Abstract
We examined the pharmacokinetic properties of vancomycin conjugated to a bone-targeting agent (BT) with high affinity for hydroxyapatite after systemic intravenous administration. The results confirm enhanced persistence of BT-vancomycin in plasma and enhanced accumulation in bone relative to vancomycin. This suggests that BT-vancomycin may be a potential carrier for the systemic targeted delivery of vancomycin in the treatment of bone infections, potentially reducing the reliance on surgical debridement to achieve the desired therapeutic outcome.
TEXT
Osteomyelitis is defined as any inflammatory process in bone, the most common cause of which is infection. Although many bacterial pathogens have been associated with osteomyelitis, Staphylococcus aureus is the predominant cause and the pathogen responsible for the most serious forms of bone infection (1). Given the increasing prevalence of S. aureus strains resistant to methicillin (2), vancomycin remains the most commonly used antibiotic for the treatment of these infections (3). While true vancomycin resistance is rare, S. aureus strains with reduced susceptibility are common and often arise as a consequence of the prolonged periods of vancomycin therapy required to treat bone infections (1, 4). Vancomycin acts by inhibiting bacterial cell wall biosynthesis (5, 6) and is a large hydrophilic molecule that has limited penetration into bone and therefore low bone bioavailability when administered systemically (7). These factors emphasize the need to develop methods to enhance delivery of vancomycin to bone in the treatment of osteomyelitis. One way to accomplish this is to employ local antibiotic delivery, which while useful suffers from inherent limitations, not the least being the ability to gain direct access to the infection site (8–16). Thus, one of the major challenges to improve therapeutic outcomes for osteomyelitis patients is to develop methods for the systemic delivery of vancomycin, and potentially other antibiotics, in sufficient concentrations to achieve the desired therapeutic effect.
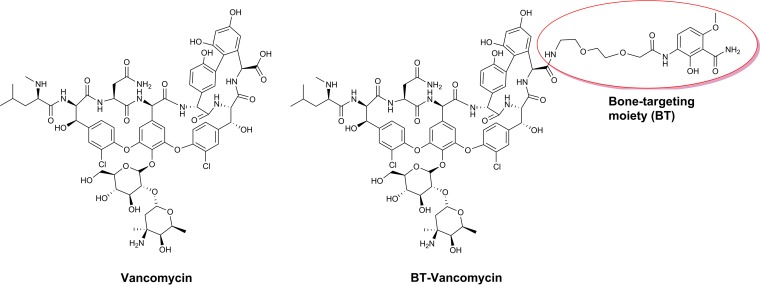
Previous studies in our laboratories have led to the development of bone-targeting agents (BT) based on their high affinity for hydroxyapatite and an enhanced tendency to accumulate in bone (17). We demonstrated that these compounds can be conjugated to vancomycin via a modified polyethylene glycol (PEG) linker (Fig. 1) to form BT-2-minipeg-2-vancomycin (BT-vancomycin) (18–20). Previous in vitro studies confirmed that the MICs of BT-vancomycin against methicillin-resistant and methicillin-susceptible S. aureus are comparable to those of vancomycin alone and that BT-vancomycin binds to hydroxyapatite to a greater extent than vancomycin (21). The objective of the present study was to define the pharmacokinetic (PK) profiles of vancomycin and BT-vancomycin after systemic administration via intravenous (i.v.) or intraperitoneal (i.p.) routes and to determine the plasma and bone content of vancomycin versus BT-vancomycin.
FIG 1.
Structures of vancomycin (left) and BT-vancomycin (right).
All experimental animal protocols were in strict accordance with the NIH “Guide for the Care and Use of Laboratory Animals” (24) and were approved by the Institutional Animal Care and Use Committees at the University of Kentucky, Lexington, KY, and Mayo Clinic, Rochester, MN. Thirty-five rats received a single i.v. injection via the tail vein of either vancomycin HCl (50 mg/kg of body weight) or BT-vancomycin (63.85 mg/kg; molar equivalent of 50 mg/kg of vancomycin HCl). Twenty rats were given an i.p. injection of either vancomycin HCl (50 mg/kg) or BT-vancomycin (63.85 mg/kg) twice daily for a total of seven doses. BT-vancomycin and vancomycin levels in plasma and bone were determined by liquid chromatography-tandem mass spectrometry (LC/MS-MS).
Bone samples (frozen tibiae) were pulverized, and the crushed bones were weighed, placed into 2-ml tubes, and stored at −80°C for further analysis. Analysis of vancomycin and BT-vancomycin was carried out using a Shimadzu LC unit coupled to an ABI 4000-Qtrap hybrid linear ion trap triple-quadrupole mass spectrometer in the multiple reaction monitoring (MRM) mode. Teicoplanin was used as an internal standard.
PK analysis was performed using data from individual rats, for which the mean and standard error of the mean (SEM) were calculated for each group. PK parameters were estimated using a noncompartmental model (Phoenix WinNonlin, Professional, version 6.2; Pharsight, Mountain View, CA). The levels of vancomycin and BT-vancomycin in plasma peaked at 13.00 ± 1.96 and 41.22 ± 8.71 μM, respectively, 1 h after administration (Fig. 2). The concentration of BT-vancomycin in plasma declined to its lowest levels (0.07 ± 0.02 μM) at 168 h, while vancomycin reached its lowest level 12 h after i.v. administration (Fig. 2). Compared to the peak concentrations in plasma, peak concentrations in bone were delayed, with peak concentrations occurring 6 h after i.v. administration (Fig. 3). The amount of BT-vancomycin in bone was approximately 5-fold higher than that of vancomycin during the initial 12-h period but increased progressively to approximately 47-fold at 168 h (Table 1).
FIG 2.

Plasma concentration-time profile of vancomycin (circles) and BT-vancomycin (squares) after i.v. administration of 50 mg/kg vancomycin or 63.85 mg/kg BT-vancomycin (molar equivalent to 50 mg/kg vancomycin). Results are the mean ± SEM (n = 5 rats). *, significantly higher than results for vancomycin, P < 0.05; **, significantly higher than results for vancomycin, P < 0.01; ***, significantly higher than results for vancomycin, P < 0.0001.
FIG 3.

Concentration-time profile in bone of vancomycin (circles) and BT-vancomycin (squares) after i.v. administration of vancomycin (50 mg/kg) or BT-vancomycin (63.85 mg/kg). Results are the mean ± SEM (n = 5 rats per group). *, significantly higher than results for vancomycin, P < 0.05; **, significantly higher than results for vancomycin, P < 0.01; ***, significantly higher than results for vancomycin, P < 0.001.
TABLE 1.
Comparative concentrations of vancomycin and BT-vancomycin in bone after i.v. administration of 50 mg/kg vancomycin or 63.85 mg/kg BT-vancomycina
| Time (h) | Concn (μM) (mean ± SEM)a |
BT-vancomycin/vancomycin ratio | |
|---|---|---|---|
| Vancomycin | BT-vancomycin | ||
| 1 | 1.04 ± 0.14 | 4.89 ± 1.08b | 4.7 |
| 6 | 1.73 ± 0.13 | 11.41 ± 1.79c | 6.6 |
| 12 | 1.51 ± 0.15 | 8.06 ± 1.46c | 5.3 |
| 24 | 0.97 ± 0.09 | 3.15 ± 0.49b | 3.3 |
| 72 | 0.35 ± 0.10 | 4.31 ± 0.63c | 12.3 |
| 168 | 0.08 ± 0.05 | 3.73 ± 0.61c | 46.6 |
n = 5 rats per group.
Significantly higher than results for vancomycin (P < 0.01).
Significantly higher than results for vancomycin (P < 0.001).
Increased accumulation of BT-vancomycin was also confirmed after i.p. administration of seven doses of 50 mg/kg of vancomycin or the molar equivalent of BT-vancomycin at 12-h intervals. The ratios of BT-vancomycin to vancomycin were 7.8, 7.4, and 47.7 at 1, 6, and 12 h after the last i.p. administration (Table 2). PK parameters obtained after i.v. administration are detailed in Table 3.
TABLE 2.
Comparative concentrations of vancomycin and BT-vancomycin in plasma and bone after i.p. administration of 50 mg/kg vancomycin or 63.85 mg/kg BT-vancomycin
| Time (h) | Concn (μM) (mean ± SEM)a in plasma |
Concn (μM) (mean ± SEM)a in bone |
BT- vancomycin/vancomycin ratio in bone | ||
|---|---|---|---|---|---|
| Vancomycin | BT-vancomycin | Vancomycin | BT-vancomycin | ||
| 1 | 15.6 ± 2.0 | 21.1 ± 2.1b | 7.3 ± 0.4 | 56.6 ± 8.4c | 7.8 |
| 6 | 1.6 ± 0.2 | 37.7 ± 5.1c | 7.9 ± 1.5 | 58.4 ± 9.2d | 7.4 |
| 12 | 0.4 ± 0.04 | 27.4 ± 3.1c | 0.9 ± 0.1 | 42.9 ± 11.1c | 47.7 |
n = 5 rats per group.
Not significantly different from results for vancomycin.
Significantly higher than results for vancomycin (P < 0.0001).
Significantly higher than results for vancomycin (P < 0.01).
TABLE 3.
PK parameters in rats following i.v. administration of a single bolus of 50 mg/kg vancomycin or 63.85 mg/kg BT-vancomycin
| Parametera | Result (mean ± SEM) for: |
|
|---|---|---|
| Vancomycin | BT-vancomycin | |
| t1/2 (h) | 1.44 ± 0.09 | 21.14 ± 4.86d |
| Tmax (h) | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Cmax (μM) | 12.63 ± 2.38 | 43.82 ± 9.45b |
| AUC (h · μM) | 58.71 ± 10.33 | 631.39 ± 95.13c |
| Vz (liters/kg) | 1.10 ± 0.18 | 1.13 ± 0.21 |
| CL (liters/h/kg) | 0.65 ± 0.12 | 0.048 ± 0.01c |
| AUMC (h · h · μM) | 162.63 ± 25.67 | 14,225.62 ± 3,012.66e |
| MRT (h) | 1.93 ± 0.17 | 12.45 ± 1.63d |
| Vss (liters/kg) | 1.32 ± 0.35 | 0.80 ± 0.25 |
Tmax, time to maximum concentration of drug in serum; Vz, volume of distribution; CL, clearance; AUMC, area under the first moment of the concentration-time curve; Vss, volume of distribution at steady state.
Significantly higher than results for vancomycin (P < 0.05).
Significantly higher than results for vancomycin (P < 0.01).
Significantly higher than results for vancomycin (P < 0.001).
Significantly higher than results for vancomycin (P < 0.0001).
These data demonstrate that vancomycin and BT-vancomycin exhibit significant differences in their PK profiles. A decrease in total clearance (CLtot) of 13.5-fold was observed for BT-vancomycin compared to vancomycin, with a 14.7-fold increase in half-life (t1/2) allowing for a 10.8-fold enhancement in the area under the concentration-time curve (AUC). The significant changes in the AUC indicate a higher degree of in vivo exposure to BT-vancomycin, facilitating the accumulation of drug in bone due to an enhanced permeation and retention effect. Consequently, BT-vancomycin shows a longer systemic mean residence time (MRT) than vancomycin (P < 0.001). The higher MRT value of BT-vancomycin could be due in part to a more protracted steady state in vivo, resulting in improved delivery, dramatically increased access into bones, and prolonged exposure in bone tissue (Table 3).
The estimates of the maximum concentration of drug in serum (Cmax) of vancomycin and BT-vancomycin determined in the present study were in agreement with previously published data, which include therapeutic peak and trough serum concentrations of 20.7 to 27.6 μM and 3.5 to 6.9 μM, respectively (22).
In our experiments, levels of BT-vancomycin in bone were above the MIC of vancomycin for up to 168 h after administration. These findings predict good antimicrobial outcomes, since the antimicrobial activity of vancomycin is time dependent and not concentration dependent (23).
In conclusion, our previously published work with BT-vancomycin showed that this novel molecule had in vitro activity similar to that of vancomycin against both methicillin-resistant and methicillin-susceptible S. aureus strains isolated from bone infections (21). Additionally, BT-vancomycin was shown to be more efficacious than an equimolar dose of vancomycin in a rat osteomyelitis model. However, the most efficacious dosing regimen used in these studies (i.p. injection every 12 h for 21 days) not only was associated with high BT-vancomycin levels in plasma but also caused a decrease in body weight, an elevation in white blood cell count, renal dysfunction, and evidence of tubulointerstitial nephritis. Although we did not examine toxicity in the current studies, we have demonstrated enhanced accumulation in bone, even after a single i.v. dose of an amount of BT-vancomycin equivalent to that used in the previous study (21). Thus, with further dose optimization, this toxicity can likely be minimized, making BT-vancomycin a useful BT therapy for the treatment of methicillin-resistant S. aureus (MRSA) osteomyelitis. More importantly, the results justify future studies to assess the utility of our promising BT agent in the context of other, less toxic antibiotics that have activity against MRSA.
ACKNOWLEDGMENTS
At the time of these studies, K.E.M. was fully employed by Pradama, Inc., and owned shares of the company. W.M.P. is the founder and Chief Scientific Officer of Pradama, Inc., and has partial ownership of the company. Pradama, Inc., holds a license to University of Louisville patents on BT-2-minipeg-2-vancomycin and related chemical entities, and a patent royalty stream to K.G.T. and W.M.P. may occur. The remaining authors declare no competing interests.
Funding Statement
This study was supported by funding from Pradama, Inc., to W.M.P. and a Department of Defense Peer-Reviewed Orthopaedic Research Program Expansion Award (W81XWH-15-1-0716) to M.S.S. and P.A.C.
REFERENCES
- 1.Cierny G., III 2011. Surgical treatment of osteomyelitis. Plast Reconstr Surg 127(Suppl 1):190S–204S. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Rodvold KA, McConeghy KW. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58(Suppl 1):S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 5.Darley ES, MacGowan AP. 2004. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother 53:928–935. doi: 10.1093/jac/dkh191. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein E, Keynan Y. 2014. Vancomycin revisited—60 years later. Front Public Health 2:217. doi: 10.3389/fpubh.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massias L, Dubois C, de Lentdecker P, Brodaty O, Fischler M, Farinotti R. 1992. Penetration of vancomycin in uninfected sternal bone. Antimicrob Agents Chemother 36:2539–2541. doi: 10.1128/AAC.36.11.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cevher E, Orhan Z, Mülazimoğlu L, Sensoy D, Alper M, Yildiz A, Ozsoy Y. 2006. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int J Pharm 317:127–135. doi: 10.1016/j.ijpharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Smith JK, Bumgardner JD, Courtney HS, Smeltzer MS, Haggard WO. 2010. Antibiotic-loaded chitosan film for infection prevention: a preliminary in vitro characterization. J Biomed Mater Res B Appl Biomater 94:203–211. doi: 10.1002/jbm.b.31642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker AC, Beenken KE, Jennings JA, Hittle L, Shirtliff ME, Bumgardner JD, Smeltzer MS, Haggard WO. 2015. Characterization of local delivery with amphotericin B and vancomycin from modified chitosan sponges and functional biofilm prevention evaluation. J Orthop Res 33:439–447. doi: 10.1002/jor.22760. [DOI] [PubMed] [Google Scholar]
- 11.Yang CC, Lin CC, Liao JW, Yen SK. 2013. Vancomycin-chitosan composite deposited on post porous hydroxyapatite coated Ti6Al4V implant for drug controlled release. Mater Sci Eng C Mater Biol Appl 33:2203–2212. doi: 10.1016/j.msec.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Shinsako K, Okui Y, Matsuda Y, Kunimasa J, Otsuka M. 2008. Effects of bead size and polymerization in PMMA bone cement on vancomycin release. Biomed Mater Eng 18:377–385. doi: 10.3233/BME-2008-0554. [DOI] [PubMed] [Google Scholar]
- 13.Beenken KE, Bradney L, Bellamy W, Skinner RA, McLaren SG, Gruenwald MJ, Spencer HJ, Smith JK, Haggard WO, Smeltzer MS. 2012. Use of xylitol to enhance the therapeutic efficacy of polymethylmethacrylate-based antibiotic therapy in treatment of chronic osteomyelitis. Antimicrob Agents Chemother 56:5839–5844. doi: 10.1128/AAC.01127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beenken KE, Smith JK, Skinner RA, Mclaren SG, Bellamy W, Gruenwald MJ, Spencer HJ, Jennings JA, Haggard WO, Smeltzer MS. 2014. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J Biomater Appl 29:514–523. doi: 10.1177/0885328214535452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giavaresi G, Bertazzoni Minelli E, Sartori M, Benini A, Della Bora T, Sambri V, Gaibani P, Borsari V, Salamanna F, Martini L, Nicoli Aldini N, Fini M. 2012. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J Orthop Res 30:348–355. doi: 10.1002/jor.21531. [DOI] [PubMed] [Google Scholar]
- 16.Jiang JL, Li YF, Fang TL, Zhou J, Li XL, Wang YC, Dong J. 2012. Vancomycin-loaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflamm Res 61:207–215. doi: 10.1007/s00011-011-0402-x. [DOI] [PubMed] [Google Scholar]
- 17.Nasim S, Vartak AP, Pierce WM Jr, Taylor KG, Smith N, Crooks PA. 2010. 3-O-Phosphate ester conjugates of 17-β-O-{1-[2-carboxy-(2-hydroxy-4-methoxy-3-carboxamido)anilido]ethyl}1,3,5(10)-estratriene as novel bone-targeting agents. Bioorg Med Chem Lett 20:7450–7453. doi: 10.1016/j.bmcl.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Neale JR, Richter NB, Merten KE, Taylor KG, Singh S, Waite LC, Emery NK, Smith NB, Cai J, Pierce WM Jr. 2009. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg Med Chem Lett 19:680–683. doi: 10.1016/j.bmcl.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Pierce WM Jr, Waite LC, Taylor KG. July 2008. Bone targeting compounds for delivering agents to bone for interaction therewith.US patent 7,399,789.
- 20.Pierce WM Jr, Waite LC, Taylor KG. December 2011. Bone targeting compounds for delivering agents to bone for interaction therewith (generation 2). US patent 8,071,575.
- 21.Karau MJ, Schmidt-Malan SM, Greenwood-Quaintance KE, Mandrekar J, Cai J, Pierce WM Jr, Merten K, Patel R. 2013. Treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis with bone-targeted vancomycin. SpringerPlus 2:329. doi: 10.1186/2193-1801-2-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundstrom TS, Sobel JD. 2004. Antibiotics for gram-positive bacterial infections: vancomycin, quinupristin-dalfopristin, linezolid, and daptomycin. Infect Dis Clin North Am 18:651–668. doi: 10.1016/j.idc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]