Abstract
The choice of an antimicrobial agent must balance optimization of efficacy endpoints with the minimization of safety events. The risk versus benefit of daptomycin for patients with Staphylococcus aureus bacteremia with or without infective endocarditis receiving daptomycin at 6, 8, and 10 mg/kg of body weight/day was assessed. The relationships between the area under the concentration-time curve over 24 h (AUC)/MIC ratio and both clinical response and time to decreased susceptibility were evaluated using data from patients with such infections who received daptomycin at 6 mg/kg/day. Using these relationships, plus the previously identified relationship between the minimum concentration and an elevation in the creatine phosphokinase (CPK) concentration (CPK elevation) (S. M. Bhavnani, C. M. Rubino, P. G. Ambrose, and G. L. Drusano, Clin Infect Dis 50:1568–1574, 2010) and Monte Carlo simulation, the probability of each outcome by MIC for daptomycin at 6, 8, and 10 mg/kg/day was calculated. The function for exposure-response relationships for clinical response (P = 0.06) and time to decreased susceptibility (P = 0.01) resembled U and inverted U shapes, respectively. Multivariable analyses demonstrated AUC/MIC ratio, creatinine clearance, albumin concentration, and disease category to be predictors of clinical response. The results of simulations failed to demonstrate large improvements in the probabilities of clinical success among cohorts of simulated patients defined by the above-described predictive factors or the probability of decreased susceptibility at 30 days when the daptomycin dose was increased from 6 to 10 mg/kg/day. The probability of CPK elevation increased from 0.073 to 0.156 over this dose range. These data can be used to inform risk-versus-benefit decisions for daptomycin dose selection in patients with S. aureus bacteremia with or without infective endocarditis. The risk of CPK elevation, which is reversible, should be weighed in the context of the mortality and severe morbidity associated with these types of serious staphylococcal infections.
INTRODUCTION
As with all drugs, the choice of antimicrobial agent must balance efficacy with safety. Safety considerations may have an impact upon the choice of dosing regimen and the duration of therapy. This is the case with aminoglycoside antimicrobial agents, for which clinicians must explicitly balance the need for efficacy against the risk of nephrotoxicity (1). On the other hand, some drug toxicities are of less concern relative to the risk of clinical failure in patients with serious and life-threatening infections. Thus, there is more flexibility with the use of higher doses and longer treatment durations. For daptomycin-treated patients with Staphylococcus aureus infective endocarditis, the need for efficacy may supersede the concern regarding an elevation in the creatine phosphokinase (CPK) concentration (referred to here as CPK elevation), a sensitive biochemical signal of potential muscle toxicity which has been observed during daptomycin therapy but which has been demonstrated to be reversible upon drug discontinuation (2).
When evaluating the risk of safety events for antimicrobial agents, the risk of the development of antimicrobial resistance should also be considered. Antimicrobial resistance can affect not only an individual patient but also society as a whole. In the late 1980s, development of on-therapy Pseudomonas aeruginosa resistance was recognized not long after the introduction of ciprofloxacin (3, 4). The impact of this resistance, the rate of which was likely accelerated by the initial suboptimal dosing of ciprofloxacin, has limited the use of this agent in patient populations which would have benefited the most.
Given that the likelihood of efficacy, the emergence of resistance, and certain safety events can each be related to drug exposure, the understanding of such relationships allows for optimization of dosing regimens for which the probability of good outcomes are maximized while the likelihood of suboptimal outcomes are minimized. A previous analysis of phase 3 data from patients with Staphylococcus aureus bacteremia with or without infective endocarditis who received daptomycin at 6 mg/kg of body weight once every 24 h for 10 to 42 days (5) demonstrated relationships between daptomycin exposure and the probability of CPK elevation (6). In an effort to assess risk-benefit considerations for daptomycin at 6 and 8 mg/kg/day in patients with S. aureus bacteremia with or without infective endocarditis, the analyses described herein were undertaken to evaluate exposure-response relationships for efficacy and decreased susceptibility. Additionally, using the relationships for these endpoints and that for a decreased probability of CPK elevation, the probability of each of these outcomes independently and jointly was also evaluated.
MATERIALS AND METHODS
Primary objectives.
The primary objectives of these analyses were to evaluate the relationships between daptomycin exposure and the probabilities of both clinical success and time to decreased susceptibility of Staphylococcus aureus to daptomycin using data from patients with S. aureus bacteremia with or without infective endocarditis enrolled in a previously described phase 3 study (5). Using the exposure-response relationships for these endpoints and that for CPK elevation (6), the joint probability of favorable outcomes was evaluated. A plan for the statistical analysis of each of these endpoints was written before the data from the phase 3 study became available.
Study design.
Daptomycin-treated patients from a phase 3, randomized, multicenter, open-label study of adult patients with bacteremia with or without endocarditis due to S. aureus for whom sufficient pharmacokinetic data to describe exposure measures were available were considered evaluable for these analyses. Patients who were randomized to the daptomycin treatment arm received daptomycin at 6 mg/kg intravenously every 24 h for 10 to 42 days (5). The institutional review board at each study site approved the protocol, and all patients or their authorized representatives provided written informed consent.
Patients were evaluated at baseline, at the end of therapy, and at 42 days after the end of therapy at the test-of-cure (TOC) visit. Blood samples for culture were obtained daily until they were negative, as well as at the end of therapy and at the TOC visit. Serum CPK concentrations were measured three times during each week of daptomycin treatment. Five serial blood samples for pharmacokinetic analyses were collected predosing and 0.25 to 0.5, 1 to 1.5, 3 to 5, and 9 to 12 h following the end of the infusion on day 5. Daptomycin plasma concentrations were determined by a validated high-performance liquid chromatography assay. Additional details about this study are described elsewhere (5).
Endpoints evaluated.
The two endpoints that were evaluated for the exposure-response analyses were clinical response at the TOC visit and time to decreased susceptibility of S. aureus to daptomycin. Clinical response, the primary endpoint for the above-described phase 3 study (5), was classified as success or failure. Cases that were classified as cure or improvement were considered to be a clinical success. Clinical cure was defined as the resolution of clinically significant signs and symptoms associated with the admission infection and no requirement for further antibiotic therapy for the primary infection under study. Improvement was defined as the partial resolution of clinical signs or symptoms of infection such that no further antibiotic therapy for the primary infection under study was required. Cases were considered to be a clinical failure if any of the following occurred: classification of clinical or microbiologic failure, death, failure to obtain a blood sample for culture, administration of potentially effective nonstudy antibiotics, or discontinuation of study medication due to clinical or microbiologic failure or an adverse event. Only those patients with clinical failure associated with the study drug were included in the analysis of clinical response.
The time to the decreased susceptibility of S. aureus to daptomycin during therapy was determined on the basis of the results of repeat blood cultures. Decreased susceptibility was defined as an increase in the daptomycin MIC values of S. aureus isolates of 4-fold or greater relative to the MIC value of the baseline S. aureus isolate.
Measures of daptomycin exposure.
As described previously (6), a two-compartment model with zero-order intravenous input and first-order elimination was used to describe the disposition of daptomycin on the basis of data from the population described herein. Using Bayesian post hoc pharmacokinetic parameter estimates, the area under the plasma concentration-time curve over 24 h (AUC) and the minimum (trough) concentration (Cmin) on day 5 of daptomycin therapy were estimated for each patient. AUC values were divided by the MIC value of the baseline S. aureus isolate to derive the ratio of the AUC to the MIC (AUC/MIC ratio). For exposure-response analyses of clinical response and time to decreased susceptibility, the exposure measure evaluated was AUC/MIC ratio. Since daptomycin is administered as a once daily dose, all pharmacokinetic-pharmacodynamic indices would be highly correlated. Thus, given the lack of ability to discriminate among such indices and the fact that previous dose fractionation data demonstrated that AUC/MIC ratio is most closely related to outcome (7), this index was evaluated for the analyses described herein. For the Monte Carlo simulation results that were used to evaluate the probability of CPK elevation, Cmin was evaluated, given the finding of the previously described exposure-response relationship between CPK elevation and Cmin (6), which was supported by preclinical data (8).
Exposure-response analyses.
The univariable relationship between the probability of clinical success and AUC/MIC ratio in the form of a continuous variable was evaluated using logistic regression. For categorical forms of AUC/MIC ratio, univariable relationships were examined using the chi-square test or Fisher's exact test. Categorical variables for AUC/MIC ratio consisted of two- and three-group variables which were evaluated to account for potential nonlinearity and/or nonmonotonicity. The thresholds for AUC/MIC ratio used to define categorical variables for evaluation of exposure-response relationships for clinical response were those that were optimally determined. A two-group variable for AUC/MIC ratio was constructed by using the resulting split of a classification tree for clinical response. A three-group variable for AUC/MIC ratio was constructed by determining a pair of cutoff values that minimized the likelihood ratio P value using logistic regression for clinical response.
Multivariable logistic regression with backwards stepping and a removal criterion of a P value of <0.1 was carried out to determine the effect of AUC/MIC ratio on the probability of clinical success in the context of other independent variables. Other independent variables considered included baseline demographic and disease characteristics, including patient age; renal function, as measured by creatinine clearance normalized for body surface area (CLCR) (9); albumin concentration; and disease category. Continuous independent variables were evaluated as such and as two- and three-group categorical variables, as described above for AUC/MIC ratio.
Univariable relationships for time to decreased susceptibility were examined using log rank tests for AUC/MIC ratio evaluated as a categorical variable and Cox regression for AUC/MIC ratio evaluated as a continuous variable. As described above for the analysis of clinical response, categorical forms of AUC/MIC ratio consisting of two- and three-group variables were considered. The thresholds for AUC/MIC ratio used to define these categorical variables were those that were optimally determined for time to decreased susceptibility. A two-group variable for AUC/MIC ratio was constructed using the cutoff maximizing the log rank test derived from a univariable Cox proportional hazard regression model for time to decreased susceptibility. A three-group variable for AUC/MIC ratio was constructed by determining the pair of cutoff AUC/MIC ratio values that achieved minimization of the log rank P value derived from Cox proportional hazards regression.
Exposure-response analyses for clinical response and time to decreased susceptibility were performed using R (version 2.11.1) (10).
Monte Carlo simulation.
Using mean parameter estimates and associated standard deviations based on the previously developed population pharmacokinetic model for daptomycin (6) and ADAPT II (11), Monte Carlo simulation was carried out to generate 5,000 simulated steady-state AUC and Cmin values following the administration of daptomycin at 6, 8, and 10 mg/kg once daily.
Using the simulated AUC values associated with each dosing regimen, mean model-predicted probabilities of clinical success for MIC values ranging from 0.015 to 4 mg/liter and clinical scenarios based on the categories of independent variables retained in the multivariable model for clinical response were determined for each patient. Likewise, the mean model-predicted probability of reduced susceptibility at 30 days posttherapy was determined for the above-described MIC values. Mean probabilities by MIC were evaluated in the context of the MIC distribution for methicillin-resistant S. aureus (MRSA) isolates based on recent surveillance data (12).
Using simulated Cmin values associated with each dosing regimen and the previously described relationship between the probability of CPK elevation and Cmin (6), the mean model-predicted probability of a CPK elevation was also determined. Briefly, univariable analyses based on the data from 108 patients described herein revealed a relationship between CPK elevation and a daptomycin Cmin of ≥24.3 mg/liter. CPK elevation was defined using one of the two clinical scenarios: (i) no CPK elevations at baseline followed by CPK elevations to ≥3 times the upper limit of normal (ULN) based on two sequential measurements during the period from day 4 (after three doses) to 3 days after the end of therapy with one or two CPK elevations of ≥5 times the ULN and (ii) a baseline CPK concentration greater than the ULN followed by CPK elevations to ≥5 times the ULN on the basis of two sequential measurements during the period from day 4 to 3 days after the end of therapy.
Mean model-predicted joint probabilities of favorable outcomes (clinical success, no change in susceptibility, and no CPK elevation) by MIC were determined for the simulated patients in each of the clinical scenarios on the basis of the categories of independent variables retained in the multivariable model for clinical response. Mean joint probabilities, which assumed the independence of each outcome, were calculated by multiplying the probabilities of individual favorable outcomes.
RESULTS
Patient population.
Data from a total of 120 patients who received 6 mg/kg/day of daptomycin were available for evaluation of exposure-response relationships for clinical response at the TOC visit and time to decreased susceptibility. Of these, 108 patients had sufficient pharmacokinetic and CPK data for analysis; 101 had baseline and follow-up S. aureus isolates for which MIC values were available and were evaluable for the analysis of time to decreased susceptibility; 78 of the 108 patients were evaluable for the analysis of clinical response. Table 1 shows the demographic characteristics of the three patient populations, consisting of 108 (the original analysis population for the exposure-response analysis of CPK elevation [6]), 101, and 78 patients.
TABLE 1.
Demographic characteristics for the analysis populations evaluated in the CPK elevation, time to decreased susceptibility, and efficacy analyses
| Characteristic | CPK elevation (n = 108) | Time to decreased susceptibility (n = 101) | Clinical response (n = 78) |
|---|---|---|---|
| Median (minimum, maximum) value for: | |||
| Age (yr) | 50 (21, 87) | 52 (21, 87) | 50 (21, 87) |
| Albumin concn (g/dl) | 3.0 (1.6, 4.3) | 3.0 (1.6, 4.3) | 3.0 (1.6, 4.3) |
| Baseline wt (kg) | 80.5 (52.0, 129) | 81.0 (52.0, 129) | 79.9 (52.0, 129) |
| Baseline CLCR (ml/min/1.73 m2)a | 87.9 (27.3, 241) | 83.3 (27.3, 213) | 91.5 (33.7, 213) |
| % patients with the following diagnosis (no. with the diagnosis/total no. tested): | |||
| Uncomplicated bacteremia | 28.7 (31/108) | 29.7 (30/101) | 30.8 (24/78) |
| Complicated bacteremia | 48.1 (52/108) | 48.5 (49/101) | 47.4 (37/78) |
| Uncomplicated right-sided endocarditis | 4.6 (5/108) | 5.0 (5/101) | 6.4 (5/78) |
| Complicated right-sided endocarditis | 11.1 (12/108) | 8.9 (9/101) | 6.4 (5/78) |
| Left-sided endocarditis | 7.4 (8/108) | 7.9 (8/101) | 9.0 (7/78) |
| % patients of the following race (no. of the indicated race/total no. of patients tested): | |||
| Asian | 0.93 (1/108) | 0.99 (1/101) | 1.3 (1/78) |
| Black | 27.8 (30/108) | 27.7 (28/101) | 28.2 (22/78) |
| Caucasian | 61.1 (66/108) | 63.4 (64/101) | 64.1 (50/78) |
| Hispanic | 6.5 (7/108) | 5.0 (5/101) | 3.8 (3/78) |
| Other | 3.7 (4/108) | 3.0 (3/101) | 2.6 (2/78) |
| % male patients (no. of male patients/total no. of patients tested) | 58.3 (63/108) | 59.4 (60/101) | 57.7 (45/78) |
Estimated using the Cockcroft-Gault method (9).
Analyses for clinical response.
A relationship between the probability of clinical success and AUC/MIC ratio (P = 0.06), which resembled a U shape, was identified. Observed successful clinical responses were 100% (8/8) and 75% (24/32) for patients with AUC/MIC ratios of ≤1,081 and >2,337, respectively. For patients in the middle of the AUC/MIC ratio range (AUC/MIC ratio, >1,081 to ≤2,337), 60.5% (23/38) had a successful clinical response.
Univariable analyses for clinical response demonstrated the influence of three other independent variables: creatinine clearance normalized for body surface area (CLCR; P < 0.001), the albumin concentration (P = 0.002), and disease category (P = 0.02). The percentages of observed successful clinical responses were 20% (2/10), 66.7% (18/27), and 85.4% (35/41) for patients with CLCR values of ≤51.2, >51.2 to 88.9, and >88.9 ml/min/1.73 m2, respectively. The percentages of observed successful clinical responses were 50% (14/28) and 84.4% (38/45) for patients with albumin concentrations of <2.9 and ≥2.9 g/dl, respectively. For patients with left-sided endocarditis, complicated or uncomplicated right-sided endocarditis or complicated bacteremia, and uncomplicated bacteremia, the percentages of observed successful responses were 28.6% (2/7), 70.2% (33/47), and 83.3% (20/24), respectively. As each of these univariable relationships was considered biologically plausible, multivariable logistic regression was then performed. The results of the final model are presented in Table 2.
TABLE 2.
Multivariable logistic regression model for clinical success
| Variable and value | Parameter estimate (SE) | Odds ratio (95% CId) | Likelihood ratio P value |
|---|---|---|---|
| CLCR (ml/min/1.73 m2) | <0.001 | ||
| ≤51.2a | 1 | ||
| >51.2 to ≤88.9 | 2.59 (1.18) | 13.3 (1.32–135) | |
| >88.9 | 4.30 (1.35) | 73.7 (5.21–1,042) | |
| AUC/MIC ratio | 0.091 | ||
| ≤1,081b | NE | NE | |
| >1,081 to ≤2,334a | 1 | ||
| >2,337 | 1.29 (0.94) | 3.64 (0.57–23.2) | |
| Albumin concn (g/dl) | 0.039 | ||
| <2.9a | 1 | ||
| ≥2.9 | 1.55 (0.78) | 4.70 (1.02–21.5) | |
| Diagnosis categoryc | 0.086 | ||
| 1a | 1 | ||
| 2, 3, or 4 | 1.85 (1.28) | 6.34 (0.52–77.2) | |
| 5 | 3.03 (1.48) | 20.8 (1.14–378) |
Represents the reference group.
With a 100% observed response (8/8) in the group with AUC/MIC ratios of ≤1,081, no estimates relative to this group could be obtained with maximum likelihood estimation. NE, not estimated.
Diagnosis category definitions are as follows: 1, left-sided endocarditis; 2, 3, or 4, complicated right-sided endocarditis, uncomplicated right-sided endocarditis, or complicated bacteremia, respectively; 5, uncomplicated bacteremia.
CI, confidence interval.
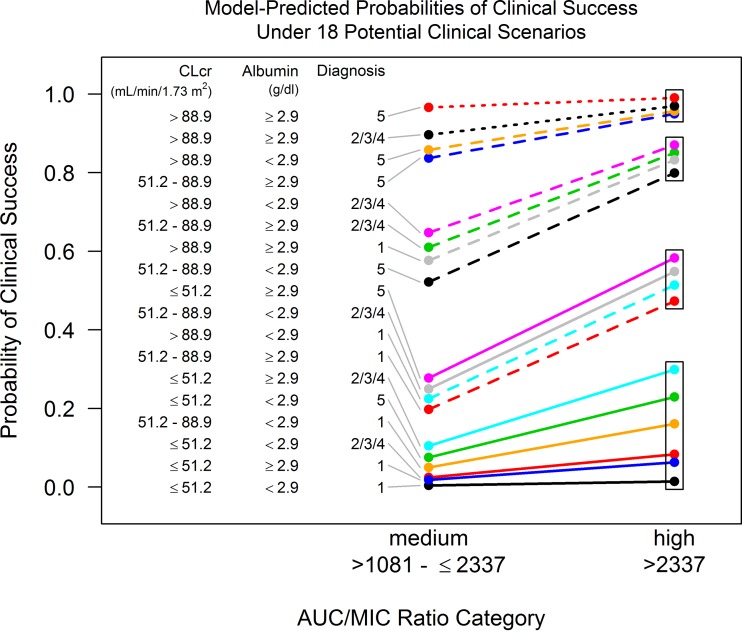
On the basis of combinations of different subcategories for the four independent variables included in the multivariable model, the model-predicted probability of clinical success was evaluated for different clinical scenarios. Model-predicted probabilities of clinical success as a function of the middle and higher AUC/MIC ratio categories (>1,081 to ≤2,337 and >2,337, respectively) for 18 clinical scenarios are shown in Fig. 1. On the basis of inspection of the four clusters of model-predicted probabilities of clinical success (denoted by the rectangular boxes in Fig. 1), it was evident that the cluster representing the highest probabilities demonstrated the minimal impact of an AUC/MIC ratio increase from the range of >1,081 to ≤2,337 to >2,337. The cluster representing the lowest probabilities demonstrated the substantial impact of this AUC/MIC ratio increase, but the probability of clinical success associated with higher AUC/MIC ratios was still low (≤0.299). The two clusters with probabilities in the middle demonstrated a clinically important change in the probability of clinical success as a function of an increase in AUC/MIC ratio, resulting in substantially higher probabilities in the higher AUC/MIC ratio group.
FIG 1.
Model-predicted probabilities of clinical success by AUC/MIC ratio category for 18 clinical scenarios. CLCR, creatinine clearance normalized for body surface area; diagnosis 1, left-sided endocarditis; diagnoses 2, 3, or 4, complicated right-sided endocarditis, uncomplicated right-sided endocarditis or complicated bacteremia, respectively; diagnosis 5, uncomplicated bacteremia.
Evaluation of the 18 specific clinical scenarios demonstrated the following patterns: (i) the model-predicted probability of clinical success was generally lower for scenarios in which patients had left-sided endocarditis; (ii) if CLCR was >88.9 ml/min/1.73 m2, the albumin concentration was ≥2.9 g/dl, and the disease category was not left-sided endocarditis, the probability of clinical success was generally high for either an AUC/MIC ratio in the range of >1,081 to ≤2,337 or an AUC/MIC ratio of >2,337; thus, an increased AUC/MIC ratio had less of an impact on the probability of clinical success (i.e., differences in probabilities, ≤0.073); and (iii) if either CLCR or the albumin concentration was lower (i.e., the CLCR was ≤51.2 or 51.2 to 88.9 ml/min/1.73 m2 or the albumin concentration was <2.9 g/dl) such that the probability of clinical success for an AUC/MIC ratio of >1,081 to ≤2,337 was not very close to either 0 or 1, then an AUC/MIC ratio increase from a value of >1,081 to ≤2,337 to one of >2,337 had an impressive impact on the probability of clinical success (i.e., with differences in probabilities being as great as 0.306).
As shown in Fig. S1 in the supplemental material, an increase in the MIC alone also influenced the mean model-predicted probability of clinical success for the simulated patients in the above-described 18 clinical scenarios evaluated; increasing MIC values shifted more patients from the high to the intermediate AUC/MIC ratio categories, for which the mean probability of clinical success was lower. The shift in the mean probability of clinical success from an MIC value of 0.5 to 0.06 mg/liter was greatest for clinical scenarios for which mean probabilities were closer to 0.5, with the shift being as great as from 0.313 to 0.576 for uncomplicated bacteremia with a CLCR of ≤51.2 ml/min/1.73 m2 and an albumin concentration of ≥2.9 g/dl. The ranges of the shifts were above 0.10 and 0.20 for 10 and 7 of the clinical scenarios, respectively.
Time to decreased susceptibility analyses.
Of the 101 patients that were evaluable for the time to decreased susceptibility, 11 (10.9%) had S. aureus isolates that had a 4-fold or greater change in the MIC from the baseline MIC value for daptomycin, and 7 of these isolates transitioned from susceptible to nonsusceptible (13). For the remaining four patients, the follow-up S. aureus isolates remained susceptible to daptomycin after the 4-fold change in the MIC. It is important to note that in all seven patients for whom nonsusceptible isolates (MIC, ≥2 mg/liter) emerged, source control (e.g., an undrained abscess) was an issue.
As shown in Fig. 2A, a significant relationship between time to decreased susceptibility and AUC/MIC ratio evaluated as a three-group variable was identified (P = 0.013). At 30 days after the start of therapy, the probabilities that the patient isolates would have decreased susceptibility were 0, 0.278, and 0.081 in patients with low (<1,480), intermediate (≥1,480 to <1,970), and high (>1,970) AUC/MIC ratios, respectively, and the pattern resembled an inverted U shape (14). A depiction of this relationship between the probability of deceased susceptibility at 30 days after the start of therapy and AUC/MIC ratio is shown in Fig. 2B. The data presented in Fig. 2B are provided in tabular format in Table S1 in the supplemental material.
FIG 2.
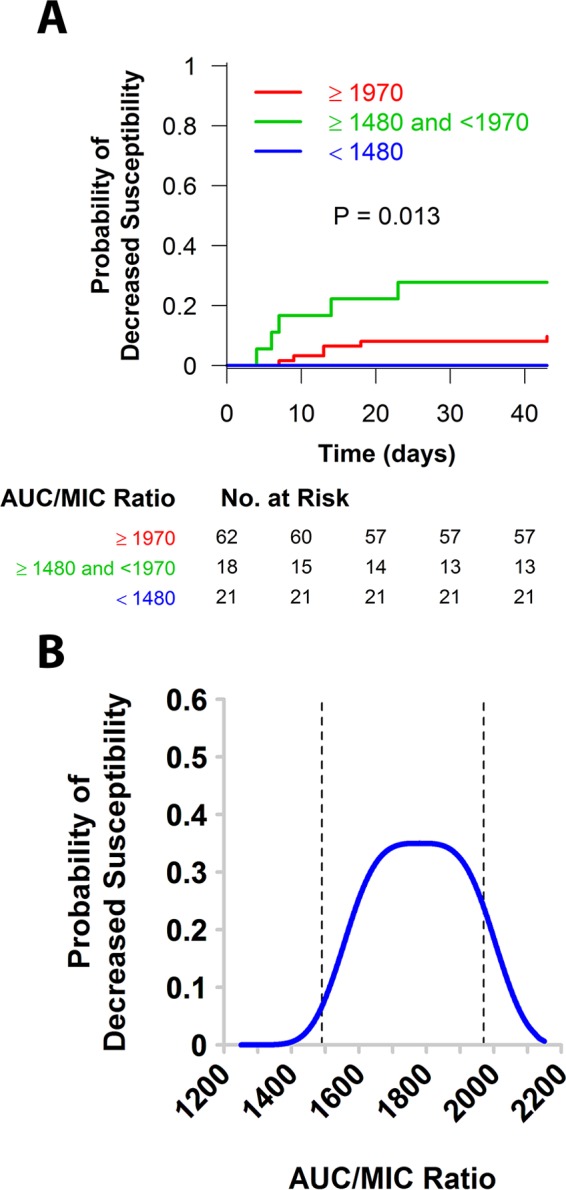
Relationship between time to decreased susceptibility and AUC/MIC ratio evaluated as a three-group variable (A) and a depiction of the relationship between the probability of decreased susceptibility at 30 days after the start of therapy and AUC/MIC ratio (B).
Monte Carlo simulation evaluating each of the outcomes.
Using the results of the above-described final model for clinical response, the exposure-response relationship for time to decreased susceptibility, and the previously identified exposure-response relationship for CPK elevation, the mean model-predicted joint probability of favorable outcomes under the 18 clinical scenarios was evaluated for simulated patients receiving daptomycin at 6 mg/kg/day. As shown in Fig. S2 in the supplemental material and similar to what was seen for the mean model-predicted probability of clinical success in Fig. S1 in the supplemental material, increasing MIC values alone also influenced the mean joint probabilities of favorable outcomes; increasing MIC values shifted more patients from the high to the intermediate AUC/MIC ratio category, for which the probability of clinical success was lower and the probability of decreased susceptibility was higher. The shift in the mean joint probabilities of favorable outcomes from an MIC value of 0.5 to 0.06 mg/liter was the greatest for clinical scenarios for which mean joint probabilities were closer to 0.5, with a shift as great as from 0.237 to 0.491 for uncomplicated bacteremia with a CLCR of ≤51.2 ml/min/1.73 m2 and an albumin concentration of ≥2.9 g/dl. The ranges of the shifts were above 0.10 and 0.20 for 13 and 8 of the clinical scenarios, respectively.
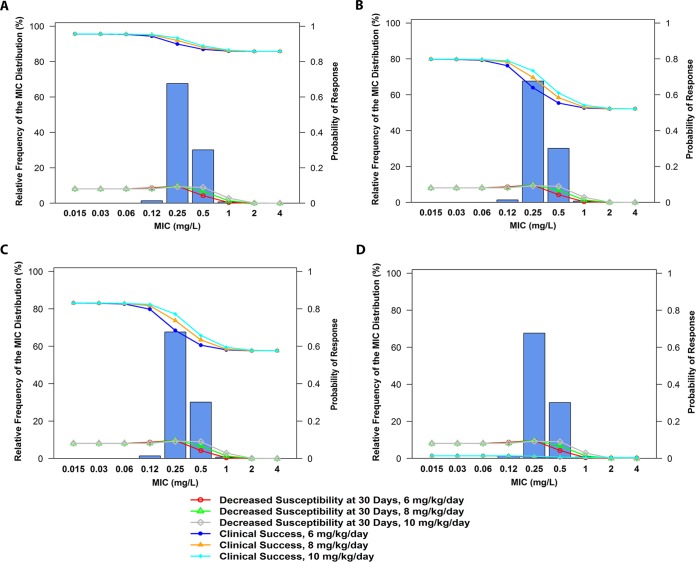
The mean probabilities of clinical success, decreased susceptibility, and increased CPK elevation by MIC value were compared for simulated patients after the administration of daptomycin at 6, 8, and 10 mg/kg/day. Of the 18 clinical scenarios, 4 were selected for closer inspection. The mean probabilities of clinical success and decreased susceptibility at days after the start of therapy by MIC for the cohorts of simulated patients described by these four clinical scenarios after administration of these daily doses of daptomycin are shown in Fig. 3 and summarized in Table S1 in the supplemental material. The four selected scenarios for which the results are shown in Fig. 3 were the following: uncomplicated bacteremia, a CLCR of >88.9 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (Fig. 3A); uncomplicated bacteremia, a CLCR of 51.2 to 88.9 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (Fig. 3B); left-sided endocarditis, a CLCR of >88.9 ml/min/1.73 m2, and an albumin concentration of ≥2.9 g/dl (Fig. 3C); and left-sided endocarditis, a CLCR of ≤51.2 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (Fig. 3D). The MIC distribution for MRSA shown was based on recent U.S. surveillance data from 2005 to 2010 (12), the MIC50 and MIC90 values for which were 0.25 and 0.5 mg/liter, respectively. At an MIC value of 0.25 mg/liter, the mean probabilities of clinical success for the simulated patients for the four scenarios when the daptomycin dose was increased from 6 to 10 mg/kg/day changed by 0.034, 0.095, 0.087, and 0.004, respectively (Fig. 3A to D). For the two dose levels, the mean probabilities of decreased susceptibility were essentially the same (0.097 versus 0.093); the mean probabilities of CPK elevation were 0.073 and 0.156, respectively.
FIG 3.
Mean probability of favorable response by MIC for simulated patients with uncomplicated bacteremia, a CLCR of >88.9 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (A), uncomplicated bacteremia, a CLCR of 51.2 to 88.9 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (B), left-sided endocarditis, a CLCR of >88.9 ml/min/1.73 m2, and an albumin concentration of ≥2.9 g/dl (C), and left-sided endocarditis, a CLCR of ≤51.2 ml/min/1.73 m2, and an albumin concentration of <2.9 g/dl (D).
DISCUSSION
Using data from daptomycin-treated patients with S. aureus bacteremia with or without infective endocarditis, the analyses described herein were undertaken to evaluate exposure-response relationships for clinical response and decreased susceptibility. Using the relationships identified for these endpoints and the relationship previously described for CPK elevation (6), the joint probability of favorable outcomes (i.e., clinical success, no change in susceptibility, and no CPK elevation) was evaluated to assess the risk versus benefit for different daptomycin dosing regimens.
An exposure-response relationship for clinical response was identified, and the shape of that relationship resembled a U. That is, patients that had AUC/MIC ratios that were lower (≤1,081) and higher (>2,337) had higher percentages of successful clinical responses (100 and 75%, respectively). Of the patients with AUC/MIC ratios in the middle of the range (>1,081 to ≤2,337), only 60.5% had successful clinical responses. When these data were examined further, it was evident that patients with the lowest daptomycin AUC/MIC ratio range also had the most favorable profile of other variables found to be predictive of clinical success, including better renal function (as measured by CLCR) and a higher albumin concentration. Among the patients in this AUC/MIC ratio range, there was also a greater percentage of patients with uncomplicated bacteremia rather than complicated bacteremia with or without infective endocarditis. Higher percentages of observed or probabilities of response in patients with low pharmacokinetic-pharmacodynamic indices and potentially other favorable factors have been reported previously (15, 16).
Application of the multivariable model to predict the probability of clinical success among cohorts of patients with combinations of these characteristics served to identify those cohorts for which optimal drug exposure had the most and the least impact. For example, the magnitude of AUC/MIC ratio had a negligible impact on the probability of clinical success in patients with reduced renal function (CLCR, ≤51.2 ml/min/1.73 m2), a lower albumin concentration (<2.9 g/dl), and left-sided endocarditis (increase in the probability of clinical success, 0.004 to 0.01) or good renal function (CLCR, >88.9 ml/min/1.73 m2), a higher albumin concentration (≥2.9 g/dl), and uncomplicated bacteremia (increase in the probability of clinical success, 0.966 to 0.990). However, for patients with left-sided endocarditis but good renal function and a higher albumin concentration, the impact of optimizing AUC/MIC ratio on the probability of clinical success was more impressive (increase in the probability of clinical success, 0.577 to 0.832). The impact of improved drug exposure was also impressive for patients with intermediate renal function (CLCR 51.2 to 88.9 ml/min/1.73 m2), a lower albumin concentration, and uncomplicated bacteremia (increase in the probability of clinical success, 0.522 to 0.799). Understanding the clinical scenarios on which drug exposure can have the most favorable influence allows the clinician to better consider the implications of underdosing or treating infections caused by isolates with higher MIC values on clinical response.
Just as with clinical response, the shape of the exposure-response relationship for time to decreased daptomycin susceptibility during therapy was nonmonotonic. At 30 days after the start of therapy, the probabilities of decreased susceptibility were 0, 0.278, and 0.081 in patients with AUC/MIC ratios that were low (<1,480), intermediate (≥1,480 to <1,970), and high (>1,970), respectively, thus resembling an inverted U shape. On the basis of these findings, it was apparent that low drug pressure did not select for a change in the MIC, but at an AUC/MIC ratio of ≥1,480 but <1,970, changes in MIC occurred. At an AUC/MIC ratio of ≥1,970, the probability of decreased susceptibility was substantially reduced. It is interesting to note that the thresholds for the AUC/MIC ratio associated with decreased susceptibility (AUC/MIC ratio, 1,970) and a higher probability of clinical response (AUC/MIC ratio, 2,337) for the patient population described herein were closely similar to that associated with a reduction in the bacterial count from the baseline count of approximately 3 log10 CFU for S. aureus in a neutropenic murine thigh infection model (AUC/MIC ratio, >2,000, on the basis of data comodeled for four isolates) (17).
While exposure-response relationships are generally monotonic (i.e., more therapeutic intensity leads to a greater effect, up to a maximum value), results from studies conducted using in vitro and murine infection models have shown that resistance suppression is a distinctly nonmonotonic function and has a shape that resembles an inverted U (14). Data from such models evaluating the activity of levofloxacin against P. aeruginosa served to show that suboptimal drug exposures amplify the growth of less susceptible bacterial populations until maximal amplification is obtained (14, 18). Until now, the observation of such a function for the exposure-response relationship for decreased susceptibility has been limited to those based on nonclinical rather than clinical data. The lack of reports based on clinical data describing this phenomenon is perhaps not surprising, given that detection of such relationships would require a priori knowledge of the nonmonotonic nature of exposure-response relationships for the emergence of resistance.
In addition to considering exposure-response relationships for clinical response and decreased susceptibility, a previously identified relationship for CPK elevation was also considered. These data showed relationships between the probability of CPK elevation and Cmin evaluated as a continuous and categorical variable. In the case of the latter, the probability of CPK elevation for patients with daptomycin Cmin values of ≥24.3 mg/liter was 0.5, whereas it was 0.029 for patients with Cmin values of <24.3 mg/liter (6). Using this relationship and the observed proportion of musculoskeletal adverse events among patients with CPK elevations (0.33), the percent probability of musculoskeletal adverse events among simulated patients was forecasted for daptomycin dosing regimens of 8 and 10 mg/kg/day. The probabilities of musculoskeletal adverse events of 3.6% and 5.1% for daily doses of 8 and 10 mg/kg, respectively (6), were similar to observed rates of 4.2% and 6.3%, respectively, based on recently published data from randomized controlled studies (19, 20). Thus, these recent data provide support for the predictive utility of these earlier findings.
Using exposure-response relationships for clinical response, time to decreased susceptibility, and CPK elevation, and the daptomycin MIC value for the infecting S. aureus isolate, one can understand the impact of higher MIC values on the probability of a favorable outcome after administration of daptomycin at 6 mg/kg/day. For example, for patients who have intermediate renal function, a lower albumin concentration, and uncomplicated bacteremia and who have achieved the targeted AUC/MIC ratio of ≥2,337, the mean joint probability of a favorable outcome decreased from 0.643 to 0.427 as the MIC increased from 0.12 to 0.5 mg/liter. In such cases where the probability of achieving the desired AUC/MIC ratio is too low, the impact of which would be increased probabilities of clinical failure and decreased susceptibility, a higher daily dose of daptomycin should be considered. If the clinician decides that the likelihood of a good clinical outcome is too low or the probability of decreased susceptibility is too high, a higher daily daptomycin dose could be considered and the impact of a higher probability of CPK elevation could be factored into the decision as to whether or not to alter the dosing regimen.
However, as shown by the results of simulations evaluating the mean probabilities of clinical success and no change in susceptibility, increases in the daily dose from 6 to 10 mg/kg did not result in large improvements in the proportion of simulated patients achieving clinical success or reduce the proportion of simulated patient isolates with decreased susceptibility. The mean probability of CPK elevation increased modestly from 0.073 to 0.156 with this dose increase. The lack of an impressive change in the mean probabilities of clinical success and decreased susceptibility was expected, as the daptomycin AUC and, thus, dose would need to more than double (e.g., the dose would need to increase from 6 to 12 mg/kg/day) to provide exposures adequate to cover a 1-dilution increase in the MIC. Increasing numbers of reports and amounts of data from clinical studies provide support for the use of higher doses of daptomycin (8 to 12 mg/kg/day) to improve outcomes among patients with serious and life-threatening infections (21–26). The results of simulations conducted to evaluate the mean probabilities of favorable outcomes with daptomycin at 8 and 10 mg/kg/day should be interpreted with some caution since the exposures and model-predicted probabilities estimated were at or beyond those for the exposure range studied; thus, some degree of extrapolation was required.
In summary, we have described relationships between daptomycin exposure and the probability of clinical success, decreased susceptibility, and CPK elevation. Using such relationships to predict the probability of good outcomes, clinicians can make informed decisions regarding the risk versus the benefit of the administration of a given daily dose of daptomycin for patients with S. aureus bacteremia with and without endocarditis. The risk of CPK elevation, which is reversible, needs to be weighed in the context of the mortality and severe morbidity associated with these types of serious infections. Lastly, understanding the relationship between AUC/MIC ratio and the change in the MIC protects the future of daptomycin for all patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kim A. Charpentier from ICPD (Latham, NY, USA) for her assistance with preparation of the manuscript.
This study was sponsored by Cubist Pharmaceuticals Inc., Lexington, MA.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02967-15.
REFERENCES
- 1.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 2.Merck & Co., Inc. 2015. Cubicin® (daptomycin for injection) for intravenous use full prescribing information. Merck & Co., Inc., Whitehouse Station, NJ. [Google Scholar]
- 3.Peloquin CA, Cumbo TJ, Nix DE, Sands MF, Schentag JJ. 1989. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med 149:2269–2273. [PubMed] [Google Scholar]
- 4.Echols RM. 1993. The selection of appropriate dosages for intravenous ciprofloxacin. J Antimicrob Chemother 31:783–787. doi: 10.1093/jac/31.5.783. [DOI] [PubMed] [Google Scholar]
- 5.Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Savor Price C, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 6.Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 50:1568–1574. doi: 10.1086/652767. [DOI] [PubMed] [Google Scholar]
- 7.Louie A, Kaw P, Liu W, Jumbe N, Miller MH, Drusano GL. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob Agents Chemother 45:845–851. doi: 10.1128/AAC.45.3.845-851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oleson FB Jr, Berman CL, Kirkpatrick JB, Regan KS, Lai JJ, Tally FP. 2000. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob Agents Chemother 44:2948–2953. doi: 10.1128/AAC.44.11.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 10.Development Core Team R. 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org Accessed 3 October 2013. [Google Scholar]
- 11.D'Argenio DZ, Schumitzky A. 1997. ADAPT II user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA. [Google Scholar]
- 12.Sader HS, Moet GJ, Farrell DJ, Jones RN. 2011. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: trend analysis of a 6-year period in US medical centers (2005-2010). Diagn Microbiol Infect Dis 70:412–416. doi: 10.1016/j.diagmicrobio.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing. 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Tam VH, Louie A, Deziel MR, Liu W, Drusano GL. 2007. The relationship between quinolone exposures and resistance amplification is characterized by an inverted U: a new paradigm for optimizing pharmacodynamics to counter-select resistance. Antimicrob Agents Chemother 51:744–747. doi: 10.1128/AAC.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhavnani SM, Rubino CM, Ambrose PG, Babinchak TJ, Korth-Bradley JM, Drusano GL. 2010. Impact of different factors on the probability of clinical response in tigecycline-treated patients with intra-abdominal infections. Antimicrob Agents Chemother 54:1207–1212. doi: 10.1128/AAC.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thwaites GE, Bhavnani SM, Hong Chau TT, Hammel JP, Torok ME, Van Wart SA, Mai PP, Reynolds DK, Caws M, Dung NT, Hien TT, Kulawy R, Farrar J, Ambrose PG. 2011. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 55:3244–3253. doi: 10.1128/AAC.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother 48:63–68. doi: 10.1128/AAC.48.1.63-68.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jumbe N, Louie A, Leary R, Liu W, Deziel MR, Tam VH, Bachhawat R, Freeman C, Kahn JB, Bush K, Dudley MN, Miller MH, Drusano GL. 2003. Application of a mathematical model to prevent in-vivo amplification of antibiotic-resistant bacterial populations during therapy. J Clin Invest 112:275–285. doi: 10.1172/JCI200316814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byren I, Rege S, Campanaro E, Yankelev S, Anastasiou D, Kuropatkin G, Evans R. 2012. Randomized controlled trial of the safety and efficacy of daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother 56:5626–5632. doi: 10.1128/AAC.00038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz DE, Lindfield KC, Steenbergen JN, Benziger DP, Blackerby KJ, Knapp AG, Martone WJ. 2008. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by Gram-positive bacteria. Int J Clin Pract 62:1455–1464. doi: 10.1111/j.1742-1241.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 21.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. 2009. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann Pharmacother 43:1211–1219. doi: 10.1345/aph.1M085. [DOI] [PubMed] [Google Scholar]
- 22.Le J, Bookstaver PB, Rudisill CN, Hashem MG, Igbal R, James CL, Sakoulas G. 2010. Treatment of meningitis caused by vancomycin-resistant Enterococcus faecium: high-dose and combination daptomycin therapy. Ann Pharmacother 44:2001–2006. doi: 10.1345/aph.1P333. [DOI] [PubMed] [Google Scholar]
- 23.Lai CC, Sheng WH, Wang JT, Cheng A, Chuang YC, Chen YC, Chang SC. 2013. Safety and efficacy of high-dose daptomycin as salvage therapy for severe Gram-positive bacterial sepsis in hospitalized adult patients. BMC Infect Dis 13:66–72. doi: 10.1186/1471-2334-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crompton JA, North DS, McConnell SA, Lamp KC. 2009. Safety and efficacy of daptomycin in the treatment of osteomyelitis: results from the CORE® Registry. J Chemother 21:414–420. doi: 10.1179/joc.2009.21.4.414. [DOI] [PubMed] [Google Scholar]
- 25.Lamp KC, Friedrich LV. 2009. Safety and clinical outcomes of high-dose daptomycin (>6 mg/kg), abstr P1790. Abstr 19th Eur Congr Clin Microbiol Infect Dis, Helsinki, Finland. [Google Scholar]
- 26.Carugati M, Bayer AS, Miro JM, Park LP, Guimaraes AC, Skoutelis A, Fortes CQ, Durante-Mangoni E, Hannan MM, Nacinovich F, Fernandez-Hidalgo N, Grossi P, Tan RS, Holland T, Fowler VG Jr, Corey RG, Chu VH, International Collaboration on Endocarditis. 2013. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Antimicrob Agents Chemother 57:6213–6222. doi: 10.1128/AAC.01563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.