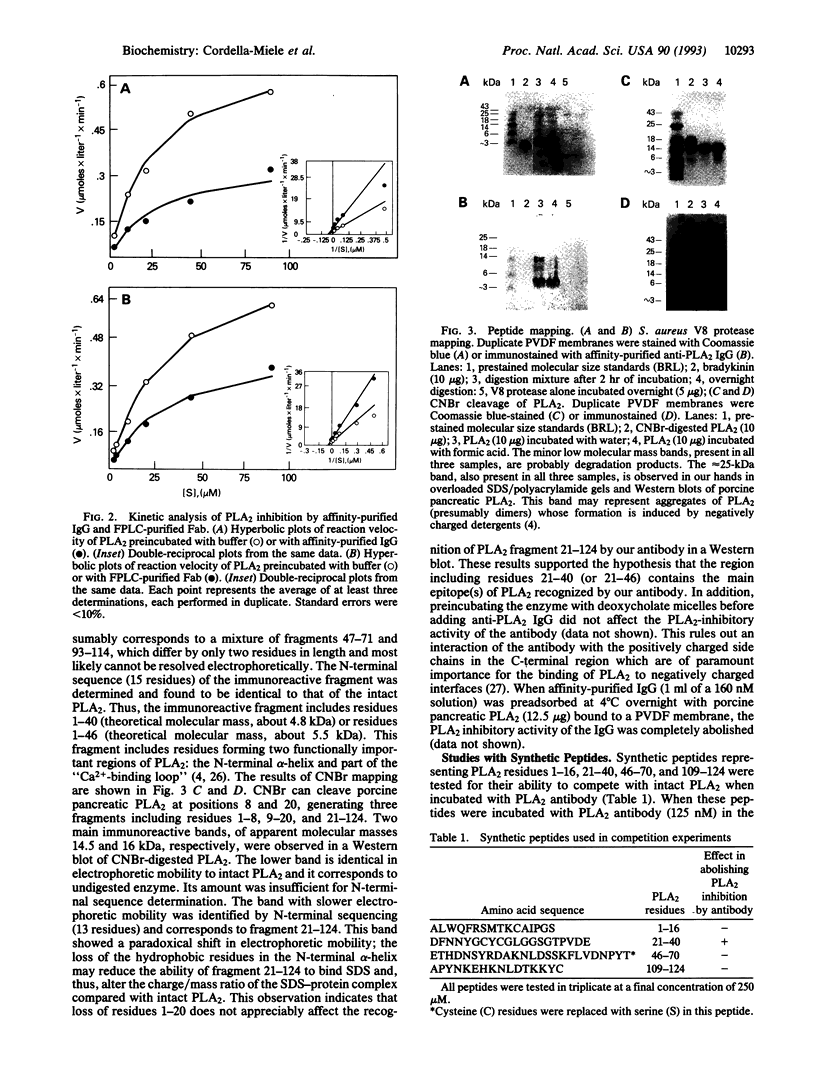
We have identified a specific region of porcine pancreatic phospholipase A2 (residues 21-40) which interacts with a neutralizing antibody causing a dramatic inhibition of its enzymatic activity (Ki in the order of 10(-8) M). The binding equilibrium of the antibody-phospholipase A2 complex is reached in < 3 min at 37 degrees C. Fab fragments are equally effective phospholipase A2 inhibitors, as are intact IgG molecules. The inhibition is virtually complete and noncompetitive with respect to phosphatidylcholine substrate. The formation of precipitating immunocomplexes is not involved in the inhibition. The region of phospholipase A2 (residues 21-40) recognized by this antibody includes a highly conserved sequence which contains several functionally important residues of both group I and group II phospholipases A2. These data suggest that amino acid residues in this region of porcine pancreatic phospholipase A2 are accessible for interaction with inhibitors such as neutralizing antibodies and that agents specifically interacting with this region may have potent phospholipase A2 inhibitory activity. Thus, this conserved region of low molecular weight, extracellular phospholipases A2 is a potential target for structure-based design of specific noncompetitive inhibitors of these enzymes. Since these extracellular phospholipases A2 are suggested to play a pathogenic role in several important human diseases, the development of such pharmacologic inhibitors is of potential clinical importance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Dixon J. E. A procedure for in situ alkylation of cystine residues on glass fiber prior to protein microsequence analysis. Anal Biochem. 1987 Mar;161(2):524–528. doi: 10.1016/0003-2697(87)90484-2. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Yoshida K., Sasaki Y., Nishizuka Y., Murakami M., Kudo I., Inoue K. Possible role of mammalian secretory group II phospholipase A2 in T-lymphocyte activation: implication in propagation of inflammatory reaction. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):716–719. doi: 10.1073/pnas.90.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burch R. M. G protein regulation of phospholipase A2: partial reconstitution of the system in cells. Adv Exp Med Biol. 1990;279:185–195. doi: 10.1007/978-1-4613-0651-1_12. [DOI] [PubMed] [Google Scholar]
- Cordella-Miele E., Miele L., Mukherjee A. B. A novel transglutaminase-mediated post-translational modification of phospholipase A2 dramatically increases its catalytic activity. J Biol Chem. 1990 Oct 5;265(28):17180–17188. [PubMed] [Google Scholar]
- Crowl R. M., Stoller T. J., Conroy R. R., Stoner C. R. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem. 1991 Feb 5;266(4):2647–2651. [PubMed] [Google Scholar]
- Crowl R., Stoner C., Stoller T., Pan Y. C., Conroy R. Isolation and characterization of cDNA clones from human placenta coding for phospholipase A2. Adv Exp Med Biol. 1990;279:173–184. doi: 10.1007/978-1-4613-0651-1_11. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981 Mar 25;147(1):97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Facchiano A., Cordella-Miele E., Miele L., Mukherjee A. B. Inhibition of pancreatic phospholipase A2 activity by uteroglobin and antiflammin peptides: possible mechanism of action. Life Sci. 1991;48(5):453–464. doi: 10.1016/0024-3205(91)90501-2. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kuipers O. P., van den Bergh C. J., Verheij H. M., de Haas G. H. Probing the mechanism of pancreatic phospholipase A2 with the aid of recombinant DNA techniques. Adv Exp Med Biol. 1990;279:65–84. doi: 10.1007/978-1-4613-0651-1_5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutz R. A., Bull C., Rodbard D. Computer analysis of enzyme-substrate-inhibitor kinetic data with automatic model selection using IBM-PC compatible microcomputers. Enzyme. 1986;36(3):197–206. doi: 10.1159/000469292. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Marshall L. A. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993 Feb 1;7(2):339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Cordella-Miele E., Miele L. Regulation of extracellular phospholipase A2 activity: implications for inflammatory diseases. DNA Cell Biol. 1992 Apr;11(3):233–243. doi: 10.1089/dna.1992.11.233. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L. Biology and biochemistry of platelet-activating factor. Clin Rev Allergy. 1983 Sep;1(3):353–367. doi: 10.1007/BF02991226. [DOI] [PubMed] [Google Scholar]
- Oka S., Arita H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. Two distinct pathways of the gene expression. J Biol Chem. 1991 May 25;266(15):9956–9960. [PubMed] [Google Scholar]
- Pruzanski W., Vadas P. Soluble phospholipase A2 in human pathology: clinical-laboratory interface. Adv Exp Med Biol. 1990;279:239–251. doi: 10.1007/978-1-4613-0651-1_17. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986 Oct;55(4):391–404. [PubMed] [Google Scholar]
- Wery J. P., Schevitz R. W., Clawson D. K., Bobbitt J. L., Dow E. R., Gamboa G., Goodson T., Jr, Hermann R. B., Kramer R. M., McClure D. B. Structure of recombinant human rheumatoid arthritic synovial fluid phospholipase A2 at 2.2 A resolution. Nature. 1991 Jul 4;352(6330):79–82. doi: 10.1038/352079a0. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Moss D. A., Kieber-Emmons T., Cohen J. A., Myers J. N., Weiner D. B., Greene M. I. Development of biologically active peptides based on antibody structure. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5537–5541. doi: 10.1073/pnas.86.14.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]