Abstract
New strategies to develop novel broad-spectrum antiviral drugs against influenza virus infections are needed due to the emergence of antigenic variants and drug-resistant viruses. Here, we evaluated C646, a novel p300/CREB-binding protein-specific inhibitor of histone acetyltransferase (HAT), as an anti-influenza virus agent in vitro and in vivo and explored how C646 affects the viral life cycle and host response. Our studies highlight the value of targeting HAT activity for anti-influenza drug development.
TEXT
Vaccination and antiviral drugs are effective ways to prevent influenza virus infection (1, 2); however, there is an urgent need to screen novel broad-spectrum antiviral drugs with the emergence of antigenic variants and drug-resistant viruses (3, 4). Influenza viruses commandeer the host cellular machinery for their propagation (5). Targeting of host factors that are critical for viral replication by using small molecular analogues or chemical inhibitors has shown great promise in the development of novel antivirals with broad-spectrum coverage (6).
Histone acetylation levels are regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) (7). Several HDAC inhibitors that show therapeutic potential for human cancers (8) and nonmalignant diseases (9) have been developed. Roles for HATs in cancer, asthma, chronic obstructive pulmonary disease, and viral infection have been demonstrated (10–12), which indicates that specific HAT inhibitors are potential tools for pharmacological research and may have clinical applications (13). The transcriptional coactivators p300 and CREB-binding protein (CBP) are important members of HAT families possessing HAT activity to influence chromatin activity. p300/CBP HATs are associated with tumorigenesis (14) and the development of many viral diseases (15–18). Several small molecules have been shown to possess p300/CBP HAT inhibitory activity (19–21) and anti-influenza virus properties (22–24). Recently, the compound C646 was identified as the first selective inhibitor of p300/CBP HATs (25). Here, we evaluated the antiviral effects of C646 on influenza A viruses.
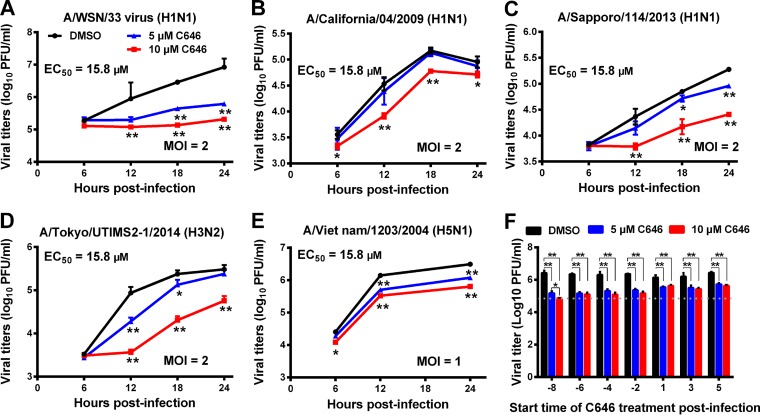
C646 showed a 50% cytotoxic concentration (CC50) of 107 μM in A549 cells (ATCC). The antiviral potency of C646 in vitro was evaluated based on C646-induced suppression of viral replication. A549 cells were treated with C646 (dimethyl sulfoxide [DMSO] as vehicle) for 10 h and then infected with viruses at an multiplicity of infection (MOI) of 2 or 1. C646 was present throughout the infection. The concentration of DMSO was kept at 0.1%, and 0.4 μg/ml tosyl phenylalanyl chloromethyl ketone (TPCK)-trypsin was used to cleave the hemagglutinin for multiple cycles of replication. The cell supernatants were collected for viral titration at the indicated times (26). C646 dose dependently suppressed the replication of different influenza A viruses, including A/WSN/33 (H1N1), A/California/04/2009 (H1N1), oseltamivir/peramivir-resistant A/Sapporo/114/2013 (H1N1) (27), A/Tokyo/UTIMS2-1/2014 (H3N2), and A/Vietnam/1203/2004 (H5N1) viruses, at 12, 18, and 24 h postinfection (hpi) with a 50% effective concentration (EC50) of 15.8 μM (Fig. 1A to E). These findings demonstrate that C646 inhibition of influenza virus replication is not subtype specific.
FIG 1.
C646 significantly suppresses the in vitro replication of influenza A viruses. A549 cells were treated with C646 (DMSO as vehicle) for 10 h and then were infected with viruses at an MOI of 2 or 1. The cell supernatants were collected for viral titration at the indicated times. Viral titers were measured in MDCK cells by using a plaque assay. Effect of C646 on the replication of A/WSN/33 (H1N1) (A), A/California/04/2009 (H1N1) (B), oseltamivir- and peramivir-resistant pandemic A/Sapporo/114/2013 (H1N1) (C), A/Tokyo/UTIMS2-1/2014 (H3N2) (D), and A/Vietnam/1203/2004 (H5N1) (E) in A549 cells. (F) Required start time of C646 treatment to affect viral replication. *, P < 0.05; **, P < 0.01 (Student's t test). Data are presented as means ± standard deviations (SD) (n = 3).
We tested whether C646 directly affects influenza virus infectivity by comparing viral titers in MDCK cells (ATCC) after incubation with virus and C646 and found that C646 does not directly affect viral infectivity (data not shown). To confirm whether C646 pretreatment before infection is necessary to suppress viral replication, A549 cells were treated with C646 at different start times such as −8, −6, −4, −2, 1, 3, and 5 hpi. The cells were infected with WSN virus at an MOI of 2, and supernatants were collected for viral titration at 24 hpi. C646 significantly suppressed viral replication even when the cells were treated at 5 hpi; however, C646 pretreatment had a greater inhibitory effect on viral replication (Fig. 1F), suggesting that C646 may have preventive and therapeutic effects on influenza virus infection.
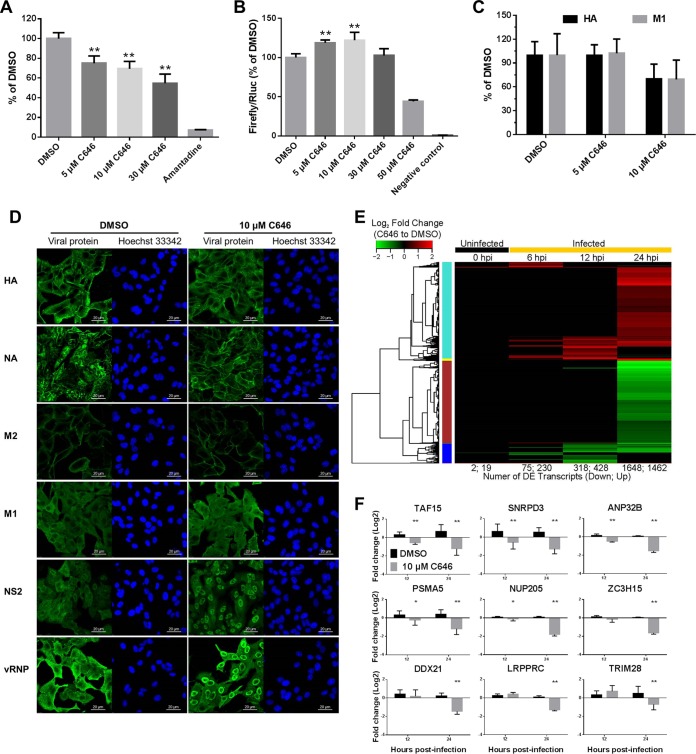
We then attempted to determine at what step(s) C646 affects influenza virus replication. To determine whether C646 affects the early steps of the viral life cycle, we infected C646-treated cells with a replication-incompetent PB2-knockout virus, whose PB2-coding region is replaced with that of Renilla luciferase (28). The expression of the Renilla luciferase is an indicator of virus binding, internalization, and limited replication (6). C646 decreased reporter expression in a dose-dependent manner (Fig. 2A), suggesting that C646 influences the early steps of the viral life cycle. To assess the role of C646 in viral genome replication and transcription, we measured viral polymerase activity by using a mini-replicon assay (29). C646 at 10 μM did not inhibit polymerase activity but enhanced it by 20% compared with that in the control group (Fig. 2B). Viral polymerase activity does not always correlate with viral pathogenicity (30–32), and it is unclear whether C646 treatment creates more pathogenic viruses.
FIG 2.
C646 affects the influenza virus life cycle and host gene expression in vitro. The effect of C646 on the viral life cycle was evaluated by using a PB2 KO virus assay (A), a mini-genome assay (B), assessment of VLP formation (C), and localization of viral proteins at 24 h postinfection in A549 cells (D). Rabbit polyclonal anti-NS2 (R-5023) and anti-RNP antibodies and mouse monoclonal antibodies against HA (WS3-54), NA (WS5-29), M1 (WS27-52), and M2 (SS23R15-1) were used. The detailed protocols for the above-described experiments were described previously (6). (E and F) Transcriptional changes induced by C646 in A549 cells. (E) Differentially expressed (DE) transcripts were identified by comparing gene expression levels in C646-treated A549 cells with those in cells treated with DMSO at the indicated times postinfection. Unsupervised clustering was used to identify genes with similar expression dynamics. A heat map of the clustered transcripts for each condition is displayed, and the color bar on the left identifies clustered gene sets: turquoise, negative regulation of cell death, cytokine production, regulation of NF-κB signaling, CREB and ATF3 transcription factor sequences; brown, poly(A) RNA binding, chromatin remodeling, cell division, ribonucleoprotein complex, NRF1 and YY1 transcription factor sequence, zinc finger C2H2-type; blue, cell division, several domains related to histones and histone cores, nucleosome and chromatin assembly. The number of DE transcripts in each comparison is provided below each column of the heat map. (F) Selected DE genes evaluated by using real-time PCR. Values represent fold changes of selected genes after C646 treatment compared with the time-matched DMSO-treated group on a log2 scale. Data are presented as means ± SD (n = 3). *, P < 0.05; **, P < 0.01 (Student's t test, compared with the DMSO-treated group). Rluc, Renilla luciferase.
Next, we evaluated the effect of C646 on the later steps of the viral life cycle, namely, virion formation. We treated 293 cells (ATCC) with C646 and then examined the effects on the formation of influenza virus-like particles (VLPs) composed of the M1, HA, and NA proteins. The efficiency of VLP production was determined based on the amounts of M1 and HA from the VLPs in the supernatant compared with those in the cell lysates (6). Although 5 μM C646 did not affect the efficiency of VLP production, 10 μM C646 decreased VLP production (Fig. 2C). We next examined the effect of C646 on the localization of viral proteins in A549 cells by using an immunofluorescence assay (6) and found that 10 μM C646 did not affect the localization or expression of HA, M1, or M2 (Fig. 2D; see also Fig. S1 to S3 in the supplemental material); however, NA protein was found both in the cytoplasm and at the plasma membrane in the DMSO group, although it mainly localized to the plasma membrane after C646 treatment (Fig. 2D; see also Fig. S4 in the supplemental material). The alterations in NA localization and expression may affect subsequent virus budding or virion formation. In addition, C646 markedly suppressed the nuclear export of NS2 and viral RNP (vRNP) (Fig. 2D; see also Fig. S5 and S6 in the supplemental material). Together, these data show that C646 affects several steps of the influenza virus life cycle, including genome transcription/translation, VLP formation, localization of viral proteins, and the nuclear export of NS2 and vRNP.
To identify the host genes affected by C646 during influenza virus infection, we investigated the transcriptional profiles of A549 cells with a microarray analysis. Using time-matched comparisons of the C646-treated group and the DMSO-treated group, we identified a total of 3,556 differentially expressed (DE) genes and analyzed them by using hierarchical clustering at different time points (Fig. 2E; see also Table S1 in the supplemental material). Upregulated genes were correlated with the negative regulation of cell death, cytokine production, and NF-κB signaling, whereas downregulated genes were mainly involved in chromatin remodeling, cell division, and nucleosome and chromatin assembly (Fig. 2E). Watanabe et al. identified 323 host genes that affect influenza virus replication and explored the mechanism of the inhibitory effects on viral replication of 91 selected host factors (top hits) (6). We compared these 323 host factors with the C646-related DE genes and found 88 overlapping genes, 21 of which were included among the 91 top hits (see Table S2 in the supplemental material). Nine of these 21 overlapping genes (ANP32B, DDX21, LRPPRC, NUP205, PSMA5, SNRPD3, TAF15, TRIM28, and ZC3H15) were downregulated in C646-treated A549 cells (see Table S2), which was confirmed by using real-time PCR at 12 hpi and 24 hpi (Fig. 2F). These 9 genes significantly suppressed viral replication when knocked down by their specific siRNAs (6). Interestingly, these 9 proteins interacted with different viral proteins and affected different steps of the viral life cycle (6), thus supporting our finding that C646 affects several steps of the viral life cycle.
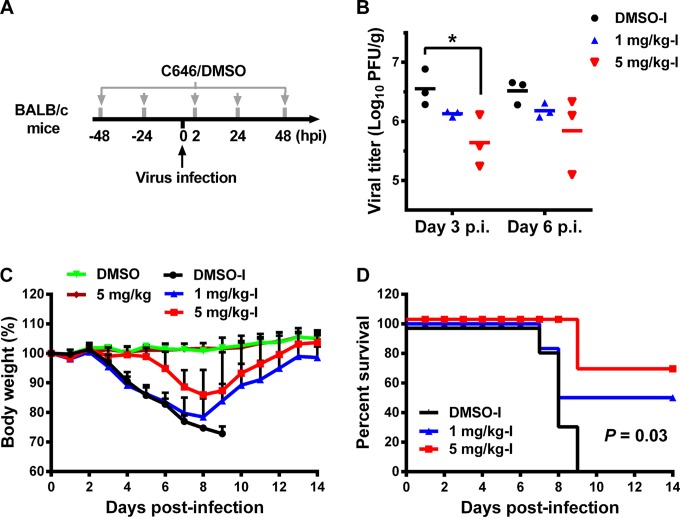
We next examined the in vivo effect of C646 in a murine model of influenza virus infection. BALB/c mice were intranasally treated with C646 and infected with 5 × 103 PFU of WSN virus (Fig. 3A). C646 treatment (1 mg/kg-I [mice were inoculated with 1 mg/kg C646 and infected with virus] and 5 mg/kg-I) dose dependently suppressed viral replication in the lungs on days 3 and 6 postinfection (Fig. 3B). Importantly, C646 dose dependently suppressed weight loss and improved mouse survival (Fig. 3C and D). Mice were also inoculated with DMSO or 5 mg/kg C646 without viral infection to evaluate the toxicity of C646 in mice; all of the mice survived and experienced minimal weight change (Fig. 3C), which suggests that C646 has no or little toxicity in vivo even at 5 mg/kg. Inhibition of p300/CBP HATs by C646 has been reported to have little effect on an immortalized murine cell line (25); however, HATs are also involved in the expression of inflammatory genes during inflammatory lung diseases (12, 33, 34). C646 may improve survival by modulating the inflammatory response in the lung. It might be worthwhile to examine whether protection against influenza virus is further enhanced by combining C646 treatment with other anti-influenza compounds.
FIG 3.
Intranasal inoculation of mice with C646 significantly suppresses viral replication in the lungs and improves survival following influenza virus infection. (A) BALB/c mice were intranasally inoculated with C646 or DMSO as shown in the schematic diagram. (B) Mice were then infected with 5 × 103 PFU of the WSN virus. On days 3 and 6 postinfection, three mice from each group were euthanized, and lung samples were collected and titrated for virus in MDCK cells. *, P < 0.05 (Student's t test). Mouse body weight (C) and survival (D) were monitored for 14 days postinfection. DMSO-I, mice were inoculated with DMSO and also infected with virus; 1 mg/kg-I, mice were inoculated with 1 mg/kg C646 and also infected with virus; 5 mg/kg-I, mice were inoculated with 5 mg/kg C646 and also infected with virus; DMSO, mice were inoculated with just DMSO and not infected with virus; 5 mg/kg, mice were inoculated with just 5 mg/kg C646 and not infected with virus. Data are presented as means ± SD (n = 6); the P value is based on the Mantel-Cox test.
In summary, our findings suggest that C646 is a promising anti-influenza virus candidate and highlight the value of targeting HAT activity with specific inhibitors as a novel antiviral strategy.
Microarray data accession number.
Microarray data were deposited in the GEO database (GSE72503).
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Watson for editing the manuscript.
Funding Statement
This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by grants-in-aid from the Ministry of Health, Labour and Welfare, Japan, by ERATO, by grants from the Strategic Basic Research Program of the Japan Science and Technology Agency, and by the Advanced Research & Development Programs for Medical Innovation from the Japan Agency for Medical Research and Development (AMED).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02055-15.
REFERENCES
- 1.Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, Cheung A, Hovhannisyan G, Ivanova L, Flottorp SA, Saeterdal I, Wong AD, Tian J, Uyeki TM, Akl EA, Alonso-Coello P, Smaill F, Schunemann HJ. 2012. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med 156:512–524. doi: 10.7326/0003-4819-156-7-201204030-00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert LC, Fauci AS. 2010. Influenza vaccines for the future. N Engl J Med 363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 3.Hayden FG, de Jong MD. 2011. Emerging influenza antiviral resistance threats. J Infect Dis 203:6–10. doi: 10.1093/infdis/jiq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka Y, Matsumae H, Katoh M, Eisfeld AJ, Neumann G, Hase T, Ghosh S, Shoemaker JE, Lopes TJ, Watanabe T, Watanabe S, Fukuyama S, Kitano H, Kawaoka Y. 2013. A comprehensive map of the influenza A virus replication cycle. BMC Syst Biol 7:97. doi: 10.1186/1752-0509-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Kawakami E, Shoemaker JE, Lopes TJ, Matsuoka Y, Tomita Y, Kozuka-Hata H, Gorai T, Kuwahara T, Takeda E, Nagata A, Takano R, Kiso M, Yamashita M, Sakai-Tagawa Y, Katsura H, Nonaka N, Fujii H, Fujii K, Sugita Y, Noda T, Goto H, Fukuyama S, Watanabe S, Neumann G, Oyama M, Kitano H, Kawaoka Y. 2014. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe 16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang XJ, Seto E. 2007. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 8.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. 2005. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 9.Dokmanovic M, Clarke C, Marks PA. 2007. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 10.Mantelingu K, Reddy BA, Swaminathan V, Kishore AH, Siddappa NB, Kumar GV, Nagashankar G, Natesh N, Roy S, Sadhale PP, Ranga U, Narayana C, Kundu TK. 2007. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol 14:645–657. doi: 10.1016/j.chembiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Kitabayashi I, Aikawa Y, Yokoyama A, Hosoda F, Nagai M, Kakazu N, Abe T, Ohki M. 2001. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with a t(8;22) (p11;q13) chromosome translocation. Leukemia 15:89–94. doi: 10.1038/sj.leu.2401983. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ, Adcock IM, Ito K. 2005. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J 25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 13.Dekker FJ, Haisma HJ. 2009. Histone acetyl transferases as emerging drug targets. Drug Discov Today 14:942–948. doi: 10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Marshall CB, Ikura M. 2013. Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition. Cell Mol Life Sci 70:3989–4008. doi: 10.1007/s00018-012-1254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Jiang Y, Geiser V, Zhou J, Jones C. 2006. Bovine herpesvirus 1 immediate-early protein (bICP0) interacts with the histone acetyltransferase p300, which stimulates productive infection and gC promoter activity. J Gen Virol 87:1843–1851. doi: 10.1099/vir.0.81766-0. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian C, Hasan S, Rowe M, Hottiger M, Orre R, Robertson ES. 2002. Epstein-Barr virus nuclear antigen 3C and prothymosin alpha interact with the p300 transcriptional coactivator at the CH1 and CH3/HAT domains and cooperate in regulation of transcription and histone acetylation. J Virol 76:4699–4708. doi: 10.1128/JVI.76.10.4699-4708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Pise-Masison CA, Fletcher TM, Schiltz RL, Nagaich AK, Radonovich M, Hager G, Cole PA, Brady JN. 2002. Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol Cell Biol 22:4450–4462. doi: 10.1128/MCB.22.13.4450-4462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang SE, Hearing P. 2003. The adenovirus E1A oncoprotein recruits the cellular TRRAP/GCN5 histone acetyltransferase complex. Oncogene 22:2836–2841. doi: 10.1038/sj.onc.1206376. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. 2004. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. 2003. Small molecule modulators of histone acetyltransferase p300. J Biol Chem 278:19134–19140. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. 2004. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem 279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 22.Chen TY, Chen DY, Wen HW, Ou JL, Chiou SS, Chen JM, Wong ML, Hsu WL. 2013. Inhibition of enveloped viruses infectivity by curcumin. PLoS One 8:e62482. doi: 10.1371/journal.pone.0062482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D-Y, Shien J-H, Tiley L, Chiou S-S, Wang S-Y, Chang T-J, Lee Y-J, Chan K-W, Hsu W-L. 2010. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem 119:1346–1351. doi: 10.1016/j.foodchem.2009.09.011. [DOI] [Google Scholar]
- 24.Hatakeyama D, Shoji M, Yamayoshi S, Hirota T, Nagae M, Yanagisawa S, Nakano M, Ohmi N, Noda T, Kawaoka Y, Kuzuhara T. 2014. A novel functional site in the PB2 subunit of influenza A virus essential for acetyl-CoA interaction, RNA polymerase activity, and viral replication. J Biol Chem 289:24980–24994. doi: 10.1074/jbc.M114.559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowers EM, Yan G, Mukherjee C, Orry A, Wang L, Holbert MA, Crump NT, Hazzalin CA, Liszczak G, Yuan H, Larocca C, Saldanha SA, Abagyan R, Sun Y, Meyers DJ, Marmorstein R, Mahadevan LC, Alani RM, Cole PA. 2010. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol 17:471–482. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisfeld AJ, Neumann G, Kawaoka Y. 2014. Influenza A virus isolation, culture and identification. Nat Protoc 9:2663–2681. doi: 10.1038/nprot.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takashita E, Kiso M, Fujisaki S, Yokoyama M, Nakamura K, Shirakura M, Sato H, Odagiri T, Kawaoka Y, Tashiro M. 2015. Characterization of a large cluster of influenza A(H1N1)pdm09 viruses cross-resistant to oseltamivir and peramivir during the 2013-2014 influenza season in Japan. Antimicrob Agents Chemother 59:2607–2617. doi: 10.1128/AAC.04836-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa M, Victor ST, Taft AS, Yamada S, Li C, Hatta M, Das SC, Takashita E, Kakugawa S, Maher EA, Neumann G, Kawaoka Y. 2011. Replication-incompetent influenza A viruses that stably express a foreign gene. J Gen Virol 92:2879–2888. doi: 10.1099/vir.0.037648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. 2010. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J Virol 84:10918–10922. doi: 10.1128/JVI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao D, Fukuyama S, Yamada S, Lopes TJ, Maemura T, Katsura H, Ozawa M, Watanabe S, Neumann G, Kawaoka Y. 2015. Molecular determinants of virulence and stability of a reporter-expressing H5N1 influenza A virus. J Virol 89:11337–11346. doi: 10.1128/JVI.01886-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, Shi J, Tian G, Liu L, Liu J, Guan Y, Bu Z, Chen H. 2013. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 340:1459–1463. doi: 10.1126/science.1229455. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Qin K, Wang J, Pu J, Tang Q, Hu Y, Bi Y, Zhao X, Yang H, Shu Y, Liu J. 2011. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc Natl Acad Sci U S A 108:4164–4169. doi: 10.1073/pnas.1019109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. 2002. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 166:392–396. doi: 10.1164/rccm.2110060. [DOI] [PubMed] [Google Scholar]
- 34.Cosío BG, Mann B, Ito K, Jazrawi E, Barnes PJ, Chung KF, Adcock IM. 2004. Histone acetylase and deacetylase activity in alveolar macrophages and blood monocytes in asthma. Am J Respir Crit Care Med 170:141–147. doi: 10.1164/rccm.200305-659OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.