Abstract
Melanoma is an aggressive disease with limited therapeutic options. Here, we determined the effects of honokiol (HNK), a biphenolic natural compound on melanoma cells and stemness. HNK significantly inhibited melanoma cell proliferation, viability, clonogenicity and induced autophagy. In addition, HNK significantly inhibited melanosphere formation in a dose dependent manner. Western blot analyses also demonstrated reduction in stem cell markers CD271, CD166, Jarid1b, and ABCB5. We next examined the effect of HNK on Notch signaling, a pathway involved in stem cell self-renewal. Four different Notch receptors exist in cells, which when cleaved by a series of enzymatic reactions catalyzed by Tumor Necrosis Factor-α-Converting Enzyme (TACE) and γ-secretase protein complex, results in the release of the Notch intracellular domain (NICD), which then translocates to the nucleus and induces target gene expression. Western blot analyses demonstrated that in HNK treated cells there is a significant reduction in the expression of cleaved Notch-2. In addition, there was a reduction in the expression of downstream target proteins, Hes-1 and cyclin D1. Moreover, HNK treatment suppressed the expression of TACE and γ-secretase complex proteins in melanoma cells. To confirm that suppression of Notch-2 activation is critical for HNK activity, we overexpressed NICD1, NICD2, and performed HNK treatment. NICD2, but not NICD1, partially restored the expression of Hes-1 and cyclin D1, and increased melanosphere formation. Taken together, these data suggest that HNK is a potent inhibitor of melanoma cells, in part, through the targeting of melanoma stem cells by suppressing Notch-2 signaling.
Keywords: Cancer stem cells, autophagy, cell cycle arrest, Notch-1, Notch-2
INTRODUCTION
The incidence of melanoma has increased dramatically over the last few decades, making it one of the fastest growing malignancies in the United States [1,2]. Melanoma is an aggressive form of skin cancer that has limited therapeutic options. Furthermore, melanoma expresses a plastic and multipotent phenotype similar to embryonic stem cells [3]. Unlike embryonic stem cells, however, melanoma cells lack major regulatory “checks and balance” mechanisms due to the aberrant activation of stem cell signaling pathways, which trigger their plastic phenotype, uncontrolled growth, and aggressive behavior [3]. Within the past decade, the paradigm for tumor development has evolved, with our increased understanding that tumors, like normal adult tissues, contain stem cells, termed cancer stem cells (CSCs), which initiate and support tumor growth and maintenance. Therefore, understanding the signals and the regulatory pathways within CSCs lends to a significant potential for more effective treatment of melanomas.
In the context of disrupting signaling pathways with CSCs, aberrant activation of the Notch signaling pathway has been implicated in a number of malignancies [4]; however, there is little known about the role of Notch signaling in melanoma. Notch signaling is an evolutionarily conserved pathway that has been implicated in normal embryonic development, organ development and the regulation of self-renewal in adult stem cells, thereby maintaining tissue homeostasis [5,6]. In mammals, the Notch receptor family consists of four receptors (Notch-1–-4) and five ligands (Delta-like-1, Delta-like-3, Delta-like–4, Jagged-1, and Jagged–2). All four Notch receptors show some common structural similarity. Each Notch receptor can be activated by cell membrane-associated ligands [7]. Activation and maturation of Notch receptors involve different proteolytic cleavage events. The first cleavage is catalyzed by ADAM-family metalloprotease TACE [8] followed by second cleavage mediated by γ-secretase, an enzyme complex that contains presenilin, nicastrin, presenilin enhancer 2 (PEN2), and anterior pharynx-defective 1 (APH1) [9]. This coordinated cleavage results in the release and translocation of the NICD into the nucleus. The active NICD can bind to activator proteins, such as recombination signaling binding protein-J (RBPJ) and mastermind-like proteins (MAML), and form a nuclear transcriptional activator complex to regulate transcription of downstream target genes, such as the hairy and enhancer of split (Hes) gene, Hey family genes, c-myc, cyclin D1, and p21/Waf1 [7,10,11]. Recent studies have reported elevation in Notch signaling in melanoma cell lines and melanoma patient tissue samples as compared to common melanocytic lesions [12]. In addition, activation of Notch signaling was also shown to promote the survival of melanocyte precursor cells, melanoblasts (Mbs) and melanoma stem cells (MSC) [13]. Therefore, Notch signaling appears to be a promising potential therapeutic target for the treatment of melanoma.
New agents that target the Notch signaling pathway, effectively killing the CSC population, could, as a single therapy or in combination with current anti-tumor drugs, be more effective in controlling tumor growth, halting tumor progression, and improving patient outcomes. Honokiol (HNK), a biphenolic compound derived from Magnolia officianalis, is used in traditional Chinese and Japanese medicine for the treatment of various ailments including ulcers, allergies, and bacterial infections. It is also used as a muscle relaxant and possesses antithrombotic activity [14,15]. Additionally, recent studies have shown that it has anti-tumor activity with low toxicity to normal tissue [16]. In this article, we have determined the effects of HNK on melanoma cells and melanoma stem cell growth, as mediated through the Notch signaling pathway.
MATERIALS AND METHODS
Cells and Reagents
B16/F-10 and SKMEL-28 melanoma cell lines were procured from American Type Culture Collection (ATCC, Manassas, VA). B16/F-10 (a highly metastatic) and SKMEL-28 cells (a malignant melanoma with homozygous BRAFV600E mutation) were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with FBS (Sigma–Aldrich., St. Louis, MO) and antibiotic-antimycotic solution (Mediatech Inc., Manassas, VA) at 37°C in a humidified atmosphere containing 5% CO2. Cells used in this study were within 18 passages after receipt or renewal. Growth medium was changed after every three days and cells were split in 1:6 ratios when they reached 70–80% of confluence. For HNK (Sigma Aldrich) treatment, stock solution of HNK was prepared in DMSO, stored at −20°C in aliquots, and diluted with fresh medium immediately before use. Other general chemicals were purchased from Sigma–Aldrich.
Cell Proliferation Assay in Two-Dimensional Culture
Hexosaminidase assay was used to study the effects of HNK on proliferation of melanoma cells [17]. In brief, cells were plated in 96 well plates, grown over night and treated next day with increasing concentrations of HNK (0–60 μM) for up to 72 h. Cell proliferation was calculated as percent proliferation rate = [(A/B)× 100], where A and B are the absorbance of treated and control cells, respectively. The best fit was used for further processing of data.
Cell Viability Assay
Cell viability of melanoma cells after HNK treatment was studied by Ghost Red 780 Dye staining, detected by flow cytometry. Ghost Dyes bind irreversibly to amine groups and are resistant to subsequent washing, fixation and permeabilization. Dead cells with compromised membranes allow Ghost Dye to permeate and bind amine groups of intracellular proteins resulting in fluorescence much brighter than live cells which are impermeant to Ghost Dye. In brief, cells were plated and grown over night in six well culture plates. Cells were treated with increasing concentrations of HNK (0–50 μM) for different time intervals. After HNK treatment, cells were washed twice with 2 ml of sodium azide and protein/serum free PBS. Cells were centrifuged at 400 g for 5 min at room temperature and re-suspended in sodium azide and protein/serum free PBS. Appropriate amount of Ghost dye was added to 1 ml of cell suspension and vortexed immediately. Cells were incubated for 30 min a 4 °C. Cells were washed twice with 1 ml of stain buffer (1X PBS with 2% FBS and 0.9% sodium azide). Finally cells were subjected to flow cytometry in FACSVerse (BD Biosciences., San Jose, CA), capturing 10,000 events for each sample. Results were analyzed with BD FACSuite software (BD Biosciences.). Ghost dye was also used to determine the viability of cells isolated from primary spheroids.
Clonogenicity Assay
To study the long-term effects of HNK on melanoma cells, colony formation assay was done [18]. In this assay, cells grown in six well plates were treated with different concentrations of HNK (0–50 μM) for different time intervals. Subsequently, medium was removed, and cells were replenished with fresh medium lacking the compound and allowed to grow for 7–8 d to form colonies. The colonies were formalin fixed and stained with 0.4% (w/v) crystal violet dye. Plates were washed and dried for further counting. Colonies were counted using CellCounterv0.2.1 by Nghia Ho available online. The colonies were counted and compared with their respective controls.
Cell-Cycle Analyses
Effect of HNK treatment on cell cycle progression in melanoma cell lines was determined by Propidium Iodide (PI)/RNase staining method detected by flow cytometry. Cells were treated with increasing concentrations of HNK (0–40 μM) for up to 48 h. After HNK treatment, cells were washed with PBS, trypsinized, washed twice with ice cold PBS, fixed in 70% ethanol (in PBS) and stored at −20°C until further use. For staining, cells were washed with ice cold PBS and finally stained with PI/RNase staining buffer for 30 min at 4°C. After 30 min, cells were centrifuged, washed and re-suspended in ice cold PBS. Finally, cells were subjected to flow cytometry in BD LSR II (BD Biosciences.), capturing 10,000 events for each sample. Results were analyzed with ModFit LT software (Verity Software House, Topsham, ME).
Transmission Electron Microscopy (TEM)
Cells treated with HNK for 48 h were fixed in 0.1 M sodium cacodylate buffer containing 2% glutaraldehyde for 4 h at room temperature. The samples were post-fixed in 1% osmium tetroxide for 1.5 h and washed with 0.1 M sodium cacodylate buffer, followed by dehydration in a series of ethanol dilutions (50–100%) for 15 min each. The cells were finally incubated with propylene oxide for 15 min. Subsequently, cells were treated with a solution of half propylene and half EmBed 812 resin medium (Electron Microscopy Sciences., Hatfield, PA). Finally, 80 nm sections were cut using a Leica UC-7 ultramicrotome. Sections were stained first with 4% uranyl acetate followed by Sato's Lead stain and finally observed on a JEOL JEM-1400 TEM at 80 KV for at 1000–4000× magnifications [19]. To capture the double wall of autopgahosomes, images were taken at higher magnification (≤20,000×).
Spheroid Formation Assay
Spheroid formation assay was performed as previously described elsewhere [20]. In brief, cells were plated in ultralow attachment plates (Corning Inc., Corning, NY) at a density of 5000 cells/ml in DMEM supplemented with 1% N2 supplement (Life Technologies., Grand Island, NY), 2% B27 supplement (Life Technologies.), 20 ng/ml human platelet growth factor (Sigma–Aldrich) 100 ng/ml epidermal growth factor (Life Technologies.) and 1% antibiotic-antimycotic (Mediatech Inc.) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Primary spheroids were photographed and counted after 6–7 days of HNK (0–40 μM) treatment. For secondary spheroid culture, primary spheroids were collected, dissociated into single cell suspension, filtered through cell strainer, counted by Millipore cell counter and re-plated in ultra-low attachment plates. Ghost dye was also used to determine the viability of cells isolated from primary spheroids. Secondary cultures were grown in the absence of HNK, and processed as above. Secondary spheroids were photographed and counted.
Immunoblot Analyses
Cell lysates were prepared after HNK treatment and quantified by using Pierce BCA protein assay kit (Thermo Scientific., Chicago, IL). Cell lysates were subjected to SDS-PAGE and blotted onto nitro cellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and proteins of interest were detected by using enhanced chemiluminescence system (GE Healthcare Bio-Sciences Corp.). Antibodies for immunoblotting were purchased from Abcam (Cambridge, MA) [CD166: Cat# ab109215; CD271: Cat# ab8875; Jarid1B: Cat# ab56759], Genscript Inc. (Piscataway, NJ) [Nicastrin: Cat# A00883–40; PEN2: Cat# A00882; Presenilin 2: Cat# A00943], Bioss Inc. (Woburn, MA) [ABCB5: Cat# bs-1604R] Cell Signaling Technology Inc. (Danvers, MA) [Caspase-3: Cat# 9665; LC3B: Cat# 3868; CyclinD1: Cat# 2978; Hes-1: Cat# 11988S; Nicastrin: Cat# 5665; Notch1: Cat# 4380; Notch2: Cat# 5732; Presenilin 2: Cat# 9979; TACE: Cat# 6978], Santa Cruz Biotechnology Inc. (Dallas, TX) [β-Actin: Cat# sc-1616; Cat# sc-5274; Nestin: Cat# sc-20978]. For demonstrating equal loading of protein, the blots were normalized for β-actin levels.
Immunofluorescence Staining
Cells were plated overnight on sterile glass coverslips in six well plates. After 48 h of HNK (30–40 μM) treatment cells were fixed with 4% paraformaldehyde for 15 min, rinsed with PBS, and incubated with 2% BSA in PBS for 30 min. The cells were then incubated overnight with anti-α-tubulin, anti-LC3B, anti-Notch-2 and anti-cyclin D1 antibodies respectively. After washing with PBS, the cells were incubated with fluorophore-conjugated secondary antibody for 30 min and washed with PBS. Cell images were observed under a fluorescent microscope.
Hes-1 Reporter Assay
Cells were plated and transfected with Hes-1A/B-Luc, a kind gift of Dr. Kimberly Foreman, Loyola University, Chicago [21], which encode firefly luciferase gene under the control of a single Hes1 (HES1 BS) binding site using Lipofectamine 2000 (Invitrogen, NY). Renilla luciferase expressing pRL-TK plasmid (Clontech, Mountain View, CA) was used as internal control. Luciferase levels in the cell lysates were determined using Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI) Cells were treated with HNK (0–40 μM) for 24 h after 48 h of transfection.
Plasmids and Transfections
For NICD overexpression, B16/F-10 cells were transfected with 3XFlagNICD1 or 3XFlagNICD2 plasmids (purchased from Addgene Inc., Cambridge, MA) and subsequently treated with HNK. For internal control, cells were transfected with p3XFLAG-CMV-7 (an empty vector) (gifted by Dr. Chris Lau, Department of Medicine VA Medical Center, San Francisco). After HNK treatment, cells were used for various experiments to study the NICD overexpression effects on HNK treated cells.
Statistical Analyses
All values are expressed as the mean ± SD. Data was analyzed using an unpaired 2-tailed t test. A P-value of <0.05 was considered statistically significant.
RESULTS
Honokiol Affects Proliferation, Viability and Clonogenic Potential of Melanoma Cells
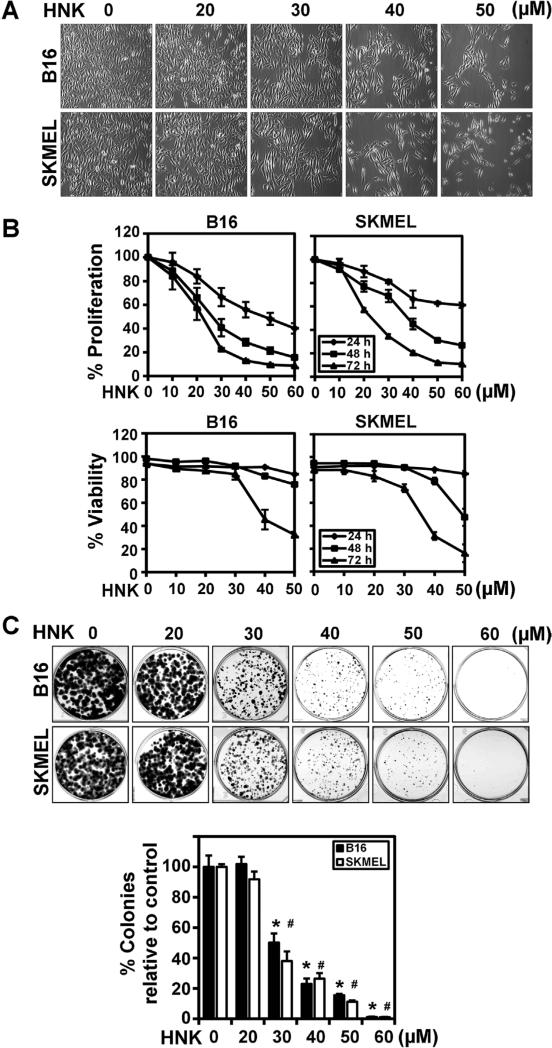
To evaluate the potential use of HNK in treatment of melanoma, we initially determined the effects on morphology, proliferation rate and viability of B16/F-10 and SKMEL-28 melanoma cells. HNK treatment resulted in a dose-dependent (0–50 μM) change in cellular morphology with a stretched morphology at lower concentrations and rounding of cells at higher HNK concentrations (Figure 1A). These changes were accompanied by a decrease in cell proliferation rate with significant effects observed at 48 and 72 h (Figure 1B). We further studied the effects of HNK on viability of melanoma cells measured by Ghost Red 780 Dye staining followed by flow cytometry (Figure 1B). HNK showed dose-dependent decrease in viability of melanoma cells with significant cell death observed after 48 h. HNK effects on cell proliferation appeared to be higher than on cell viability. We next sought to identify the mechanism by which HNK affects melanoma cell growth, exploring issues such as loss of reproductive integrity and ability to proliferate. A cell that has acquired the potential to divide and proliferate endlessly can produce a colony of cells and is referred to as “clonogenic”. To confirm that the effects observed following HNK treatment were not transient, we determined the long-term effects of HNK on melanoma cell lines by performing a clonogenicity assay. For this, cells were treated with varying concentrations of HNK for up to 72 h and then allowed to grow and form colonies. HNK significantly inhibited the clonogenic potential of both melanoma cell lines in a dose (Figure 1C) and time dependent manner (data not shown). These data suggest that HNK not only inhibited the growth of melanoma cells by decreasing cell proliferation rate but also induced the cell death.
Figure 1.
HNK inhibit growth and clonogenic potential of melanoma cells. (A) Representative photomicrographs of B16/F-10 and SKMEL-28 cells after 24 h of HNK treatment (magnification 20×). Cells were seeded in 6 well plates, allowed to grow overnight and treated with increasing concentrations of HNK (0–50 μM) for 24 h. Significant changes in cell morphology were observed in both the cell lines. (B) Cell proliferation assay showed HNK induced growth inhibitory effects on melanoma cells. Cells were treated with increasing concentrations of HNK (0–60 μM) for 24, 48, and 72 h. Experiments were conducted at n = 6, and repeated at least three times. The data was analyzed as percent of control, where the control wells were treated with equivalent amounts of DMSO alone, and the analyzed data was presented as average ± SD. The differences among mean values were deemed significant at P < 0.05. HNK decrease cell proliferation in dose and time-dependent manner (P < 0.05). Cell proliferation assay showed HNK mediated decrease in viability of melanoma cells. Cells were treated with increasing concentrations of HNK (0–50 μM) for 24, 48, and 72 h. The data was analyzed as percent of control, where the control wells were treated with equivalent amounts of DMSO alone, and the analyzed data was presented as average ± SD. The differences among mean values were deemed significant at P < 0.05. (C) HNK inhibited the clonogenic potential of melanoma cells. Cells were incubated with increasing concentrations of HNK for 72 h. HNK significantly inhibited colony formation in cells in a dose and time dependent manner (data not shown). Results are representative of three independent experiments. Colonies were counted, analyzed and data was presented as an average ± SD. The differences among mean values were deemed significant at P < 0.05. (* #, P < 0.05).
Honokiol Induces Cell Cycle Arrest and Autophagy in Melanoma Cells
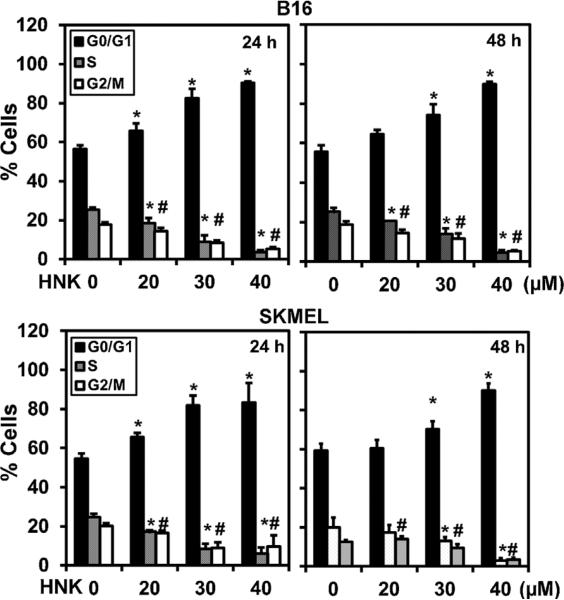
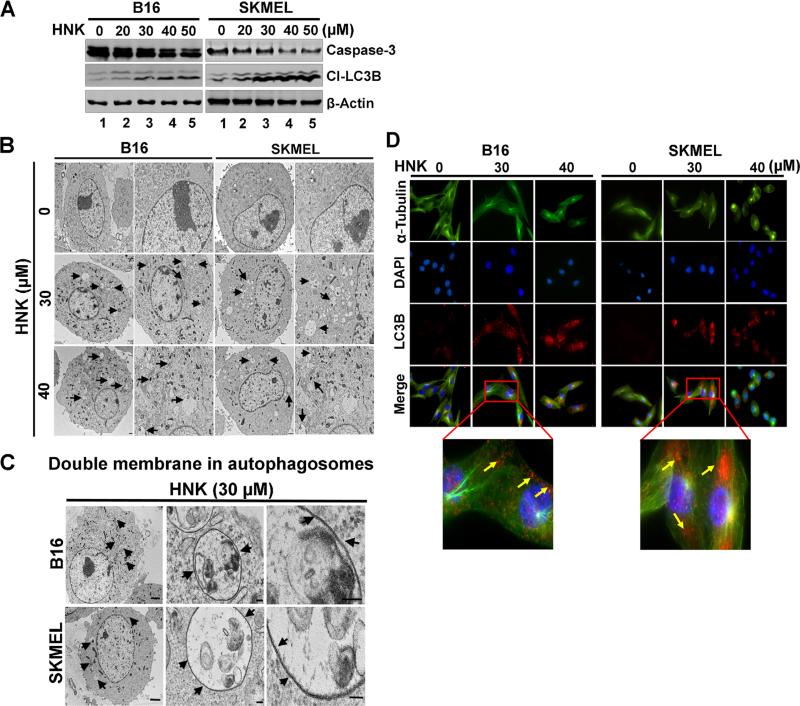
Due to the potent inhibitory effects of HNK on melanoma cell proliferation and survival, we next determined the mechanism of its action. First, we studied the effect of HNK on cell cycle progression. Flow cytometry analyses demonstrated that HNK arrested both cell lines in the G0/G1 phase of cell cycle (Figure 2). Inhibition of the cell cycle in G0/G1 was most effective at ≥30 μM concentrations (Figure 2). This was coupled with a reduction of cells in S-phase of cell cycle in both cell lines. Next, we explored the type of cell death induced by HNK in melanoma cells. First, we studied whether cell death occurred through the apoptotic pathway. Key effector molecules for apoptosis are activated caspase-3, and poly (ADP) ribosyl polymerase (PARP), which initiates events including DNA laddering and cellular morphologic changes. However, following HNK treatment, there was no cleaved caspase-3 (Figure 3A) or PARP-γ (data not shown), suggesting that HNK did not induce apoptotic cell death. Another potential mechanism of cell death is autophagy, which can be demonstrated by the presence of autophagosomes containing organelles, intact lamellar structure, cytoplasmic structure, and/or residual digested materials. Electron microscopy demonstrated the presence of these autophagic vacuoles following HNK treatment (Figure 3B). The micrographs at higher magnification further confirmed the presence of double walled autophagic vacuoles in both cells lines after HNK treatment (Figure 3C).
Figure 2.
HNK induce G0/G1 cell cycle arrest in melanoma cells. After 24 and 48 h of HNK (0–40 μM) treatment, cells were stained by PI/RNase staining buffer and analyzed by flow cytometry to quantify the cellular DNA content in cells. Data is percentage change normalized to control and the average of three replicate assays. HNK treatment significantly induced G0/G1 cell cycle arrest with 24 h of HNK treatment in both the cell types (*P < 0.05).
Figure 3.
HNK induce autophagy in melanoma cells. (A) Effect of HNK on cleavage of LC3B and caspase-3 in melanoma cells. Cells after 48 h of HNK (0–40 μM) treatment were lysed and used for western blot to study the cell death markers in melanoma cells. HNK induced autophagic cell death in melanoma cells that were evident from cleavage of LC3B in cells. However, cleavage of apoptotic marker, like caspase-3 and PAPR-γ (data not shown) was not detected after HNK treatment in any of the cell lines. (B) Transmission electron micrographs (TEM) of melanoma cells treated with HNK. After HNK (0–40 μM) treatment for 48 h, cells showed formation of numerous pre-autophagosomal, and autophagosomal vacuoles in cells. All images were taken at 2000 and 4000 magnifications. (C) Transmission electron micrographs [at higher magnification (≤20,000×)] of cells after HNK treatment showed double wall in autophagosomes. (D) Cells treated with 30 μM HNK for 48 h were subjected to immuno-fluorescent staining using specific antibodies for α-tubulin and cleaved LC3B proteins in cells. HNK treatment showed cytoplasmic accumulation of cleaved LC3B in both the cell types. Yellow arrows in lower panel showing LC3 staining in both the cells after HNK treatment (30 μM).
Another hallmark of autophagy is the activation of LC3B, an ubiquitin-like protein. Western blot and immunofluorescence analyses demonstrated a significant dose-dependent increase in cleaved LC3B in melanoma cells following HNK treatment (Figure 3A and D). The immunofluorescence analyses demonstrated increased cytoplasmic accumulation of cleaved LC3B (Figure 3D). These results suggest that HNK induces autophagic cell death in melanoma.
Honokiol Affects Melanoma Stem Cells
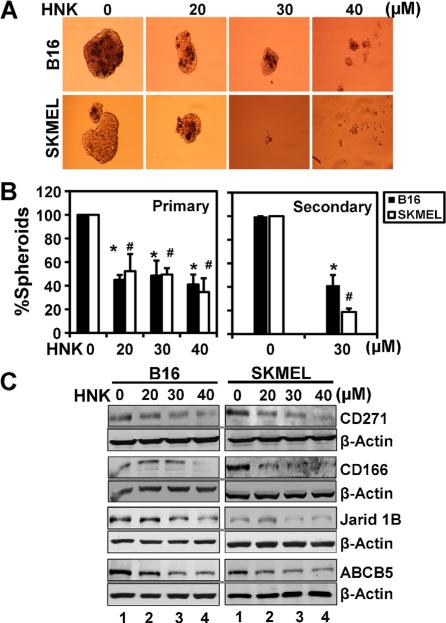
A small number of cells within a tumor, termed CSCs are undifferentiated and have an increased capacity for self-renewal [22]. Effect on CSCs can be determined by growth of melanospheres, which are non-adherent 3D spheroid bodies. Previous study from our group has demonstrated that HNK can affect CSCs in colon cancers [20]. Hence, we next determined whether HNK affects the growth of melanospheres. As shown in Figure 4A, HNK significantly inhibited the growth of primary melanospheres in a dose dependent manner. Cells isolated from primary spheroids were counted using Millipore automated cell counter and then plated to develop secondary melanospheres, but in the absence of any additional HNK. Cell viability after primary spheroid cultures was also assessed using Ghost Red 780 Dye staining by Flow cytometer (Supplementary Figure S1). The data shows that HNK suppressed the growth of secondary melanospheres (Figure 4B). Further confirmation of HNK effects on CSCs in melanoma cells was obtained by western blot analyses for markers of stem cells in melanoma CD271, CD166, Jarid1B, and ABCB5. Expression of melanoma stem cells markers was markedly reduced in cells by HNK treatment in dose dependent manner (Figure 4C). Together, these data suggest that HNK is a potent inhibitor of the melanoma stem cells in both cell lines.
Figure 4.
HNK affects melanoma stem cells. (A) to perform melanosphere formation assay, cells were grown in ultralow attachment plates and treated with increasing concentrations of HNK (0–50 μM). After 6–7d, the spheroids were photographed and counted. (B) HNK (30 μM) treatment significantly inhibited primary and secondary melanospheres in 3D culture (* #, P < 0.05). Primary melanospheres were counted and performed bar diagram. For secondary spheroids, primary melanospheres were collected after 6–7 d of HNK treatment and dissociated into single cell suspension and replated for secondary spheroid formation without HNK treatment. (C) HNK decreased the expression of melanoma stem cell markers in both the cell types. Western blot analyses of B16/F-10 and SKMEL-28 cells after HNK treatment showed decreased levels of CD271, CD166, Jarid1b, and ABCB5.
Honokiol Inhibits Notch Signaling in Melanoma Cells
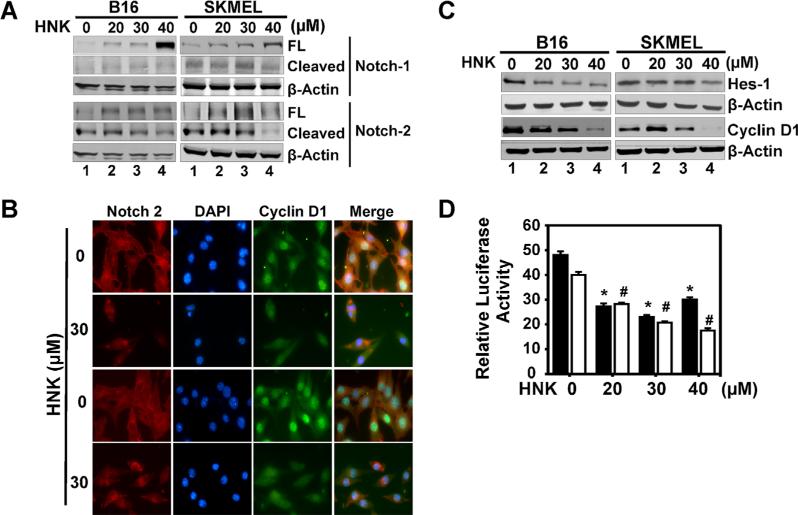
Aberrant activation of embryonic signaling pathways in cancer has been well documented. One critical pathway is the Notch signaling pathway, wherein specific ligands bind to one of four cell surface Notch receptors (Notch-1–Notch-4) and activate a series of proteolytic cleavage of the receptor resulting in release of an intracellular domain (NICD) that translocate to the nucleus and induces transcription of target genes. Western blot analyses demonstrated that HNK treatment resulted in reduced levels of cleaved Notch particularly Notch-2 receptor in the melanoma cells (Figure 5A). This was further confirmed by immunofluorescence staining of Notch-2 protein in melanoma cells. HNK (30 μM) significantly decreased Notch-2 staining in cells (Figure 5B). We also confirmed that the pathway is inhibited by western blot analyses for downstream target genes, Hes-1 and cyclin D1. HNK treatment resulted in significant reduction in both proteins in the two cell lines (Figure 5C). These results were further confirmed by NICD/Hes-1 promoter reporter assay (Figure 5D). HNK inhibited the Hes-1 reporter activity in a dose dependent manner in both cell lines (Figure 5D).
Figure 5.
HNK affects Notch signaling in melanoma cells. (A) HNK inhibited Notch signaling by decreasing the levels of cleaved Notch-2 receptor at 48 h of HNK treatment in melanoma cells in a dose dependent manner. (B) Cells treated with 30 μM of HNK for 48 h were subjected to immuno-fluorescent staining using antibodies for Notch-2 and cyclin D1. HNK treatment resulted in reduced levels of Notch-2 and cyclin D1 expression in cells. (C) Western blot analyses further showed decreased levels of Hes-1 and cyclin D1 Notch target proteins in cells. (D) Melanoma cells, transfected with HES-1 responsive luciferase plasmid, showed a HNK dependent reduction of luciferase activity in melanoma cells at 48 h of HNK treatment (* #, P < 0.05).
Inhibiting Notch Activation is Essential for Honokiol-Mediated Suppression of Melanoma Cells
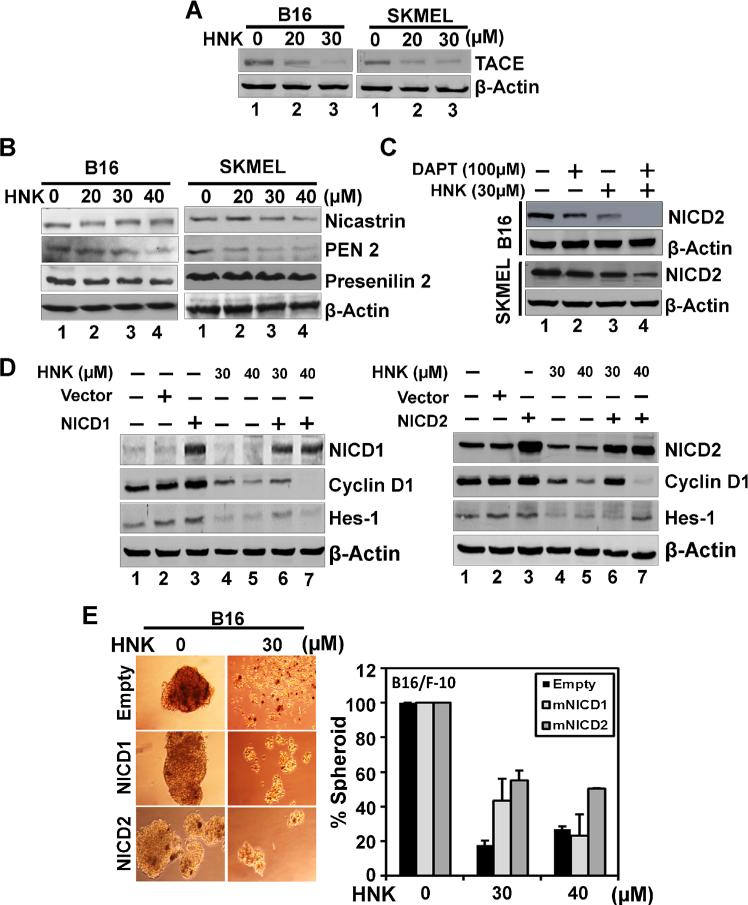
Two critical steps are essential for releasing NICD, the first being the cleavage of the extracellular domain by TACE enzyme resulting in a conformational change to the Notch receptor. This opens up the receptor to intracellular cleavage immediately inside the plasma membrane by the γ-secretase complex [8,9]. Given the suppression of Notch activation, we next determined the effect of HNK on the expression of these enzymes. Western blot analyses demonstrated that HNK treatment (0–30 μM) at 48 h significantly decreased the levels of both TACE (Figure 6A) and γ-secretase complex proteins (presenilin-1, presenilin-2, nicastrin, and PEN2) (Figure 6B). Specifically, HNK downregulated the expression of nicastrin and PEN2 in SKMEL-28 cells, while it affected presenilin-2 and PEN2 in B16/F-10 cells (Figure 6B). We further used DAPT (γ-secretase inhibitor) in both cells lines to support above data. DAPT alone decreased the levels of cleaved Notch2 receptor in both the cell lines. This change was more in B16/F-10 cells as compared to SKMEL-28 cells (Figure 6C). Interestingly, HNK in combination with DAPT was more potent in inhibiting the cleavage of Notch2 receptor. These data suggest that HNK–mediated down regulation of the Notch signaling cascade occurs, in part, through the inhibition of the TACE and γ-secretase complex.
Figure 6.
Mechanism involved in HNK induced changes in melanoma cells. (A) and (B) Western blot analyses showed levels of proteins involved in S2 and S3 cleavage of Notch receptors in cells. HNK showed reduced levels of TACE and γ-secretase complex proteins (presenilin-1 and 2, nicastrin, and PEN2) involved in S2 and S3 cleavage of Notch receptors in melanoma cells. (C) Western blot analyses showed effect of DAPT and/or HNK on cleavage of Notch2 receptor in both cell lines. DAPT showed reduced levels of NICD2 in both the cells line with more robust change in B16/F-10 cells. HNK alone showed more promising effect in inhibiting Notch2 receptor cleavage. However, both DAPT and HNK in combination further enhanced inhibition of cleavage of Notch2 receptor in melanoma cells. (D) ectopic overexpression of NICD1 and 2 in B16/F-10 melanoma cells. NICD1 and 2 overexpression overcomes HNK-mediated suppression of Hes-1 and cyclin D1 expression in cells. Cells transiently expressing NICD were treated with honokiol for 48 h. Lysates were analyzed by Western blotting. Hes-1 and cyclin D1 levels were increased in the NICD-expressing cells when compared with vector-transfected controls. (E) NICD overexpression recapitulates HNK-inhibited melanosphere formation. Melanoma cells transfected with NICD overexpressing plasmid were grown in melanosphere culture media in Ultra Low attachment plates for 6–7 d in the presence and absence of HNK. Ectopic expression of NICD rescued HNK-mediated inhibition of melanosphere formation of B16/F-10 cells (*, P < 0.05).
To further support our findings, we ectopically overexpressed NICD1 and NICD2 proteins in B16/F-10 cells following HNK treatment. Western blot analyses showed an increase in basal levels of Hes-1 and cyclin D1 in NICD1 and NICD2 ectopic overexpression in B16/F-10 cells (Figure 6D). In addition, ectopic expression of NICD2 suppressed HNK-mediated inhibition of cyclin D1 expression (Figure 6D). More importantly, overexpression of both NICDs suppressed HNK-mediated inhibition of melanosphere formation (Figure 6E). Together, these data suggest that HNK suppresses Notch signaling by affecting the two cleavage events by affecting the expression of TACE and γ-secretase complex proteins resulting in reduced levels of NICD and thereby affecting stem cell viability.
DISCUSSION
The current systemic therapeutic modalities for melanoma have limited effectiveness resulting in poor response and patient survival rates in more advanced disease. Hence, there is a dire need for novel agents that can be utilized for therapeutic interventions. There are many reasons for the lack of effective treatments, including the malfunctioning of vital signaling pathways and the presence of heterogeneous cell populations in tumors [23,24]. Recent studies have also focused on the presence of quiescent CSCs, and unique signaling pathways such as the Notch pathway within these cells are activated [24,25]. Here, we have demonstrated that HNK significantly inhibited rapidly proliferating melanoma cells and the relatively rare CSCs, in part through suppressing the Notch signaling pathway.
Given our results, we believe HNK is a putative candidate for both chemoprevention and therapeutic interventions for melanoma. Previous studies have demonstrated that HNK has a good bioavailability profile in preclinical models with good absorption, and rapid excretion [16]. Here, we have demonstrated that HNK inhibits melanoma cell proliferation and induced autophagic cell death within 24 h of treatment, with maximal effects observed at 48 and 72 h. Additionally, inhibition of clonogenic potential of melanoma cells reflect long term effects of HNK on melanoma cells growth. Our data is in accordance with recent studies published by our group and others that showed the higher efficacy of HNK against several types of cancers without any reported toxicity in normal cells or tissues [18,26–29].
Previous studies have shown that HNK induces apoptosis along with G0/G1 phase cell cycle arrest in cancer cells [30–33]. However, in the current study, we do not observe apoptosis induction after HNK treatment. Rather, in melanoma cells HNK induces autophagy along with G0/G1 phase cell cycle arrest. However, this may not be unique to melanoma. Similar induction of autophagic cell death was recently demonstrated in glioblastoma cells, although there was also apoptosis [34]. The only difference between melanoma cells and glioblastoma is that while melanoma cells only show autophagy, glioblastoma cells had both autophagy and apoptosis [34]. No reason was given on the presentation of both apoptosis and autophagy in glioblastoma, but this would be an interesting area to further investigate. In other cancer types, however, HNK has only been shown to induce apoptosis, including mesothelioma, pancreatic and colon cancer [20,31,35]. The possibility exists that certain cancers such as glioblastoma and mesothelioma have a greater susceptibility to apoptotic cell death, while melanoma being highly aggressive may require a less direct mechanism of cell death and more stealthy mechanisms such as autophagy, which is a result of breakdown of cellular components that ensure cellular survival during stress. It would be interesting to determine whether HNK can also induce melanoma to also undergo apoptosis in unique circumstances, although this might require the suppression of autophagy.
Existence of highly dynamic phenotypic heterogeneity in melanomas leads to regeneration of CSCs from non-CSC population [23,36]. Therefore, targeting of both CSCs and the bulk population should be considered during treatment and development of new anticancer therapy against melanoma. Our results strongly suggest that the HNK is a potent inhibitor of CSCs based on melanoshpere forming assay and western blotting analyses of melanoma stem cell markers. HNK was very effective in inhibiting the growth of melanospheres suggesting that HNK target the CSCs. These results were further strengthened by inhibiting the expression of melanoma stem cell markers especially CD271, CD166, Jarid1B, and ABCB5 in melanoma cells. Our results are also in accordance to recent studies that suggested that natural compounds such as curcumin and sulforaphane target cancer stem cells [37–40].
A critical aspect of our study is the demonstration that HNK affects the growth of CSCs in the melanoma cell lines. Understanding the mechanisms that underlie the self-renewal behavior of CSCs is of greatest importance for discovery and development of anticancer drugs targeting CSCs. A number of reports have demonstrated up-regulated Notch receptors activity in a variety of human cancer tissues [41–44]. Recent microarray data comparing the gene expression pattern of normal melanocytes to human melanomas revealed up-regulation of Notch receptors, ligands, and downstream target genes [12,45]. It has been also demonstrated that activation of the Notch signaling pathway is important to preserve the melanocyte stem cells and may also play a role in melanoma progression [12,45]. Our data demonstrate that HNK inhibited Notch signaling in melanoma cell lines, and that this pathway is essential for stemness. Moreover, we have demonstrated that the pathway is inhibited by suppressing the two key cleavage events, especially the intracellular cleavage by the TACE and γ-secretase enzyme since overexpression of NICD1 and NICD2 was able to suppress HNK-mediated suppression of melanospheres. However role of individual NICDs was not clearly predicted in our case and need further investigation. From our point of view, both NICD1 and NICD2 depend on each other for their functions and required to affect any vital pathways in melanoma. However, we have seen higher activity of NICD2 as compared to NICD1 in these melanoma cell lines. Recent microarray studies have also suggested that Notch-2 at mRNA levels is overexpressed in melanoma cells as compared to nevi and normal melanocytes [12]. Therefore, the role of Notch signaling in tumorigenesis is highly context dependent, and still far from completely elucidation. Various studies have demonstrated that inhibiting γ-secretase activity is a good strategy for designing anticancer drugs. In fact, γ-secretase inhibitors (GSI) are in preclinical trial. However, in our studies, incubation with GSI alone (DAPT and DBZ) (data not shown) did not demonstrate any effect on the cells in terms of inhibiting proliferation and inducing cell death in melanoma cells. On the other hand, we did see decreased levels of NICD2 following DAPT treatment in both the cell lines. Interestingly, there was a significant effect when cells were treated with the combination of HNK with DAPT. There was a further reduction in NICD2 levels in melanoma cells when compared to HNK or GSI alone. These data suggest that while, HNK alone is a good strategy for inhibiting melanoma stem cells, the combination of HNK with GSI could be a better inhibitor of the γ-secretase enzyme, in addition to its ability to suppress TACE activity.
We also observed that HNK inhibit the expression of cyclin D1 protein, which is a target of activated Notch-dependent expression. Previous studies have also demonstrated that HNK can inhibit cancer cell progression through the cell cycle at G0/G1 phase, depending on the cancer cell type [31,46,47]. In the current studies, we have demonstrated that HNK induced a G0/GI cell cycle arrest of melanoma cells confirmed by the reduction in cyclin D1 levels. Cyclin D1, often found to be overexpressed in human melanomas, is an important positive regulator of the G1–S cell-cycle transition through its binding and activation of cyclin-dependent kinases 4/6 [48]. Notch signaling has also been shown to affect cyclin D1 expression and activation of CDK2 during melanoma transformation [49]. Cyclin D1 has to interact with four cyclin dependent kinases, activation of cyclin-dependent kinases further inactivate retinoblastoma protein, blocks its growth-inhibitory activity, and promotes release of bound E2F, which in turn can induce the expression of the next set of genes including cyclin E that are important for S phase progression [48]. A number of studies have focused on the possible involvement of the Notch signals in the deregulation of components of the cell cycle machinery [50]. Similarly, in hepatocellular carcinoma Notch1 signaling was found to downregulate the expression of cyclins (D, E) and CDK2, induce p53 expression, and thus apoptosis after a significant growth inhibition related to G0/G1 cell cycle [51]. Further studies are required to determine whether HNK also affects the expression of the cyclin dependent kinases, and whether the suppression of cyclin D1 is a critical factor in the G0/G1 arrest. Another interesting aspect of the studies that is yet to be explored is whether HNK affects only the activation of Notch receptors or whether it can also affect the expression of the proteins. In this regard, further studies, ongoing in the lab is focused on determining whether HNK affects the transcription of the various genes in the Notch signaling pathway such as ligands Jagged1, Jagged2, DLL1, DLL3 and DLL4, and the Notch receptors Notch1 and Notch2 in melanoma cells. In addition, our current studies are focused on whether translational machinery is affected by HNK treatment resulting in reduced mRNA translation of these genes. These are potentially the focus of our future publications in relation to HNK and suppression of melanoma stem cells.
Previous studies using a combination of gene expression and chromatin immunoprecipitation assays revealed the existence of a large number of genes that can directly be regulated by the Notch signaling pathway [52–53]. It would be interesting to determine whether HNK is able to affect the expression of all or just a cohort of these genes, and whether the expression patterns are specific for all or only a subset of melanoma cells. This could not only give a better understanding of HNK's mechanism of action but also might be a way of classifying the cancer into subtypes for future precision medication strategies.
In conclusion, the current studies demonstrate that HNK is a potent inhibitor of melanoma cells, especially the stem cells. Moreover, synthetic derivatives generated from it continue to be excellent source for novel therapeutic agents. While additional studies are required to demonstrate in vivo efficacy of the compound for the treatment of melanoma, the ability to target both rapidly growing cells and the relatively rare quiescent stem cells makes the compound an attractive novel potential agent and warrants further investigation.
Supplementary Material
ACKNOWLEDGMENT
We thank the Anant lab members for help throughout the course of these studies. We wish to acknowledge The Electron Microscopy Research facility for assistance with the electron microscopy. The EMRL is supported in part by the NIH grant 9P20GM104936.
Grant sponsor: NIH; Grant numbers: CA109269; CA182872; CA168524
Abbreviations
- ADAM17
a disintegrin and metalloproteinase
- BCA
bicinchoninic acid
- CDK2
cyclin-dependent kinase 2
- CDK4
cyclin-dependent kinase 4
- CDK6
cyclin-dependent kinase 6
- DAPT
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- DBZ
dibenzazepine
- ERK
extracellular signal-regulated kinases
- Hes
hairy and enhancer of split
- HNK
honokiol
- LC3
light chain 3
- mTOR
mammalian target of rapamycin
- NICD
notch intracellular domain
- PARP-γ
poly (ADP) ribosyl polymerase
- PEN2
presenilin enhancer 2
- PTEN
phosphatase and tensin homologue deleted from chromosome 10
- RBPJ
recombination signal binding protein for immunoglobulin kappa J region
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web-site.
REFERECNES
- 1.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlis C, Herlyn M. Recent advances in melanoma biology. Oncologist. 2004;9:182–187. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- 3.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 4.Koch U, Radtke F. Notch signaling in solid tumors. Cur Top Develop Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- 5.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 6.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 7.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 8.Mumm JS, Kopan R. Notch signaling: From the outside in. Develop Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 9.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 10.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 11.Borggrefe T, Oswald F. The Notch signaling pathway: Transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009:1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoek K, Rimm DL, Williams KR, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 13.Pinnix CC, Herlyn M. The many faces of Notch signaling in skin-derived cells. Pigment Cell Res. 2007;20:458–465. doi: 10.1111/j.1600-0749.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Itokawa H, Sashida Y. Studies on the components of Magnolia obovata Thunb. 3. Occurrence of magnolol and honokiol in M. obovata and other allied plants. Yakugaku Zasshi. 1973;93:429–434. doi: 10.1248/yakushi1947.93.4_429. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11:1139–1148. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Method. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 18.Subramaniam D, Ponnurangam S, Ramamoorthy P, et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PloS ONE. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsko N, Mueller M. Epoxy resin as fixative during freeze-substitution. J Struct Biol. 2005;152:92–103. doi: 10.1016/j.jsb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Ponnurangam S, Mammen JM, Ramalingam S, et al. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Can Ther. 2012;11:963–972. doi: 10.1158/1535-7163.MCT-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi's sarcoma tumor cells. Oncogene. 2005;24:6333–6344. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 22.Pannuti A, Foreman K, Rizzo P, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perego M, Tortoreto M, Tragni G, et al. Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol. 2010;130:1877–1886. doi: 10.1038/jid.2010.69. [DOI] [PubMed] [Google Scholar]
- 24.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 25.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 26.Jiang QQ, Fan LY, Yang GL, et al. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer. 2008;8:242. doi: 10.1186/1471-2407-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou W, Chen L, Yang G, et al. Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytother Res. 2008;22:1125–1132. doi: 10.1002/ptr.2472. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Liu Y, Zhao X, et al. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. E J Obstet Gyn Reprod Biol. 2008;140:95–102. doi: 10.1016/j.ejogrb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Wang T, Wu YF, et al. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10:3459–3463. doi: 10.3748/wjg.v10.i23.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahm ER, Singh SV. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol Can Ther. 2007;6:2686–2695. doi: 10.1158/1535-7163.MCT-07-0217. [DOI] [PubMed] [Google Scholar]
- 31.Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PloS ONE. 2011;6:e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Beitler JJ, Wang H, et al. Honokiol Enhances Paclitaxel Efficacy in Multi-Drug Resistant Human Cancer Model through the Induction of Apoptosis. PloS ONE. 2014;9:e86369. doi: 10.1371/journal.pone.0086369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Z, Subramaniam D, Ramalingam S, et al. Honokiol radiosensitizes colorectal cancer cells: Enhanced activity in cells with mismatch repair defects. Amer J Physiol Gastrointest LPhysiol. 2011;301:G929–G937. doi: 10.1152/ajpgi.00159.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chang KH, Yan MD, Yao CJ, Lin PC, Lai GM. Honokiol-induced apoptosis and autophagy in glioblastoma multiforme cells. Oncol Let. 2013;6:1435–1438. doi: 10.3892/ol.2013.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chae JI, Jeon YJ, Shim JH. Downregulation of Sp1 is involved in honokiol-induced cell cycle arrest and apoptosis in human malignant pleural mesothelioma cells. Oncol Rep. 2013;29:2318–2324. doi: 10.3892/or.2013.2353. [DOI] [PubMed] [Google Scholar]
- 36.Sztiller-Sikorska M, Koprowska K, Majchrzak K, Hartman M, Czyz M. Natural Compounds' Activity against Cancer Stem-Like or Fast-Cycling Melanoma Cells. PloS ONE. 2014;9:e90783. doi: 10.1371/journal.pone.0090783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2:321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer stem cells: A novel paradigm for cancer prevention and treatment. Mini Rev Med Chem. 2010;10:359–371. doi: 10.2174/138955710791330954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14:779–786. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 42.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 43.Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H, Dorken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–3403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
- 44.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 45.Hu Y, Fu L. Targeting cancer stem cells: A new therapy to cure cancer patients. A J Cancer Res. 2012;2:340–356. [PMC free article] [PubMed] [Google Scholar]
- 46.Park EJ, Min HY, Chung HJ, et al. Down-regulation of c-Src/EGFR-mediated signaling activation is involved in the honokiol-induced cell cycle arrest and apoptosis in MDAMB-231 human breast cancer cells. Cancer Lett. 2009;277:133–140. doi: 10.1016/j.canlet.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Fan S, Li X, Lin J, Chen S, Shan J, Qi G. Honokiol inhibits tumor necrosis factor-alpha-stimulated rat aortic smooth muscle cell proliferation via caspase- and mitochondrial-dependent apoptosis. Inflammation. 2014;37:17–26. doi: 10.1007/s10753-013-9707-y. [DOI] [PubMed] [Google Scholar]
- 48.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 49.Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic). Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massi D, Tarantini F, Franchi A, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Modern Pathol. 2006;19:246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 51.Qi R, An H, Yu Y, et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–8329. [PubMed] [Google Scholar]
- 52.Krejci A, Bernard F, Housden BE, Collins S, Bray SJ. Direct response to Notch activation: Signaling crosstalk and incoherent logic. Science Signal. 2009;2:ra1. doi: 10.1126/scisignal.2000140. [DOI] [PubMed] [Google Scholar]
- 53.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. PNAS. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.