Abstract
Highly drug-resistant Salmonella enterica serovar Indiana became the most common serovar in broilers with diarrhea in China over the course of this study (15% in 2010 to 70% in 2014). While most S. Indiana isolates (87%, 384/440) were resistant to 13 to 16 of the 16 antibiotics tested, 89% of non-S. Indiana isolates (528/595) were resistant to 0 to 6 antibiotics. Class 1 integrons and IncHI2-type plasmids were detected in all S. Indiana isolates, but only in 39% and 1% of non-S. Indiana isolates.
TEXT
Poultry are major reservoirs of Salmonella enterica, which is an important food-borne pathogen that can cause gastroenteritis in people and animals. China is the second largest producer of broiler chickens in the world (1), and antibiotics have been widely used to prevent and treat frequently occurring enteric infections in broilers, although it has been noted that their effectiveness has decreased significantly in recent years (2). Salmonella enterica serovar Indiana is generally regarded to have a low frequency worldwide, where it has been associated with vomiting, diarrhea, fever, gastroenteritis, and acute cholecystitis in humans in North America and Europe (3–5) and an outbreak of abortion in ewes in Spain (6). The presence of S. Indiana in patients and retail raw chicken meat has been reported in China since 2009 (2, 7, 8).
In 2010, a study investigating Salmonella as a cause of enteritis in broilers was initiated by the Poultry Institute, Chinese Academy of Agricultural Sciences (PICAAS). Between January 2010 and December 2014, fecal samples from 2,758 broilers with diarrhea were submitted to our laboratory from 121 farms in the 12 major broiler breeding provinces in China: Beijing, Tianjin, Hebei, Hubei, Henan, Shandong, Jiangsu, Shanghai, Anhui, Zhejiang, Guangdong, and Guangxi. Each sample was analyzed according to the international standard ISO 6579:2002/Amd 1:2007, and isolates were serotyped as described previously (9).
Over the 5-year study, 1,023 Salmonella enterica isolates were made from broilers on 121 farms, the five most prevalent serovars being S. Indiana (n = 428; 42%), S. Typhimurium (n = 168; 16%), S. Enteritis (n = 147; 14%), S. Pullorum (n = 108; 11%), and S. Heidelberg (n = 48; 5%). At the beginning of the study in 2010, S. Indiana was the fourth most common serovar found, but it has become more prevalent over the years and represented more than 70% of the isolates made in 2014 (Fig. 1), completely replacing the traditionally dominant serovars of poultry such as S. Typhimurium and S. Enteritis.
FIG 1.
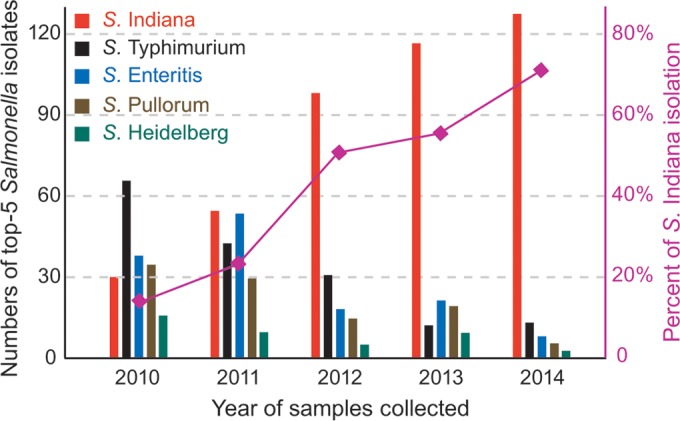
The five most prevalent Salmonella serovars from broilers with diarrhea, 2010-2014. The percentage of S. Indiana among Salmonella serovars rose from 15% in 2010 to 70% in 2014.
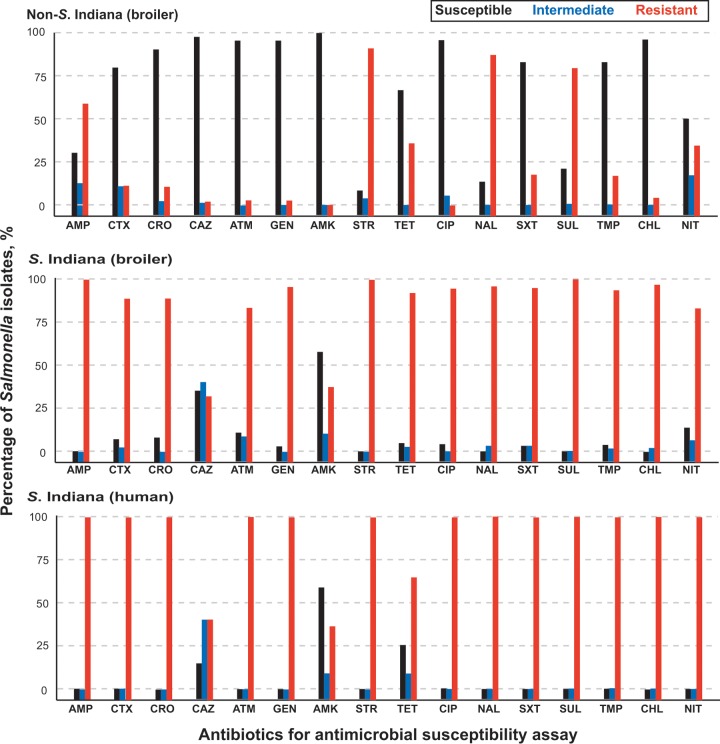
We determined the antimicrobial susceptibility of S. Indiana isolates using the disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines (10) and found high proportions of the isolates to be resistant to ampicillin (100%), streptomycin (100%), sulfafurazole (100%), chloramphenicol (99%), nalidixic acid (98%), trimethoprim-sulfamethoxazole (97%), gentamicin (97%), ciprofloxacin (97%), trimethoprim (96%), tetracycline (93%), cefotaxime (92%), ceftriaxone (92%), aztreonam (83%), and nitrofurantoin (81%) (Fig. 2). Relatively lower proportions were resistant to amikacin (34%) and ceftazidime (29%). The high levels of resistance against all antibiotics tested that we found in S. Indiana isolates contrasted with the consistently lower levels of resistance found in the non-S. Indiana isolates we obtained in this study (Fig. 2). Of particular note was the finding that 97% of the S. Indiana isolates were resistant to ciprofloxacin and 92% were resistant to ceftriaxone, which are commonly used to treat severe non-Typhi Salmonella (NTS) infections in adults and children, respectively (11). This is a major public health concern.
FIG 2.
Antimicrobial resistance to 16 antibiotics of Salmonella isolates from broilers and poultry workers with diarrhea. The columns denote the percentages of 595 non-S. Indiana isolates (top) from broilers, 428 S. Indiana isolates (middle) from broilers, and 12 S. Indiana isolates (bottom) from poultry workers that were resistant, intermediate, or susceptible to 16 common antibiotics. AMP, ampicillin; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; ATM, aztreonam; GEN, gentamicin; AMK, amikacin; STR, streptomycin; TET, tetracycline; CIP, ciprofloxacin; NAL, nalidixic acid; SXT, trimethoprim-sulfamethoxazole; SUL, sulfafurazole; TMP, trimethoprim; CHL, chloramphenicol; NIT, nitrofurantoin.
Multidrug resistance was common in our S. Indiana isolates with all strains being resistant to at least 9 of the 16 antibiotics and 6 being resistant to all 16 (Table 1). In total, we identified 26 multiple resistance patterns with isolates resistant to 13 or more antibiotics being the most prevalent (87%). In contrast, 89% of non-S. Indiana isolates (528/595) were resistant to 0 to 6 antibiotics.
TABLE 1.
Multidrug resistance patterns of avian S. Indiana isolates in this study
| No. of antibiotics | Multidrug resistance patterna | No of isolatesb | Resistant rate (%) |
|---|---|---|---|
| 9 | AMP-STR-SUL-SXT-GEN-TMP-TET-NIT-AMK | 5 | 2.1 |
| AMP-STR-SUL-CHL-NAL-SXT-CIP-TMP-TET | 4 | ||
| 10 | AMP-STR-SUL-CHL-SXT-GEN-TMP-TET-NIT-AMK | 3 | 1.2 |
| AMP-STR-SUL-CHL-NAL-SXT-CIP-TMP-TET-NIT | 1 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET | 1 | ||
| 11 | AMP-STR-SUL-CHL-NAL-GEN-CIP-TET-CTX-CRO-ATM | 3 | 4.9 |
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-ATM | 1 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-NIT | 17 | ||
| 12 | AMP-STR-SUL-CHL-NAL-GEN-CIP-TET-CTX-CRO-ATM-NIT | 9 | 4.9 |
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-CTX-CRO-ATM-NIT | 4 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-NIT-AMK | 3 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO | 5 | ||
| 13 | AMP-STR-SUL-CHL-NAL-SXT-TMP-TET-CTX-CRO-ATM-NIT-CAZ | 5 | 15.4 |
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TET-CTX-CRO-ATM-NIT | 1 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-CTX-CRO-ATM-NIT | 12 (1) | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM | 16 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-NIT | 32 | ||
| 14 | AMP-STR-SUL-CHL-NAL-SXT-CIP-TMP-TET-CTX-CRO-ATM-NIT-CAZ | 2 | 30.4 |
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-CTX-CRO-ATM-NIT-CAZ | 13 (3) | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-CAZ | 11 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-AMK | 7 | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-NIT | 97 (2) | ||
| 15 | AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-AMK-CAZ | 35 | 39.7 |
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-NIT-CAZ | 50 (2) | ||
| AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-NIT-AMK | 85 (4) | ||
| 16 | AMP-STR-SUL-CHL-NAL-SXT-GEN-CIP-TMP-TET-CTX-CRO-ATM-NIT-AMK-CAZ | 6 | 1.4 |
Abbreviations: AMP, ampicillin; STR, streptomycin; SUL, sulfafurazole; CHL, chloramphenicol; NAL, nalidixic acid; SXT, trimethoprim-sulfamethoxazole; GEN, gentamicin; CIP, ciprofloxacin; TMP, trimethoprim; TET, tetracycline; CTX, cefotaxime; CRO, ceftriaxone; ATM, aztreonam; NIT, nitrofurantoin; AMK, amikacin; CAZ, ceftazidime.
In parentheses are the resistance patterns of 12 S. Indiana isolates from poultry workers (i.e., the number of human isolates).
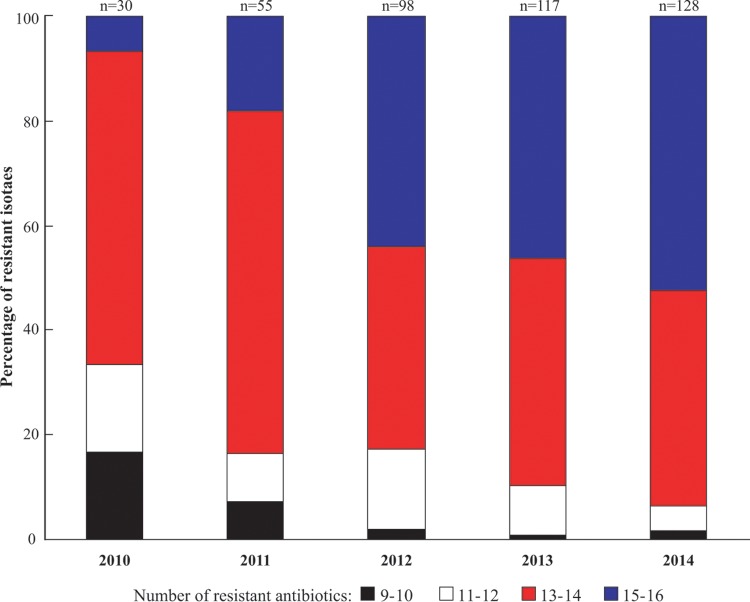
Analysis of antibiograms over the years of the study showed a trend of increasing resistance of the S. Indiana isolates to cefotaxime and ceftriaxone (67% in 2010 to 98% in 2014) and to aztreonam (43% in 2010 to 91% in 2014) and the development of more widespread multidrug-resistant strains. It was noteworthy that the proportion of S. Indiana isolates increased as the levels of antibiotic resistance rose (Fig. 3). Earlier studies showed that S. Indiana isolates were generally sensitive to antibiotics, being generally resistant to less than 6 antibiotics (6, 12, 13). The reasons for the marked increase in resistance we observed over the course of our study could not be established, but it appears likely to be driven by drug selection pressure. China is the largest user of antibiotics the in world (14), and it is known that large quantities of antibiotics are used in livestock production in China due to the lack of antibiotic control measures (15). Although veterinary use of cefotaxime, ceftriaxone, and aztreonam is prohibited, our S. Indiana isolates showed high levels of resistance to these antibiotics. It is possible that the use of antibiotics permitted in veterinary practice, mainly that of the cephalosporin ceftiofur, leads to extended-spectrum β-lactamase (ESBL) resistance that has an extended hydrolysis spectrum directed toward third-generation cephems and aztreonam (16).
FIG 3.
Changes in multidrug resistance patterns among S. Indiana isolates from poultry (2010-2014). The columns denote the percentages of 428 avian S. Indiana isolates that were resistant to 9 or 10 antibiotics, 11 or 12 antibiotics, 13 or 14 antibiotics, and 15 or 16 antibiotics.
Toward of the end of our study (March 2014), fecal samples were collected from 19 poultry workers with diarrhea who were employed to feed the broilers and clean the poultry houses on 5 S. Indiana-prevalent farms (4 in Jiangsu and 1 in Anhui). S. Indiana was isolated from 12 of 19 (63%) poultry workers with no other Salmonella serovars being found. The antimicrobial susceptibilities of the 12 S. Indiana human isolates were very similar to those of the poultry isolates, with six isolates resistant to 15 drugs, five isolates resistant to 14 drugs, and one isolate resistant to 13 drugs (Table 1, Fig. 2). The resistance patterns of the human isolates were identical to those of the 2014 poultry isolates (n = 19) from the farms where the poultry workers were employed. Genotyping of the above 31 S. Indiana isolates (12 human and 19 chicken) using multilocus sequence typing as previously described (17) showed that all 31 S. Indiana isolates belong to the sequence type ST17. This indicates the possible transmission of S. Indiana between chickens and poultry workers. To our best knowledge, this is the first report of the isolation of S. Indiana from poultry workers and chickens on the farms where they worked.
To investigate the molecular basis of the antimicrobial resistance in the Salmonella strains we isolated, PCRs and genomic sequencing were carried out to determine the presence of integrons, Salmonella genomic island 1 (SGI1) variants, and plasmids as described previously (18–20). All poultry and human S. Indiana isolates and 39% of non-S. Indiana isolates (233/595) were found to carry class 1 integrons. SGI1 variants were not identified in any S. Indiana isolates but were identified in 3% of non-S. Indiana isolates (19/595) (S. Kentucky, S. Albany, and S. Saintpaul). The PCR-based replicon typing assay showed that all S. Indiana isolates carried IncHI2-type plasmids, which are known to be transferable and play a key role in the acquisition of antibiotic resistance (21, 22). In contrast, only 1% of the non-S. Indiana isolates (6 S. Typhimurium/595) contained the IncHI2-type plasmids. The above data suggest that the high-level antimicrobial resistance that we found in S. Indiana could have been associated with the presence of class 1 integrons and IncHI2 plasmids.
In conclusion, our study shows that S. Indiana became the clearly dominant serovar found in broilers with diarrhea over the period 2010 to 2014 in China. Poultry workers were also infected with S. Indiana on some farms with both the broiler and human isolates having identical sequence types. Our S. Indiana isolates had high levels of multidrug resistance with most being resistant to the antibiotics commonly used to treat Salmonella infections. This might have been a result of the high levels of class 1 integrons and IncHI2 plasmids we found in the S. Indiana isolates. Health workers and animal workers need to be alerted to the possibility of infections with multidrug-resistant S. Indiana, and further studies are indicated to explore the mechanisms for development of multidrug resistance in S. Indiana isolates.
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers KU288613 to KU288633.
REFERENCES
- 1.Suo X, Zhang JX, Li ZG, Yang CT, Min QR, Xu LT, Liu Q, Zhu XQ. 2006. The efficacy and economic benefits of Supercox, a live anticoccidial vaccine in a commercial trial in broiler chickens in China. Vet Parasitol 142:63–70. doi: 10.1016/j.vetpar.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Zhao H, Sun J, Liu Y, Zhou X, Beier RC, Wu G, Hou X. 2014. Characterization of multidrug-resistant Salmonella enterica serovars Indiana and Enteritidis from chickens in Eastern China. PLoS One 9:e96050. doi: 10.1371/journal.pone.0096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price J Jr, Carter HR Jr. 1967. An outbreak of gastroenteritis caused by Salmonella Indiana. Public Health Rep 82:551–554. doi: 10.2307/4593068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell CW, Eckman MR. 1975. Acute acalculous cholecystitis caused by Salmonella Indiana. JAMA 233:815. doi: 10.1001/jama.1975.03260070073030. [DOI] [PubMed] [Google Scholar]
- 5.Beckers HJ, Daniels-Bosman MSM, Ament A, Daenen J, Hanekamp AWJ, Knipschild P, Schuurmann AHH, Bijkerk H. 1985. Two outbreaks of salmonellosis caused by Salmonella Indiana: a survey of the European summit outbreak and its consequences. Int J Food Microbiol 2:185–195. doi: 10.1016/0168-1605(85)90038-8. [DOI] [Google Scholar]
- 6.Luque I, Echeita A, León J, Herrera-León S, Tarradas C, González-Sanz R, Huerta B, Astorga RJ. 2009. Salmonella Indiana as a cause of abortion in ewes: genetic diversity and resistance patterns. Vet Microbiol 134:396–399. doi: 10.1016/j.vetmic.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Xia S, Hendriksen RS, Xie Z, Huang L, Zhang J, Guo W, Xu B, Ran L, Aarestrup FM. 2009. Molecular characterization and antimicrobial susceptibility of Salmonella isolates from infections in humans in Henan Province, China. J Clin Microbiol 47:401–409. doi: 10.1128/JCM.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang B, Qu D, Zhang X, Shen J, Cui S, Shi Y, Xi M, Sheng M, Zhi S, Meng J. 2010. Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shanxi, China. Int J Food Microbiol 141:63–72. doi: 10.1016/j.ijfoodmicro.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Gong J, Zhang J, Xu M, Zhu C, Yu Y, Liu X, Kelly P, Xu B, Wang C. 2014. Prevalence and fimbrial genotype distribution of poultry Salmonella isolates in China (2006 to 2012). Appl Environ Microbiol 80:687–693. doi: 10.1128/AEM.03223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK, Infectious Diseases Society of America. 2001. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 12.Vo AT, van Duijkeren E, Fluit AC, Wannet WJ, Verbruggen AJ, Maas HM, Gaastra W. 2006. Antibiotic resistance, integrons and Salmonella genomic island 1 among non-typhoidal Salmonella serovars in The Netherlands. Int J Antimicrob Agents 28:172–179. doi: 10.1016/j.ijantimicag.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Punia P, Hampton MD, Ridley AM, Ward LR, Rowe B, Threlfall EJ. 1998. Pulsed-field electrophoretic fingerprinting of Salmonella indiana and its epidemiological applicability. J Appl Microbiol 84:103–107. doi: 10.1046/j.1365-2672.1997.00325.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. 2015. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 15.Hvistendahl M. 2012. Public health. China takes aim at rampant antibiotic resistance. Science 336:795. doi: 10.1126/science.336.6083.795. [DOI] [PubMed] [Google Scholar]
- 16.Shiraki Y, Shibata N, Doi Y, Arakawa Y. 2004. Escherichia coli producing CTX-M-2 β-lactamase in cattle, Japan. Emerg Infect Dis 10:69–75. doi: 10.3201/eid1001.030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbottle H, White DG, McDermott PF, Walker RD, Zhao S. 2006. Comparison of multilocus sequence typing, pulsed-field gel electrophoresis, and antimicrobial susceptibility typing for characterization of Salmonella enterica serotype Newport isolates. J Clin Microbiol 44:2449–2457. doi: 10.1128/JCM.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doublet B, Praud K, Bertrand S, Collard JM, Weill FX, Cloeckaert A. 2008. Novel insertion sequence and transposon-mediated genetic rearrangements in genomic island SGI1 of Salmonella enterica serovar Kentucky. Antimicrob Agents Chemother 52:3745–3754. doi: 10.1128/AAC.00525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J, Wang Y, Shen J, Li R, Han J, Foley SL, Wu C. 2013. Unique class 1 integron and multiple resistance genes co-located on IncHI2 plasmid is associated with the emerging multidrug resistance of Salmonella Indiana isolated from chicken in China. Foodborne Pathog Dis 10:581–588. doi: 10.1089/fpd.2012.1455. [DOI] [PubMed] [Google Scholar]
- 20.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Liao X, Yang Y, Sun J, Li L, Liu B, Yang S, Ma J, Li X, Zhang Q, Liu Y. 2013. Spread of oqxAB in Salmonella enterica serotype Typhimurium predominantly by IncHI2 plasmids. J Antimicrob Chemother 68:2263–2268. doi: 10.1093/jac/dkt209. [DOI] [PubMed] [Google Scholar]
- 22.Cain AK, Hall RM. 2012. Evolution of IncHI2 plasmids via acquisition of transposons carrying antibiotic resistance determinants. J Antimicrob Chemother 67:1121–1127. doi: 10.1093/jac/dks004. [DOI] [PubMed] [Google Scholar]