Abstract
Staphylococcus aureus possesses exceptional virulence and a remarkable ability to adapt in the face of antibiotic therapy. We examined the in vitro evolution of S. aureus in response to escalating vancomycin exposure by evaluating bacterial killing and the progression of resistance. A hollow-fiber infection model was utilized to simulate human doses of vancomycin increasing from 0.5 to 4 g every 12 h (q12h) versus a high inoculum (108 CFU/ml) of methicillin-resistant S. aureus (MRSA) USA300 and USA400. Host-pathogen interactions using Galleria mellonella and accessory gene regulator (agr) expression were studied in serially obtained isolates. In both USA300 and USA400 MRSA isolates, vancomycin exposure up to 2 g q12h resulted in persistence and regrowth, whereas 4 g administered q12h achieved sustained killing against both strains. As vancomycin exposure increased from 0.5 to 2 g q12h, the bacterial population shifted toward vancomycin-intermediate resistance, and collateral increases in the MICs of daptomycin and televancin were observed over 10 days. Guideline-recommended exposure of a ratio of the area under the concentration-time curve for the free, unbound fraction of the drug to the MIC (fAUC/MIC ratio) of 200 displayed a 0.344-log bacterial reduction in area, whereas fAUC/MICs of 371 and 554 were needed to achieve 1.00- and 2.00-log reductions in area, respectively. The stepwise increase in resistance paralleled a decrease in G. mellonella mortality (P = 0.021) and a gradual decline of RNAIII expression over 10 days. Currently recommended doses of vancomycin resulted in amplification of resistance and collateral damage to other antibiotics. Decreases in agr expression and virulence during therapy may be an adaptive mechanism of S. aureus persistence.
INTRODUCTION
Staphylococcus aureus is a primary human pathogen capable of exceptional virulence and an array of life-threatening infections ranging from necrotizing pneumonia to endocarditis (1). S. aureus also has a remarkable ability to adapt in the face of antimicrobial therapy via a plethora of resistance mechanisms (2–6). Although there is a large body of information on the mechanisms of antibiotic resistance in S. aureus, the interplay between virulence and antibiotic resistance is not completely understood. The results of genome-wide sequencing analyses have been confounded by the discovery that multiple genetic pathways may lead to similar levels of antibiotic resistance (7–9). Also, while the sequential development of resistance mutations during the course of an infection has been analyzed, the temporal link between resistance and virulence is poorly defined (10).
Despite the successful use of vancomycin to combat community-associated (CA) methicillin-resistant S. aureus (MRSA) for several decades, the spread of vancomycin-intermediate S. aureus (VISA) has brought the utility of vancomycin into question (4, 11). The genetic basis for VISA is unknown, although reduced vancomycin susceptibility has been associated with dysfunction of the accessory gene regulator (agr), the master regulator of pathogenicity in S. aureus (12–14). Isolates of S. aureus that are defective in agr have significantly reduced virulence profiles and low-level vancomycin resistance conferred through a number of mechanisms, including biofilm formation, stationary-phase growth, and alterations in autolysis (15–17). At present, it is not known how modulating vancomycin dosing regimens will alter the time scale of agr expression or S. aureus virulence. Here, we attempted to resolve the link between vancomycin resistance and virulence by simulating human dosing in an in vitro hollow-fiber infection model (HFIM) to study the stepwise evolution of vancomycin resistance and also determine the impact that these mutations have on virulence.
MATERIALS AND METHODS
Bacterial isolates.
To represent the predominant clones of MRSA in the United States, Europe, and Canada, we selected the two pulsed-field gel electrophoresis-type community-acquired strains USA300 (FPR 3757) and USA400 (MW2), of which the complete genome sequences have been established (18–20). Both isolates were obtained from the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA).
Antibiotics and medium.
Vancomycin, ciprofloxacin, levofloxacin, gentamicin, nafcillin, and rifampin analytical-grade powders were commercially purchased (Sigma Chemical Company, St. Louis, MO). Daptomycin was obtained from Cubist (Lexington, MA), telavancin was obtained commercially from the University at Buffalo Pharmacy, and linezolid was obtained from Pfizer (Groton, CT). Brain heart infusion (BHI) broth and BHI agar (Difco Laboratories, Detroit, MI) were used for all in vitro hollow experiments.
Determining alterations in MIC.
Antimicrobial agents commonly used to treat MRSA were tested with CLSI broth microdilution methods to evaluate the potential collateral damage of vancomycin treatment to other antibiotics. Isolates were initially evaluated for MICs at baseline prior to vancomycin exposure. Selective postexposure mutants obtained from the HFIM were also evaluated for MICs. CLSI interpretive criteria were used to categorize the isolates as susceptible, intermediate, or resistant. S. aureus ATCC 29213 bacteria were utilized as quality control (QC) organisms. All QC results were within published limits.
HFIM.
As previously described, an HFIM was used to evaluate how clinically relevant vancomycin regimens alter the bacterial burden of MRSA and influence the amplification of antibiotic resistance over 240 h (21). The HFIM used a C3008 cellulosic cartridge (FiberCell Systems, Frederick, MD). Bacteria were trapped in the extracapillary space of the cartridge, and the large surface area from the hollow fibers was used to facilitate nutrient exchange and antibiotic exposure. Over 10 days, vancomycin dosing regimens were administered to achieve the same values for areas under the concentration-time curve (AUCs) as those expected in humans. The clinical scenario of a high bacterial burden infection such as a bilobar pneumonia or endocarditis was simulated using a 108-CFU/ml inoculum achieved from overnight MRSA cultures. Samples were taken from the 10-day HFIM experiment at 0, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h and subsequently incubated and quantified to assess bacterial growth.
To simulate human antibiotic dosing, four different vancomycin regimens were administered in the HFIM assuming a free (ƒ) fraction of 50% and a 6-h half-life. The following dosing schemes were chosen to target the pharmacokinetic parameters of maximal concentration (Cmax), minimum concentration (Cmin), and an area-under-the-curve/MIC (AUC/MIC) ratio expected in human plasma: (i) 500 mg every 12 h (q12h) (ƒCmax of 10 mg/liter, ƒCmin of 2.5 mg/liter, and ƒAUC/MIC of 112.5); (ii) 1,000 mg q12h (ƒCmax of 20 mg/liter, ƒCmin of 5 mg/liter, and ƒAUC/MIC of 225); (iii) 2,000 mg q12h (ƒCmax of 40 mg/liter, ƒCmin of 10 mg/liter, and ƒAUC/MIC of 450); and (iv) 4,000 mg q12h (ƒCmax of 80 mg/liter, ƒCmin of 20 mg/liter, and ƒAUC/MIC of 900).
Samples from the HFIM were also obtained serially for confirmation of vancomycin pharmacokinetics using a standard agar diffusion bioassay procedure. Mueller-Hinton agar (MHA) with Micrococcus luteus ATCC 9341 as an indicator organism was utilized to validate vancomycin concentrations as previously described (43). All observed pharmacokinetic parameters were within 12% of targeted values.
Population analysis profiles.
Quantitative cultures from the HFIM were determined in “real time” in mini-population analysis profiles (PAPs) that utilized BHI agar containing 0, 2, 4, and 6 mg/liter of vancomycin, which enabled the daily detection of the total bacterial population, as well as vancomycin-resistant subpopulations in all four dosing regimens. Full PAPs were also completed using 0, 0.5, 1, 2, 3, 4, 6, 8, and 16 mg/liter of vancomycin to quantify daily evolution of the resistant subpopulations in the 2-g q12h regimen, as well as the 240-h time point of all four dosing regimens. PAP cultures were incubated for 48 h, and the colonies were then counted and plotted against either vancomycin concentration or time.
RNA extraction and quantitative real-time PCR.
Cells were collected at 240 h from the HFIM for MRSA USA300 before and after exposure to the four vancomycin regimens. RNAIII expression was then assessed with real-time PCR as described previously (22). Briefly, the collected samples were centrifuged at 14,000 rpm for 5 min at room temperature. The supernatant was aspirated, and the pellet was immediately frozen at −80°C until RNA isolation. The total RNA was isolated from the pellet (SV total RNA isolation system; Promega, Madison, WI) according to the manufacturer's protocol for Gram-positive bacteria. From the total RNA pool, mRNA was purified (MICROBExpress; Ambion, Austin, TX). Reverse transcription (RT) of the mRNA (315 ng) was carried out using random hexamer primers and the AccuScript high-fidelity RT-PCR system (Stratagene, La Jolla, CA).
G. mellonella virulence assays.
Galleria mellonella was utilized to investigate the pathogenicity of resistant mutants which evolved in the HFIM, as detailed previously (23, 24). In the 10-day HFIM utilizing a vancomycin dose of 2 g q12h, USA300 mutants were collected daily and stored at −80°C prior to the virulence assessment. Twenty randomly chosen caterpillars 200 to 300 mg in weight in the final instar larval stage (Vanderhorst, Inc., St. Mary's, OH) were used in each group. A 10-μl Hamilton syringe was used to inject 10-μl aliquots of the inoculum into the hemocoel of each caterpillar via the last left proleg. Bacterial colony counts were used to confirm all inocula, and appropriate control arms with caterpillars receiving no injection or an injection of phosphate-buffered saline were included.
RESULTS
MRSA USA300 and USA400 HFIMs.
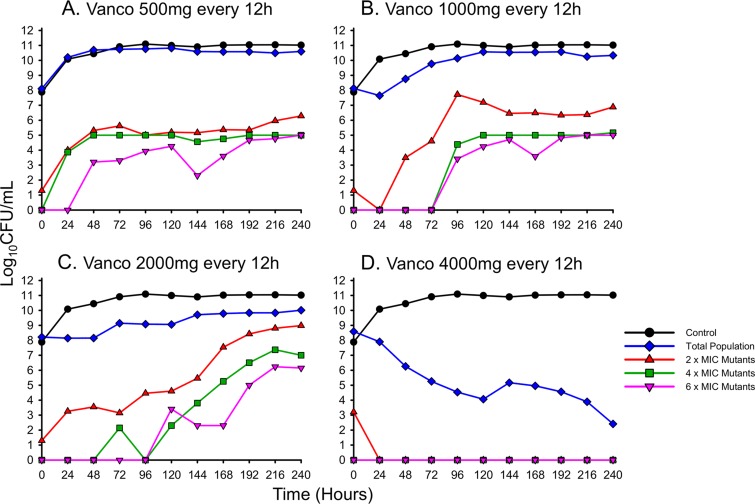
To study the temporal profile of MRSA USA300's response to increasing antibiotic exposure, an HFIM was utilized to simulate human vancomycin dosing over a 10-day period (Fig. 1). Despite high doses of up to 2 g q12h, mimicking exposure profiles in administered humans, USA300 demonstrated persistence and tolerance, maintaining bacterial counts of >1010 CFU/ml with growth similar to the control by the 240-h endpoint. The lower-dose vancomycin regimen of 500 mg q12h was nearly identical to that of the control throughout the 240 h. The 1-g q12h scheme, which is the regimen administered to the majority of patients with S. aureus bloodstream infections, demonstrated initial stasis at ∼5 × 107 CFU/ml for approximately 24 h, followed by gradual regrowth that plateaued at ∼3.5 × 1010 CFU/ml by 120 h. Similarly, the 2-g q12h regimen demonstrated stepwise increases in bacterial counts beginning with an initial stasis phase from 0 to 48 h at ∼108 CFU/ml, followed by a regrowth phase from 48 h to 96 h at ∼109 CFU/ml, followed in turn by a final regrowth phase at 144 h to ∼1010 CFU/ml that continued to 240 h. Unlike the lower-dose regimens, the 4-g q12h scheme achieved a >3-log reduction in bacterial counts by 72 h and, after 240 h of continuous killing, resulted in a final population of ∼3 × 102 CFU/ml.
FIG 1.
Humanized dosing regimens of vancomycin (Vanco) against MRSA USA300 quantifying the total population (blue) and the sequential emergence of resistance secondary to drug exposure (red, gray, and pink) over a 10-day period.
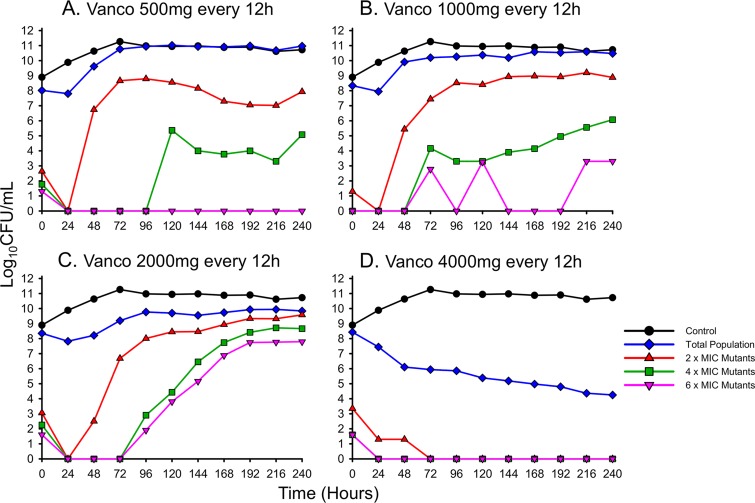
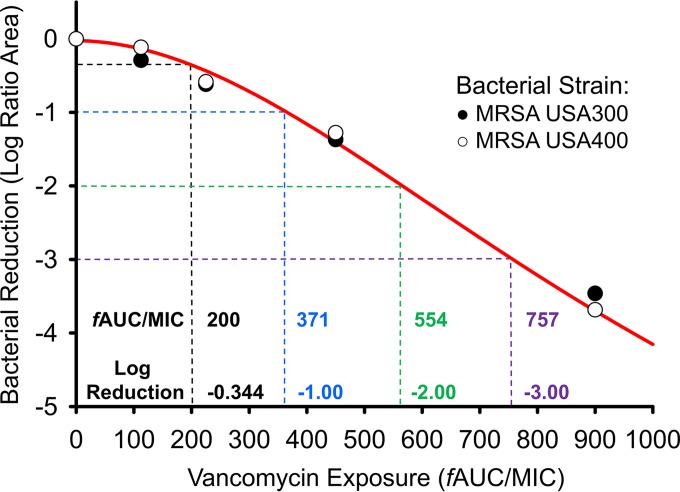
An additional HFIM analysis was conducted on USA400 to confirm that the levels of activity of vancomycin are comparable among common CA-MRSA strains (Fig. 2). Similar to USA300, vancomycin doses up to 2 g q12h were unable to prevent USA400 from regrowing by 48 h. While the 500-mg and 1-g q12h regimens mirrored the growth control by 96 h, the 2-g q12h scheme maintained counts below 1010 CFU/ml for the duration of the experiment. The 4-g q12h regimen was once again the only simulated vancomycin therapy capable of achieving bactericidal activity, with a >3-log reduction conferred by 120 h and a final population count of ∼104 CFU/ml at 240 h. Comodeling the reduction in the log ratio area of both USA300 and USA400 as a function of vancomycin exposure confirmed that the performances of vancomycin were similar for the two strains (Fig. 3). In both USA300 and USA400, ratios of the AUC for the free, unbound fraction of the drug to the MIC (fAUC24/MIC ratios) of approximately 200, 371, 554, and 757 achieved a reduction in the total population's log ratio area of 0.344, 1.00, 2.00, and 3.00, respectively (R2 = 0.990), as described by a Hill-type function utilized previously (25).
FIG 2.
Humanized dosing regimens of vancomycin against MRSA USA400 quantifying the total population (blue) and the sequential emergence of resistance secondary to drug exposure (red, gray, and pink) over a 10-day period.
FIG 3.
Bacterial reductions (represented as log ratio areas) of MRSA USA300 and USA400 are plotted as a function of vancomycin exposure. The fAUC/MICs needed to confer a reduction in the log ratio areas of 0.344, 1, 2, and 3 (black, blue, green, and purple, respectively) are listed above the corresponding vancomycin exposures. The data were described by a Hill-type function as described previously (25).
Sequential emergence of antibiotic resistance.
Throughout the 10-day HFIM experiments, subpopulations capable of growing on 2, 4, and 6 mg/liter of vancomycin were tracked for both USA300 and USA400 (Fig. 1 and 2). In both investigational strains, lower vancomycin doses of 500 mg and 1 g q12h did not substantially amplify vancomycin resistance, with <1% of the population ever growing on 2 mg/liter of vancomycin. However, when the dose of vancomycin was increased to 2 g q12h, ∼10% of USA300's population and ∼50% of USA400's total population were capable of growing on 2 mg/liter of vancomycin by 240 h. Increasing the vancomycin dose even further to 4 g q12h resulted in drastic killing of the total bacterial populations, with resistant subpopulations growing on 2 mg/liter of vancomycin comprising <1% of the population for the duration of the experiments.
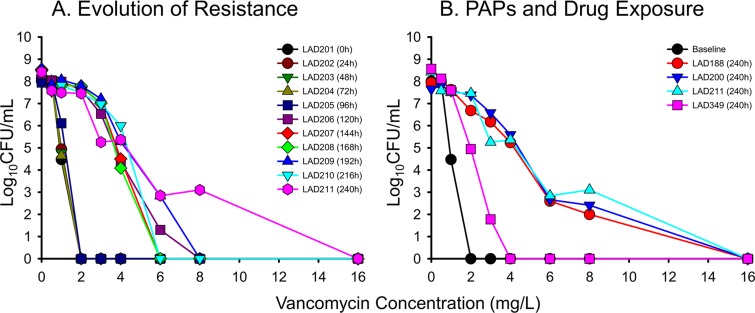
A more comprehensive PAP scheme utilizing vancomycin concentrations up to 16 mg/liter was also performed for the entire 2-g q12h regimen and the 240-h terminal time points of all the USA300 HFIMs (Fig. 4). After several days of vancomycin exposure, subpopulations of USA300 began to grow on 6 mg/liter of vancomycin beginning at 120 h. Resistance to vancomycin intensified as the 2-g q12h regimen continued, eventually resulting in isolates capable of growing on 8 mg/liter of vancomycin by 240 h. Comparing the dose responses among different regimens, doses of 500 mg up to 2 g q12h produced similar resistance profiles by 240 h, characterized by bacterial growth on 8 mg/liter of vancomycin. In contrast, the 4-g q12h regimen prevented the growth of any colonies in the presence of ≥4 mg/liter of vancomycin at 240 h.
FIG 4.
(A) Stepwise evolution of resistance, as shown in population analysis profiles of vancomycin quantified every 24 h over a 10-day period in response to simulated human exposure to vancomycin at 2 g q12h. (B) Comparative population analysis profiles among different vancomycin dosing regimens of 500, 1,000, 2,000, and 4,000 mg q12h at the 240-h study endpoint.
In order to track the emergence of resistance to other agents during vancomycin therapy, the MICs of common antibiotics were determined for USA300 isolates collected every 24 h during the 2-g q12h HFIM experiment and are presented in Table 1. Secondary to vancomycin exposure, significant alterations in the susceptibility to the lipopeptide daptomycin and lipoglycopeptide telavancin were observed. For daptomycin, sequential increases were noted from 0.125 to 0.25 mg/liter on day 4, to 0.5 mg/liter on day 7, and 1.0 mg/liter on day 10. For telavancin, sequential increases from 1.0 to 2.0 on day 2, and to 4.0 mg/liter on day 5 occurred. There was also a consistent trend of decreased rifampin susceptibility from 0.015 to 0.03 mg/liter, albeit only a 1-fold difference
TABLE 1.
Bacterial isolates of MRSA USA300 collected during the 10-day HFIM investigating a 2-g q12h vancomycin regimena
| Strain | Time (h) of VAN 2-g q12h exposure | MIC (mg/liter) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VAN | LZD | DAP | RIF | LEV | CIP | GEN | NAF | ||
| LAD201 | 0 (baseline) | 1 | 2 | 0.5 | 0.015 | 8 | 32 | 1 | 32 |
| LAD202 | 24 | 1 | 2 | 0.5 | 0.015 | 8 | 32 | 2 | 32 |
| LAD203 | 48 | 1 | 2 | 0.5 | 0.015 | 8 | 32 | 2 | 32 |
| LAD204 | 72 | 2 | 2 | 0.5 | 0.015 | 16 | 32 | 2 | 32 |
| LAD205 | 96 | 2 | 2 | 0.25 | 0.015 | 8 | 32 | 2 | 32 |
| LAD206 | 120 | 4 | 2 | 0.5 | 0.03 | 8 | 32 | 2 | 32 |
| LAD207 | 144 | 4 | 2 | 1 | 0.03 | 16 | 32 | 0.5 | 32 |
| LAD208 | 168 | 4 | 2 | 1 | 0.03 | 16 | 16 | 2 | 32 |
| LAD209 | 192 | 4 | 2 | 2 | 0.03 | 8 | 16 | 2 | 16 |
| LAD210 | 216 | 4 | 2 | 2 | 0.03 | 8 | 16 | 1 | 32 |
| LAD211 | 240 | 4 | 2 | 2 | 0.03 | 8 | 16 | 2 | 32 |
MICs to vancomycin and other antibiotic of interest are listed for each isolate. VAN, vancomycin; LZD, linezolid; DAP, daptomycin; RIF, rifampin, LEV, levofloxacin; CIP, ciprofloxacin; GEN, gentamicin; NAF, nafcillin.
G. mellonella survival.
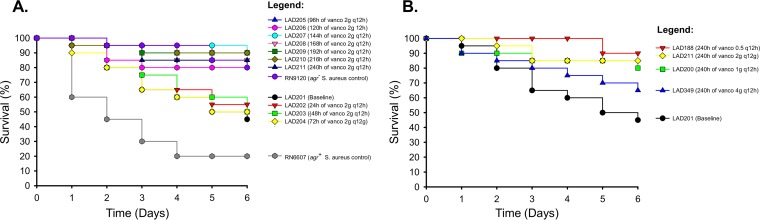
To determine what impact vancomycin resistance has on S. aureus virulence, G. mellonella moths were inoculated with USA300 isolates collected daily throughout the 2-g q12h regimen (Fig. 5A) and also isolates obtained at the 240-h terminal time point of each USA300 HFIM experiment (Fig. 5B). In the 2-g q12h regimen, isolates collected after ≥96 h of vancomycin exposure displayed attenuated virulence that paralleled the agr dysfunctional control, with significantly higher G. mellonella survival rates after 6 days compared to the results seen with the baseline isolate (G. mellonella survival of ≥80% versus 45% on day 6, P = 0.021, log-rank test). Conversely, isolates obtained prior to 96 h of vancomycin exposure demonstrated killing that mirrored the profile of the baseline isolate (G. mellonella survival of ≤60% on day 6). When all four vancomycin regimens were compared to one another, doses of 500 mg to 2 g q12h resulted in significantly better G. mellonella survival by day 6 relative to the results seen with the baseline isolate (G. mellonella survival of ≥80% versus 45% on day 6, P = 0.029), whereas the 4-g q12h regimen resulted in an isolate with comparable virulence to the baseline isolate (P = 0.231).
FIG 5.
(A) G. mellonella virulence assay in response to simulated human vancomycin exposure. Worms were inoculated with MRSA USA300 isolates collected throughout the 2-g q12h vancomycin regimen in the HFIM, and the subsequent survival of the worms was recorded. G. mellonella inoculated with isolates collected after ≥96 h of vancomycin treatment displayed significantly better survival than worms inoculated with the baseline isolate (P = 0.021, log-rank test). (B) The assay was repeated for the terminal MRSA USA300 isolates collected at the end of the 10-day HFIM experiments for each vancomycin regimen. Vancomycin doses of ≤2 g q12h resulted in significantly better survival of the G. mellonella relative to the baseline isolate (P = 0.029), whereas the isolate collected after 240 h of exposure to the 4-g q12h regimen was comparably virulent to the baseline isolate (P = 0.231).
RNAIII expression and comodeling with total counts, virulence, and resistance plots.
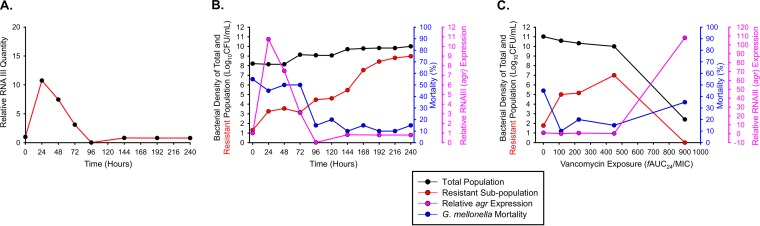
Real-time PCR of RNAIII was performed on all USA300 isolates recovered from 0 to 240 h during the 2-g q12h regimen to delineate the temporal pattern of agr expression during antibiotic therapy and its relationship to resistance and virulence in S. aureus (Fig. 6A). The quantity of RNAIII initially surged to >10× baseline between 0 and 24 h, following a steep decline to almost undetectable amounts of RNAIII at 96 h, after which agr expression hovered around baseline until 240 h. When agr expression was comodeled with bacterial counts and G. mellonella mortality, a trend was observed in which the initial surge in agr expression coincided with higher levels of G. mellonella mortality (∼50%), whereas the low levels of agr expression beginning at 96 h corresponded to attenuated mortality rates of ≤20% (Fig. 6B). The relationship between bacterial counts and agr expression was not as clearly defined in the 2 g of vancomycin q12h regimen. However, when agr expression and bacterial counts were plotted as a function of vancomycin exposure, an inverse relationship was observed in which an fAUC24/MIC of 900 mg·h/liter resulted in a bacterial count of ∼3 × 102 CFU/ml and agr expression >10× baseline at 240 h (Fig. 6C).
FIG 6.
(A) RNAIII profiling to measure activity of agr, the primary quorum-sensing response regulator of virulence in S. aureus, quantified every 24 h in response to MRSA USA300 exposed to vancomycin at 2 g q12h for 10 days. (B) Comodeling of the agr expression in panel A (pink) with MRSA USA300's total population (black), vancomycin-resistant subpopulations growing on 4.0 mg/liter of vancomycin (red), and G. mellonella mortality (blue). (C) The same analysis is repeated using the 240-h terminal time point for vancomycin doses of 500 mg to 4 g q12h.
DISCUSSION
Although vancomycin was the drug of choice for MRSA infections for several decades, increased treatment failure rates coinciding with the spread of VISA have cast doubt on the reliable use of vancomycin (26–30). As S. aureus MICs continue to creep upward for vancomycin, understanding how drug exposure influences both resistance and virulence is critical to the judicious use of anti-MRSA agents. Here, we investigated clinically relevant vancomycin regimens in a 10-day HFIM to translate how vancomycin dosing alters MRSA population dynamics and glycopeptide resistance. In both of the investigational CA-MRSA strains, administration of 500 mg or 1 g of vancomycin q12h was unable to achieve bacteriostatic activity. When the dose of vancomycin was increased to 2 g q12h, a regimen yielding twice the drug exposure of the most common vancomycin scheme, bacterial counts still rose steadily in the face of intensified antibiotic dosing. Only the 4-g q12h regimen achieved bactericidal activity, with sustained killing over 10 days for both investigational strains.
Unlike the AUC/MIC target of 400 (fAUC/MIC = 200) advocated by current vancomycin guidelines, higher vancomycin exposures were needed to kill the CA-MRSA strains investigated in the present study (31). As current guidelines recommend vancomycin use for MRSA infections in which the causative organism's vancomycin MIC is ≤1 mg/liter, the isolates investigated in the present study are an accurate representation of MRSA strains commonly treated with vancomycin (MIC = 1 mg/liter for USA300 and USA400). However, the 2-g q12h vancomycin regimen produced an fAUC/MIC >2× the suggested AUC/MIC of 400 (fAUC/MIC of 200), and negligible killing was achieved in both investigational strains. An alarmingly high fAUC/MIC of 554 was necessary to achieve a 2-log bacterial reduction in area. Vancomycin regimens commonly used in the clinic may therefore be unable to fully overcome S. aureus resistance mechanisms at high inocula, and alternative regimens may need to be considered even when an organism's vancomycin MIC is deceptively below 2 mg/liter.
Not only were the 500 mg to 2 g of vancomycin q12h regimens incapable of killing S. aureus, but the suboptimal vancomycin exposure also amplified antibiotic resistance. Similar to other investigations concerning antibiotic resistance, an inverted “U” phenomenon was observed in which rising vancomycin concentrations resulted in higher levels of antibiotic resistance until extreme concentrations were capable of killing the entire population (32). Vancomycin exposure was also found to augment resistance to other antimicrobials, including daptomycin, telavancin, and rifampin. Taken together, these results emphasize the importance of utilizing optimal vancomycin dosing practices against MRSA that is capable of being killed by vancomycin. If a high enough vancomycin exposure cannot be achieved to confer bactericidal activity, resistance to glycopeptides and other antibiotic classes will increase in a manner that is proportionate to the amount of vancomycin exposure.
Although the amplification of antibiotic resistance is a concern, agr expression and G. mellonella mortality demonstrated that the ability of S. aureus to resist glycopeptide treatment appears to come at a cost to its virulence. Lower vancomycin dosing schemes of 500 mg to 2 g q12h resulted in reduced agr activity and a higher survival rate of the G. mellonella, whereas the bactericidal 4-g q12h regimen suppressed vancomycin resistance and correspondingly did not significantly alter the survival rate of G. mellonella. The emergence of vancomycin resistance appears to be a heterogenous process, but two observations commonly made upon rising vancomycin MICs are thicker cell walls and dysfunctional agr profiles (4, 16, 33, 34). It has been proposed that alterations in the cell wall of S. aureus may interfere with the cell's ability to bind the autoinducing peptide of the agr system used for quorum sensing and activation of S. aureus toxins and other virulence factors (35). It is likely that suboptimal vancomycin dosing in the clinic will drive MRSA from a more antibiotic-susceptible and virulent state toward a more resistant but less virulent population.
Another consequence of vancomycin exposure is the potential conversion of S. aureus from a virulent phenotype into a persistent state better adapted to survive antimicrobial therapy and the host immune response. In the present study, the survival of G. mellonella improved as the expression of agr decreased, suggesting that the reduction in virulence factor release reduced the pathogenicity of S. aureus. A prior investigation that compared S. aureus isolates collected from a patient before and after 6 weeks of vancomycin treatment found that vancomycin exposure resulted in downregulated agr, slower autolysis, increased cell wall thickness, and a reduction in the secretion of alpha-toxin and phenol-soluble modulins (36). It has been established that alpha-toxin release activates the NLRP3-inflammasome and induces interleukin-1 and interleukin-6 secretion, while the VISA phenotype has been shown to provoke less tumor necrosis factor alpha and interleukin release than its vancomycin-susceptible counterparts (9, 37, 38). An investigation of the selective pressure of vancomycin on MRSA also found that vancomycin exposure amplifies the relative abundance of the small colony variant phenotype, which is a slow-growing phenotype implicated in chronic and recurrent S. aureus infections, as well as intracellular persistence (25, 39). The consequence of suboptimal vancomycin exposure is therefore not restricted to proliferating antibiotic resistance but is also seen in the shifting of the population dynamics of S. aureus toward a persistent state that is capable of enduring host countermeasures and exogenous antimicrobials.
The present study has several meaningful limitations to consider before the results can be fully translated into the clinical setting. Similar to other in vitro investigations, not only does the HFIM fail to account for host defenses against S. aureus, but the use of Mueller-Hinton broth provides a nutrient-rich environment that may improve bacterial survival relative to in vivo experiments. Most importantly, the poor performance of standard vancomycin regimens is likely ascribable to the high inoculum investigated in the present study. A previous in vitro investigation not only demonstrated a large inoculum effect for vancomycin against heteroresistant-VISA but also found that simulated doses of vancomycin up to 5 g q12h were unable to achieve sustained killing (40). A murine thigh infection model was later used to characterize the magnitude of inoculum effects for MRSA and heteroresistant-VISA exposed to several antimicrobials (41); the authors concluded that the inoculum effect was much more severe for vancomycin than for other antistaphylococcal agents, such as daptomycin and linezolid. It is therefore prudent to view the results of the current investigation as representative of a worst-case clinical scenario, since vancomycin may achieve much better activity in more favorable conditions.
In closing, clinically relevant vancomycin regimens simulated in an HFIM were largely ineffective against two common CA-MRSA strains, with increasing vancomycin exposure conferring more substantial antibiotic resistance and severe losses in virulence. The only investigational regimen capable of killing USA300 and USA400 was the 4-g q12h regimen, which is a dosing scheme neglected clinically due to the propensity of vancomycin for dose-related nephrotoxicity (42). In the model in vitro system, simply obtaining the suggested AUC/MIC of 400 was not enough to overcome the resistance mechanisms of S. aureus. Owing to the inability of vancomycin to achieve bactericidal activity at clinical concentrations, clinicians are cautioned about selecting vancomycin for MRSA infections containing S. aureus strains with questionable vancomycin susceptibility. Aggressive stewardship and the use of alternative antimicrobials may be necessary to stem the progressive upward creep of the MIC of vancomycin against S. aureus, since seemingly optimal vancomycin regimens may actually exacerbate vancomycin resistance. It will likely take many years to fully define where the niche of vancomycin now lies in the context of new anti-MRSA agents that offer clinicians a more diverse armamentarium against S. aureus.
ACKNOWLEDGMENTS
We thank Alan Forrest for insight into pharmacokinetic/pharmacodynamic and statistical analyses.
B.T.T. was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01AI111990). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. 1998. Activated cell wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother 42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. 1998. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother 42:315–320. doi: 10.1093/jac/42.3.315. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Sieradzki K, Tomasz A. 2003. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J Bacteriol 185:7103–7110. doi: 10.1128/JB.185.24.7103-7110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuji BT, von Eiff C, Kelchlin PA, Forrest A, Smith PF. 2008. Attenuated vancomycin bactericidal activity against Staphylococcus aureus hemB mutants expressing the small-colony-variant phenotype. Antimicrob Agents Chemother 52:1533–1537. doi: 10.1128/AAC.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam MT, Petit RA III, Crispell EK, Thornton TA, Conneely KN, Jiang Y, Satola SW, Read TD. 2014. Dissecting vancomycin-intermediate resistance in Staphylococcus aureus using genome-wide association. Genome Biol Evol 6:1174–1185. doi: 10.1093/gbe/evu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidaillac C, Gardete S, Tewhey R, Sakoulas G, Kaatz GW, Rose WE, Tomasz A, Rybak MJ. 2013. Alternative mutational pathways to intermediate resistance to vancomycin in methicillin-resistant Staphylococcus aureus. J Infect Dis 208:67–74. doi: 10.1093/infdis/jit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howden BP, Smith DJ, Mansell A, Johnson PD, Ward PB, Stinear TP, Davies JK. 2008. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol 8:39. doi: 10.1186/1471-2180-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, Tomasz A. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Sun X, Chang W, Dai Y, Ma X. 2015. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One 10:e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 13.Lyon GJ, Wright JS, Muir TW, Novick RP. 2002. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41:10095–10104. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 14.Ji G, Beavis R, Novick RP. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 15.Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC Jr. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis 38:1700–1705. doi: 10.1086/421092. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji BT, Rybak MJ, Lau KL, Sakoulas G. 2007. Evaluation of accessory gene regulator (agr) group and function in the proclivity towards vancomycin intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother 51:1089–1091. doi: 10.1128/AAC.00671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas G, Eliopoulos GM, Moellering RC Jr, Novick RP, Venkataraman L, Wennersten C, DeGirolami PC, Schwaber MJ, Gold HS. 2003. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis 187:929–938. doi: 10.1086/368128. [DOI] [PubMed] [Google Scholar]
- 18.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 19.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 20.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher C, Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, Mayes R, Moule S, Mungall K, Ormond D, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Sharp S, Simmonds M, Stevens K, Whitehead S, Barrell BG, Spratt BG, Parkhill J. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A 101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 190:1642–1651. doi: 10.1086/424849. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji BT, Brown T, Parasrampuria R, Brazeau DA, Forrest A, Kelchlin PA, Holden PN, Peloquin CA, Hanna D, Bulitta JB. 2012. Front-loaded linezolid regimens result in increased killing and suppression of the accessory gene regulator system of Staphylococcus aureus. Antimicrob Agents Chemother 56:3712–3719. doi: 10.1128/AAC.05453-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulman ZP, Sutton MD, Ly NS, Bulitta JB, Holden PN, Nation RL, Li J, Tsuji BT. 2015. Emergence of polymyxin B resistance influences pathogenicity in Pseudomonas aeruginosa mutators. Antimicrob Agents Chemother 59:4343–4346. doi: 10.1128/AAC.04629-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenhard JR, von Eiff C, Hong IS, Holden PN, Bear MD, Suen A, Bulman ZP, Tsuji BT. 2015. Evolution of Staphylococcus aureus under vancomycin selective pressure: the role of the small-colony variant phenotype. Antimicrob Agents Chemother 59:1347–1351. doi: 10.1128/AAC.04508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother doi: 10.1128/aac.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casapao AM, Davis SL, McRoberts JP, Lagnf AM, Patel S, Kullar R, Levine DP, Rybak MJ. 2014. Evaluation of vancomycin population susceptibility analysis profile as a predictor of outcomes for patients with infective endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:4636–4641. doi: 10.1128/AAC.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vriese AS, Vandecasteele SJ. 2014. Vancomycin: the tale of the vanquisher and the pyrrhic victory. Peritoneal Dial Int 34:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong RK, Low J, Koh TH, Kurup A. 2009. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur J Clin Microbiol Infect Dis 28:983–987. doi: 10.1007/s10096-009-0741-5. [DOI] [PubMed] [Google Scholar]
- 30.Moise PA, Schentag JJ. 2000. Vancomycin treatment failures in Staphylococcus aureus lower respiratory tract infections. Int J Antimicrob Agents 16(Suppl 1):S31–S34. [DOI] [PubMed] [Google Scholar]
- 31.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 32.Tam VH, Louie A, Deziel MR, Liu W, Drusano GL. 2007. The relationship between quinolone exposures and resistance amplification is characterized by an inverted U: a new paradigm for optimizing pharmacodynamics to counterselect resistance. Antimicrob Agents Chemother 51:744–747. doi: 10.1128/AAC.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji BT, Rybak MJ, Cheung CM, Amjad M, Kaatz GW. 2007. Community- and health care-associated methicillin-resistant Staphylococcus aureus: a comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn Microbiol Infect Dis 58:41–47. doi: 10.1016/j.diagmicrobio.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Viedma E, Sanz F, Orellana MA, San Juan R, Aguado JM, Otero JR, Chaves F. 2014. Relationship between agr dysfunction and reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus causing bacteraemia. J Antimicrob Chemother 69:51–58. doi: 10.1093/jac/dkt337. [DOI] [PubMed] [Google Scholar]
- 35.Rudkin JK, Edwards AM, Bowden MG, Brown EL, Pozzi C, Waters EM, Chan WC, Williams P, O'Gara JP, Massey RC. 2012. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J Infect Dis 205:798–806. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardete S, Kim C, Hartmann BM, Mwangi M, Roux CM, Dunman PM, Chambers HF, Tomasz A. 2012. Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin-resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLoS Pathog 8:e1002505. doi: 10.1371/journal.ppat.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onogawa T. 2002. Staphylococcal alpha-toxin synergistically enhances inflammation caused by bacterial components. FEMS Immunol Med Microbiol 33:15–21. doi: 10.1016/S0928-8244(01)00308-X. [DOI] [PubMed] [Google Scholar]
- 39.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 40.Rose WE, Leonard SN, Rossi KL, Kaatz GW, Rybak MJ. 2009. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 53:805–807. doi: 10.1128/AAC.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DG, Murakami Y, Andes DR, Craig WA. 2013. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 105 and 107 CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother 57:1434–1441. doi: 10.1128/AAC.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harigaya Y, Bulitta JB, Forrest A, Sakoulas G, Lesse AJ, Mylotte JM, Tsuji BT. 2009. Pharmacodynamics of vancomycin at simulated epithelial lining fluid concentrations against methicillin-resistant Staphylococcus aureus (MRSA): implications for dosing in MRSA pneumonia. Antimicrob Agents Chemother 53:3894–3901. doi: 10.1128/AAC.01585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]