Abstract
Ischaemic strokes resulting from atrial fibrillation (AF) constitute a devastating condition for patients and their carers with huge burden on health care systems. Prophylactic treatment against systemic embolization and ischaemic strokes is the cornerstone for the management of AF. Effective stroke prevention requires the use of the vitamin K antagonists or non-vitamin K oral anticoagulants (NOACs). This article summarises the latest developments in the field of stroke prevention in AF and aims to assist physicians with the choice of oral anticoagulant for patients with non-valvular AF with different risk factor profile.
Abbreviations: NVAF, non-valvular atrial fibrillation; TIA, transient ischaemic attack; TE, thromboembolic episode; LV, left ventricle; HF, heart failure; eGFR, estimated glomerular filtration rate; HTN, hypertension; DM, diabetes mellitus; RSM, risk stratification model; SE, systemic embolism; NICE, National institute for Health and Care Excellence; PCI, percutaneous coronary intervention; INR, international normalised ratio; TTR, time in therapeutic range; NCB, net clinical benefit; CrCl, creatinine clearance; CKD, chronic kidney disease; ESRF, end stage renal failure; ICH, intracranial haemorrhage
Keywords: Atrial fibrillation, Stroke prevention, Risk stratification, Oral anticoagulation, Non-vitamin K oral anticoagulants, Net clinical benefit
Highlights
-
•
Vitamin K antagonists (VKA) or non-VKA oral anticoagulants should be used for stroke prevention in atrial fibrillation.
-
•
Antiplatelet agents should not be prescribed solely for thromboprophylaxis in atrial fibrillation.
-
•
Stroke risk should be assessed using the CHA2DS2-VASc score, and bleeding risk with the HAS-BLED score.
-
•
The net clinical benefit favours oral anticoagulation with non-VKA oral anticoagulants in presence of at least 1 risk factor.
1. Introduction
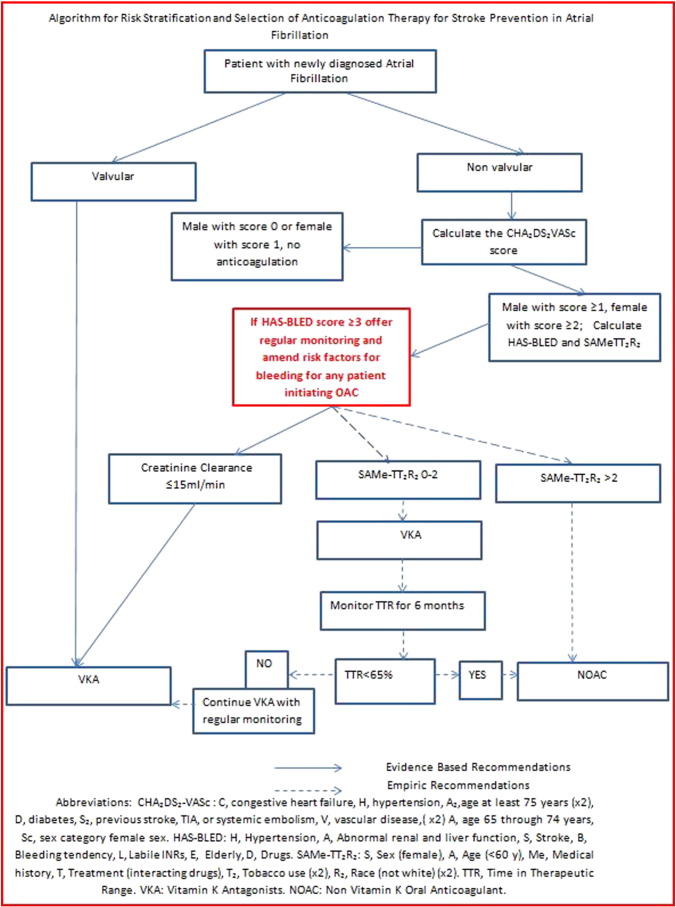
Atrial fibrillation (AF) is associated with a 3-to-5 fold increased risk ischaemic stroke (Ball et al., 2013). AF often occurs in association with other cardiac problems, such as chronic heart failure (up to 50% develop AF) and Acute Coronary Syndrome (up to 25% develop AF) leading to worse outcomes (Ball et al., 2013). Appropriate thromboprophylaxis is central for prevention of thrombotic complications, but it can cause to worrying complications, such as bleeding (Camm et al., 2012a, Kirchhof et al., 2011). (See Fig. 1.)
Fig. 1.
Algorithm for risk stratification and selection of anticoagulation therapy for stroke prevention in atrial fibrillation.
Abbreviations: CHA2DS2-VASc: C, congestive heart failure, H, hypertension, A2,age at least 75 years (× 2), D, diabetes, S2, previous stroke, TIA, or systemic embolism, V, vascular disease,(× 2) A, age 65 through 74 years, Sc, sex category female sex. HAS-BLED: H, hypertension, A, abnormal renal and liver function, S, stroke, B, bleeding tendency, L, labile INRs, E, elderly, D, drugs. SAMe-TT2R2: S, sex (female), A, age (< 60 y), Me, medical history, T, treatment (interacting drugs), T2, tobacco use (× 2), R2, race (not white)(× 2). TTR, time in therapeutic range. VKA: vitamin K antagonists. NOAC: Non-vitamin K oral anticoagulant.
The risks associated with AF are not homogeneous, and various risk factors for stroke and bleeding have been identified, leading to the development and validation of several stroke Risk Stratification Models (RSM). Recognition of the importance of establishing individual risk profiles was accompanied by pursuing an integrative approach in risk assessment with evaluation of ‘net clinical benefit’ for the proposed stratification models (Pisters et al., 2012).
Currently proposed models particularly focus on non-valvular AF, the most common type of AF, which is not related to haemodynamically significant rheumatic valvular disease (predominantly mitral stenosis) or prosthetic heart valves (Camm et al., 2010).
2. Risk Factors for Stroke in Atrial Fibrillation: A Brief Overview
The pathophysiology of thromboembolism in AF is multi-factorial. Increasing evidence points to the fulfilment of Virchow's triad. The loss of atrial systole in AF results in increased stasis of blood within the left atrium (blood flow abnormalities). At macroscopic level, left atrium and left atrium appendage enlargement are common findings in AF. Inflammatory changes in atrial tissue have been demonstrated at microscopic and molecular levels. The final part of the Virchow's triad, abnormal procoagulant blood constituents, is well recognised in AF with abnormalities of coagulation and fibrinolysis pathway resulting to a chronic hypercoagulable state (Choudhury and Lip, 2004).
The most common risk factors associated with stroke (eg, heart failure, hypertension, diabetes, age, prior stroke) were initially identified from treatment naïve cohorts of randomised trials conducted 2 decades ago (Lip & Lane, 2015a). These trials only randomised < 10% of patients screened and many common stroke risk factors were not recorded or consistently defined.
A systematic analysis from the Stroke in AF Working Group searched for independent risk factors for stroke related to AF using information from 27 studies. Of the 24 studies (although many were from trial cohorts), age was found to be an independent risk of stroke, associated with an incremental increase in risk of 1.5-fold per decade [Relative Risk (RR) 1.5 per decade; 95% Confidence Interval (CI), 1.3–1.7]. Overall stroke risk increased 2.5-fold in patients with prior stroke/TIA (RR 2.5; 95% CI, 1.8–3.5). Hypertension was independently associated with stroke in 13 of 20 studies (RR, 2.0; 95% CI, 1.6–2.5) (Pisters et al., 2012). In another systematic review, history of hypertension was present in 42% to 53% (mean of 48%) of analysed subjects and was independently related to stroke in all studies included. Diabetes mellitus was present in 14% to 18% (mean of 15%) of the study cohorts and it was a significant independent risk factor for stroke (RR 1.7, 95% CI, 1.4 to 2.0) (Fibrillation and Group, 2007). Interestingly, heart failure (HF) and coronary artery disease did not emerge as independent predictors for stroke risk in this analysis.
Other data suggest that recent decompensated HF is indeed related to higher risk of stroke in AF, whether left ventricular (LV) ejection fraction (EF) was reduced or preserved (Banerjee et al., 2012a, Banerjee et al., 2013). However, moderate-to-severe systolic LV dysfunction on cardiac imaging is associated with a higher stroke risk even if asymptomatic (Predictors of thromboembolism in atrial fibrillation, 1992, Wagstaff et al., 2014). One systematic review found that although moderate–severe LV systolic impairment on echocardiography is an independent risk factor for stroke/systemic embolism, the magnitude by which it increases the risk of stroke cannot be precisely quantified. Overall, although a clinical diagnosis of HF have not been universally predictive of stroke in AF, significant impairment of LV contractility or previous admission due to HF decompensation irrespective of LV systolic function clearly increases risk of stroke (Agarwal et al., 2014).
Female sex was also associated with an increased risk of stroke, especially in women aged > 65 or in presence of an additional stroke risk factor. Meta-analysis of 17 studies revealed a 1.31-fold (95% CIs) elevated risk of stroke in women with AF; especially in those aged ≥ 75 years (Wagstaff et al., 2014). Overall, female sex plays limited role in prognostication in AF at age of < 65 years when there no other risk factors (i.e., risk of ischaemic stroke < 1%/year) (Melgaard et al., 2014, Friberg et al., 2012a, Mikkelsen et al., 2012).
A systematic review by Anandasundaram et al. supports a significant association between a history of myocardial infarction (MI) and the risk of thromboembolism in AF (in 5 of 6 studies). Angina was reported as a stroke risk factor in AF by two studies (Anandasundaram et al., 2013). Furthermore, the presence of AF in association with peripheral artery disease (PAD) and presence of complex plaque in the descending aorta are independent predictors for stroke and thromboembolism in AF (Zabalgoitia et al., 1998).
Renal failure has been assessed as a risk factor for stroke in AF patients. A correlation between decreasing glomerular filtration rate and stroke risk in conjunction with proteinuria has been reported as an independent stroke risk factor (Olesen et al., 2012). The Swedish Atrial Fibrillation cohort study confirmed such association using the composite endpoint, that included unspecified stroke, TIA and systemic embolism but not with ischaemic stroke alone (Friberg et al., 2012b).
Standard echocardiography contributes to refinement of stroke risk stratification. Moderate–severe LV dysfunction from 2-D echocardiography was the strongest independent predictor of later thromboembolism (The Stroke Prevention in Atrial Fibrillation Investigators, 1992). LV hypertrophy, defined as a LV mass > 110 g/m2 in women and > 134 g/m2 in men, was found to be a significant independent risk factor for stroke in two studies (Pisters et al., 2012). Non-unexpectedly, demonstration of left atrial thrombi, complex aortic plaque, spontaneous echo contrast or low velocities in left atrial appendage have also been associated with an increased risk of thromboembolism in AF (Zabalgoitia et al., 1998).
Various blood-based biomarkers have been proposed to further refine estimation of stroke risk. High levels of fibrin, D-dimer and von Willebrand factor may identify AF patients at higher risk for stroke in non-anticoagulated AF (Wannamethee et al., 2012, Lip et al., 1996). Elevation in troponin I and NT-proBNP are common in patients with AF and they are independently related to increased risks of stroke and mortality even in anticoagulated patients (Hijazi et al., 2012).
3. Risk Stratification Models for Stroke
Co-existence of several risk factors for stroke potentiates the overall risk thus prompting development of various Risk Stratification Models (RSMs). Some of these models categorise AF patients into ‘high’, ‘moderate’, and ‘low’ risk strata; however, stroke risk is a continuum of risk, and thus approaches based on risk quantification appear the preferred option (Camm et al., 2010).
In 2001, an amalgamation of the AF Investigators (AFI) and Stroke Prevention in AF (SPAF) schemes from the original historical trials led to introduction of the CHADS2 score (Gage et al., 2001). The CHADS2 acronym was derived from the individual stroke risk factors: Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, and prior Stroke or TIA. Two points were given for prior stroke or TIA (hence, the subscripted “2”), and 1 point was assigned for each of the other factors (Gage et al., 2004).
The CHA2DS2-VASc score (Table 5) extends stroke prognostication in AF by including additional common stroke predictors to the older CHADS2 score (ages 65–74, vascular disease, and female sex) (Lip, 2015). The CHA2DS2-VASc score particularly improves the risk prediction in many patients deemed ‘low risk’ based on the CHADS2 schema (Table 1) (Olesen et al., 2011a, Lip, 2010). The CHA2DS2-VASc tool has been validated in multiple independent populations and is now recommended by the European (Camm et al., 2010), US (January et al., 2014) and UK (NICE) (Atrial fibrillation: management | Guidance and guidelines | NICE, 2015) guidelines.
Table 5.
Recommended risk assessment tools for stroke risk, bleeding risk and successful vitamin K antagonist-based anticoagulation in patients with atrial fibrillation.
| Risk of stroke |
Risk of bleeding |
Likelihood TTR ≥ 70% (choosing between VKA or NOAC) |
|||
|---|---|---|---|---|---|
| CHA2DS2VASc score (Lip, 2010) |
HAS-BLED score (Pisters et al., 2010) |
SAMe-TT2 R2 score (Apostolakis et al., 2013d) |
|||
| Condition or characteristic | Points | Condition or characteristic | Points | Definition | Points |
| Congestive heart failure | 1 | Hypertension | 1 | Sex (female) | 1 |
| Hypertension | 1 | Abnormal renal and liver function (1 point each) | 1 or 2 | Age (< 60 y) | 1 |
| Age ≥ 75 y | 2 | Stroke | 1 | Medical historya | 1 |
| Diabetes mellitus | 1 | Bleeding tendency/predisposition (anaemia) | 1 | Treatment (interacting drugs, eg, amiodarone for rhythm control) | 1 |
| Stroke/TIA/SE | 2 | Labile INRs (eg, TTR < 60%) | 1 | Tobacco use (within 2 y) | 2 |
| Vascular disease (prior ACS, PAD, or aortic plaque) | 1 | Elderly (eg, age > 65 y, frail condition) | 1 | Race (not white) | 2 |
| Age 65–74 y | 1 | Drugsbc or alcohol excess (1 point each) | 1 or 2 | 8 | |
| Sex category (ie, female sex)a | 1 | 9 | |||
| Maximum score | 9 | ||||
Abbreviations: VKA—vitamin K antagonist, NOAC—non-vitamin K oral antagonist, TTR—time in therapeutic range, TIA—transient ischaemic attack, SE—systemic embolism, ACS—acute coronary syndrome, PAD—peripheral arterial disease, INR—international normalised ratio.
Two of the following: hypertension, diabetes mellitus, coronary artery disease or myocardial infarctions, peripheral artery disease, congestive heart failure, previous stroke, pulmonary disease, or hepatic or renal disease.
Concomitant antiplatelet drugs, Steroids, non-steroidal anti-inflammatory drugs.
Counts only in the presence of another risk factor.
Table 1.
Event rates (95% CI) of hospital admission and death due to thromboembolism per 100 person years [based on Olesen et al. (2011a)].
| Score/risk category | 1 year's follow-up | 5 years' follow-up |
|---|---|---|
| CHA2DS2-VASc | ||
| 0 | 0.78 (0.58 to 1.04) | 0.69 (0.59 to 0.81) |
| 1 | 2.01 (1.70 to 2.36) | 1.51 (1.37 to 1.67) |
| 2 | 3.71 (3.36 to 4.09) | 3.01 (2.83 to 3.20) |
| 3 | 5.92 (5.53 to 6.34) | 4.41 (4.21 to 4.61) |
| 4 | 9.27 (8.71 to 9.86) | 6.69 (6.41 to 6.99) |
| 5 | 15.26 (14.35 to 16.24) | 10.42 (9.95 to 10.91) |
| 6 | 19.74 (18.21 to 21.41) | 12.85 (12.07 to 13.69) |
| 7 | 21.50 (18.75 to 24.64) | 13.92 (12.49 to 15.51) |
| 8 | 22.38 (16.29 to 30.76) | 14.07 (10.80 to 18.33) |
| 9 | 23.64 (10.62 to 52.61) | 16.08 (8.04 to 32.15) |
| CHA2DS2-VASc | ||
| Low risk (0) | 0.78 (0.58 to 1.04) | 0.69 (0.59 to 0.81) |
| Intermediate risk (Ball et al., 2013) | 2.01 (1.70 to 2.36) | 1.51 (1.37 to 1.67) |
| High risk (Camm et al., 2012a, Kirchhof et al., 2011, Pisters et al., 2012, Camm et al., 2010, Choudhury and Lip, 2004, Lip and Lane, 2015b, Fibrillation and Group, 2007, Banerjee et al., 2012a) | 8.82 (8.55 to 9.09) | 6.01 (5.88 to 6.14) |
CHA2DS2-VASc: C, congestive heart failure, H, hypertension, A2, age at least 75 years, D, diabetes, S2, previous stroke, TIA, or systemic embolism, V, vascular disease, A, age 65 through 74 years, Sc, sex category female sex.
Other risk stratification schemes that have been developed, such as the ATRIA (Singer et al., 2013) and the QSTROKE (Hippisley-Cox et al., 2013), have included further clinical and/or demographic factors, as well as different weighing strategies for primary or secondary prevention of stroke. However, perceived marginal benefits (at least statistically) of these scores are outweighed by their complexity and thus limited practicality for everyday use. All suggested risk stratification schemes have their own limitations but the major guidelines emphasise the role of CHA2DS2-VASc score for clinical use, in view of its better capability to identify patients with genuinely low risk who do not need any antithrombotic therapy along with ability to accurately quantify risk of stroke in subjects with multiple risk factor and additional circumstances needed to be considered decision making on individual based (e.g., risk of bleeding) (Lip & Lane, 2015a).
4. Risk of Bleeding
Despite the clinical benefit of OAC for stroke prevention in AF, major bleeding events (especially intra-cranial bleeds) may be devastating events. Identification of patients at high risk of bleeding and delineation of conditions and situations associated with bleeding risk can help to refine antithrombotic therapy in individual cases to minimise bleeding risk (Lip et al., 2011).
The first bleeding risk prediction score (ORBI score) was published in 1998 and it was developed in patients newly started on warfarin after hospitalisation for venous thromboembolism (Beyth et al., 1998). Since then various bleeding risk scores have been described but only four (HAEMORR2AGES (Gage et al., 2006), HAS-BLED (Pisters et al., 2010), ATRIA (Fang et al., 2011) and ORBIT (O'Brien et al., 2015)) were developed and verified in AF populations.
The HAS-BLED score (Table 5) demonstrated good discriminatory performance for “any clinically relevant bleeding” in anticoagulated patients with AF (Apostolakis et al., 2013a). It also performs well in predicting bleeding events compared with older bleeding scores and the ATRIA score (Apostolakis et al., 2013b). Other scores such as ATRIA or ORBIT may falsely categorise some patients as “low risk’ with no action needed whilst the HAS-BLED score would flag up those subjects, particularly if labile INRs are evident (Lip & Lane, 2015b).
Additionally, the simple HAS-BLED score was highly predictive of bleeding events in patients manages with non-vitamin K oral anticoagulants (NOACs) (Apostolakis et al., 2013c), patients on triple antithrombotic therapy post PCI (i.e., dual anti-platelets therapy and warfarin) (Smith et al., 2012) and during bridging of chronic oral anticoagulants with unfractionated or low molecular weight heparin prior to surgery (Omran et al., 2012).
A HAS-BLED score ≥ 3 indicates high risk of bleeding and suggest some caution with OAC in such patients with their regular review following the initiation of antithrombotic therapy (Camm et al., 2010). A high HAS-BLED score is not a reason to withhold anticoagulation but it emphasises the need to correct potential causes of bleeding such as uncontrolled hypertension, concomitant use of other medications that can contribute to bleeding and falls, excessive alcohol consumption, etc. (Lip & Lane, 2015a).
A large number of patients with high risk of stroke/SE (i.e., CHA2DS2-VASc Score > 3) also have a high risk of bleeding (Roldán et al., 2013). This is partly due to the fact that both scores have some characteristics in common (e.g., age, hypertension and previous stroke). Nonetheless, HAS-BLED outperforms CHADS2 and CHA2DS2-VASc for predicting bleeding (Apostolakis et al., 2013a). Thus, a bleeding risk score (HAS-BLED) should be used to predict bleeding, and stroke risk assessed using a specific stroke risk score (CHA2DS2-VASc) (Roldán et al., 2013). From a large observational study the adjusted net clinical benefit favoured OAC in patients irrespective of HAS-BLED or CHA2DS2-VASc score (Friberg et al., 2012c).
Many clinicians are reluctant to initiate appropriate antithrombotic therapy in patients with AF and risk or history of falls. In a large “real world” cohort, prior history of falls was uncommon and it was independently associated with increased risk of stroke/thromboembolism, bleeding, and mortality but not with haemorrhagic stroke in the presence of anticoagulation (Banerjee et al., 2014). In another cohort, patients on oral anticoagulants at high risk of falls did not have any significant increase in risk of major bleeds, and risk of falls is usually not a valid reason to avoid OACs in AF patients (Donzé et al., 2012). In a modelling analysis, Man-Son-Hing et al. estimated that elderly patients need to fall 295 times per year for the benefit of stroke reduction to be outweighed by serious bleeding (Man-Son-Hing et al., 1999).
5. Guidelines for Thromboprophylaxis in Atrial Fibrillation
The European Society of Cardiology (ESC) in 2010 (Camm et al., 2010) with an update in 2012 (Camm et al., 2012a), the American College of Chest Physicians (ACCP) in 2012 (You et al., 2012), the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) in 2014 (January et al., 2014), the Canadian Cardiovascular Society (CCS) in 2014 (Verma et al., 2014) and the National Institute for Health and Care Excellence (NICE) in 2014 (Atrial fibrillation: management | Guidance and guidelines | NICE, 2015) published their guidelines for the management of stroke prevention in AF. Table 2 provides a summary of the guidelines regarding the antithrombotic treatment for patients with NVAF.
Table 2.
Recommendations of guidelines.
| Guidelines | Risk factors | Treatment |
|---|---|---|
| AHA/ACC/HRS 2014 (January et al., 2014) | CHA2DS2-VASc score | |
| ≥ 2 | VKAs or NOACs | |
| 1 | Nothing, aspirin or NOAC | |
| 0 | No OAC | |
| NICE 2014 (Atrial fibrillation: management | Guidance and guidelines | NICE, 2015) | CHA2DS2-VASc score | |
| For males ≥ 1 or females ≥ 2 | VKAs or NOACs | |
| For males 0 or females 1 | Nothing | |
| CCS 2014 (Verma et al., 2014) | CHADS2 score | |
| Identify those aged ≥ 65 y and CHADS2 score risk factors | VKAs or NOACs | |
| No risk factors (ie, age < 65 y with no CHADS2 risk factors or vascular disease) | Nothing | |
| No risk factors, ie, age < 65 y with no CHADS2 risk factors with vascular disease | Aspirin | |
| ACCP 2012 (You et al., 2012) | CHADS2 score | |
| 0 plus additional non-CHADS2 risk factors (eg, age 65–74 y, woman, and vascular disease) | Warfarin or dabigatran | |
| ≥ 1 | Warfarin or dabigatran | |
| No risk factors | Nothing | |
| ESC 2012 (Camm et al., 2012a) | CHA2DS2-VASc | |
| For males ≥ 1 or females ≥ 2 | VKAs if TTR > 70% or NOACs. Antiplatelet therapy with aspirin-clopidogrel combination therapy or, less effectively, aspirin monotherapy is recommended only when patients refuse any form of OAC | |
| For males 0 or females 1 | Nothing | |
Abbreviations: AHA/ACC/HRS: American Heart Association/American College of Cardiology/Heart Rhythm Society, ESC: European Society of Cardiology, ACCP: American College of Chest, CCS: Canadian Cardiovascular Society, NICE: National Institute for Health and Care Excellence, CHA2DS2-VASc: C, congestive heart failure, H, hypertension, A2,age at least 75 years, D, diabetes, S2, previous stroke, TIA, or systemic embolism, V, vascular disease, A, age 65 through 74 years, Sc, sex category female sex. CHADS2: C, congestive heart failure, hypertension, A, age at least 75 years, D, diabetes, and S2, previous stroke, transient ischemic attack (TIA), or systemic embolism; VKAs: vitamin K antagonists, NOAC: non-vitamin K oral anticoagulant, OAC: oral anticoagulation, TTR: time in therapeutic range.
5.1. Vitamin K Antagonists
Effective stroke prevention for patients with AF requires the use of OACs, whether a VKA or a NOAC. The commonly used VKAs are 4-hydroxycoumarins and include warfarin, phenprocoumon and acenocoumarol (De Caterina et al., 2013). In six trials involving 2900 patients with a total of 186 strokes, anticoagulation with the VKAs was compared with placebo or control. Meta-analysis showed that therapy with adjusted dose warfarin reduced the relative risk for stroke by 64% (95% CI, 49% to 74%). All-cause mortality decreased in participants who received warfarin compared to placebo/control [RR 26% (CI, 3% to 43%)] (Hart et al., 2007).
The prothrombin time assay has been used for decades to monitor the intensity of therapy. To promote standardisation, the World Health Organization introduced a prothrombin time standardisation expressed as international normalised ratio (INR) in 1983 (De Caterina et al., 2013). Suboptimal anticoagulation with frequent episodes of INR < 2.0 is a cause of many cases of thromboembolism, while those with an INR > 3.0 make up many of the cases of major haemorrhage (Mearns et al., 2014). Numerous studies have shown that an estimated time spent in the therapeutic INR range (TTR) to be an important determinant of the quality of anticoagulation with VKA and risk of on treatment thrombotic events (Mearns et al., 2014). TTR was also an independent risk factor for major bleeding following initiation of VKA therapy (Gallego et al., 2013). Anticoagulation services should aim for a TTR > 70% to maximise benefits and minimise risk of complications in patients receiving VKA (Wan et al., 2008).
5.2. Non-vitamin K Antagonist Oral Antagonists (NOACs)
For over 60 years, VKAs were the only available agents for long-term oral anticoagulation. They have revolutionised management of AF but they do have shortcomings. These include a slow onset of action, variable dose requirement that reflect, at least in part, common polymorphisms influencing the pharmacokinetics and pharmacodynamics of VKAs, variations in dietary vitamin K intake, and multiple drug–drug interactions (De Caterina et al., 2013). These limitations have restricted the use of warfarin and have led to the development of NOACs.
Currently used NOACs fall into 2 major classes, the oral direct thrombin inhibitors (eg. dabigatran) and oral factor Xa inhibitors (e.g. rivaroxaban, apixaban, edoxaban) (Lip & Lane, 2015a). Routine measurement of the anticoagulant effect of the NOACS is not necessary because their pharmacokinetic and pharmacodynamic characteristics are predictable and the therapeutic window is sufficiently large to allow fixed dose administration without the need for routine monitoring of response (Table 3) (Hankey, 2014).
Table 3.
Pharmacokinetic properties and other profile features of non-vitamin K oral antagonists.
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|
| Mechanism of action | Direct thrombin inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor |
| Fixed dose | Twice daily | Once daily | Twice daily | Once daily |
| Half-life (h) | 12–17 | 5–9 | 9–12 | 8–11 |
| Time to peak effect (h) | 2 | 2–3 | 1–2 | 1–2 |
| Renal elimination | 80% | 33% | 25% | 35% |
| Drug interactions | Strong P-gp inhibitors and inducers | Strong CYP3A4 inducers, strong inhibitors of both CYP3A4 and P-gp | Strong inhibitors and inducers of CYP3A4 and P-gp | Strong P-gp inhibitors |
| Coagulation assay | aPTT, dTT Ecarin clotting time | PT Chromogenic anti-Xa assay | Chromogenic anti-Xa assay | Chromogenic anti-Xa assay |
Abbreviations: P-gp—permeability glycoprotein, aPTT—activated partial thromboplastin time, dTT—dilute thrombin time, PT—prothrombin time.
Four large multicentre Phase III, randomised clinical trials (RE-LY (Connolly et al., 2009), ROCKET-AF (Patel et al., 2011), ARISTOTLE (Granger et al., 2011), ENGAGE-AF (Giugliano et al., 2013)) have compared the NOACs against warfarin for stroke prevention in AF. In RE-LY and ENGAGE AF, two doses of dabigatran and edoxaban, respectively, were compared with warfarin in a 3 arm trial design (Skjøth et al., 2014). Dabigatran was administered, in a blinded fashion, in capsules containing either 110 mg or 150 mg of the drug (Connolly et al., 2009). In ENGAGE-AF, the high-dose edoxaban group received 60 mg, and the low-dose group 30 mg (Giugliano et al., 2013). In the ROCKET-AF trial, which was a 2-arm trial, patients were randomly assigned to receive either fixed-dose rivaroxaban (20 mg daily with a dose adjustment to 15 mg daily in patients with a creatinine clearance of 30 to 49 ml per minute) or adjusted-dose warfarin (Patel et al., 2011). In ARISTOTLE trial, warfarin was compared to apixaban 5-mg bid, in a two-arm trial; however, there was a dose adjustment where 2.5-mg bid was used in a patient with two or more of the following criteria: age ≥ 80 years, a body weight < 60 kg, or serum creatinine level of 133 μmol/L or higher (Granger et al., 2011). All four NOACs were at least non-inferior to warfarin regarding prevention of stroke and systemic thromboembolism and risk of major bleeding and they consistently caused less intracranial bleeding than warfarin.
In a recent meta-analysis of the four trials (Ruff et al., 2014), 42,411 participants received a NOAC and 29,272 participants received warfarin. NOACs significantly reduced stroke or SE events by 19% compared with warfarin (RR 0.81, 95% CI 0.73–0.91, p < 0.0001), which was mainly driven by a reduction in haemorrhagic stroke (RR 0.49, 95% CI 0.38–0.64, p < 0.0001). NOACs also significantly reduced all-cause mortality (RR 0.90, 0.85–0.95, p = 0.0003) and intracranial haemorrhage (RR 0.48, 95% CI 0.39–0.59; p < 0.0001), but increased gastrointestinal bleeding (RR 1.25, 95% 1.01–1.55, p = 0.04). No heterogeneity were noted for stroke or systemic embolic events in important subgroups, but there was a greater relative reduction in major bleeding with new oral anticoagulants when the centre-based time in therapeutic range was less than 66% than when it was 66% or more (RR 0.69, 95% CI 0.59–0.81 vs RR 0.93, 95% CI 0.76–1.13, p for interaction 0.022).
Low-dose NOACs regimens tested in the trials above showed similar overall reductions in stroke or systemic thromboembolic events to warfarin (RR 1.03, 95% CI 0.84–1.27, p = 0.74), and a more favourable bleeding profile (RR 0.65, 95% CI 0.43–1.00, p = 0.05), but significantly more ischaemic strokes (RR 1.28, 95% CI 1.02–1.60, p = 0.045). Low dose regimens might be an appealing option for frail patients or for those who have a high risk for bleeding with full-dose anticoagulation (Ruff et al., 2014).
Among patients with AF who are at high risk for stroke and for whom VKA therapy is unsuitable or refused by patients, apixaban substantially reduced the risk of stroke, as compared with aspirin, with no significant increase in the risk of major bleeding or intracranial bleeding (Connolly et al., 2011). No head-to-head comparisons of NOACs have been done and comparisons of NOACs based on the results of large randomised trials of NOACs versus warfarin are problematic due to differences in the participants CHADS2 score, TTR and rates of stroke and systemic embolism and haemorrhage in the warfarin arms of the trials. Predetermined subgroup and post hoc analyses, as well as meta-analyses, have examined the risk and benefits of NOACs in different patient subgroups. These analyses can be helpful in guiding NOAC choices in challenging clinical situations (Table 4) (Shields and Lip, 2015).
Table 4.
Suggested patient groups in which specific non-VKA oral anticoagulants (NOACs) may be relatively advantageous.
| Individual patient groups and characteristics | NOACs with characteristics beneficial to target group | |
|---|---|---|
| Elderly patients | Consider comorbidities and agents with lower extracranial haemorrhage among elderly (age > 75) | Apixaban Edoxaban |
| Renal impairment (45 ml/min > CrCl > 15 ml/min) |
Consider agents with lower haemorrhagic complications in moderate to severe renal impairment | Apixaban |
| Previous GI haemorrhage | Consider agents with no increased risk of GI haemorrhage | Apixaban Dabigatran 110 mg |
| High bleeding risk (HAS-BLED ≥ 3) | Consider agents with lower incidence of extracranial haemorrhage | Apixaban Dabigatran 110 mg Edoxaban |
| Recurrent stroke despite well managed VKA | Consider agent with demonstrable benefit in reducing both ischaemic AND haemorrhagic stroke | Dabigatran 150 mg |
| Preference for low pill burden | Consider once daily formulations | Edoxaban Rivaroxaban |
Abbreviations: CrCl creatinine clearance, HAS-BLED: H, hypertension, A, abnormal renal and liver function, S, stroke, B, bleeding tendency, L, labile INRs, E, elderly, D, drugs. GI gastrointestinal, VKA vitamin K antagonist.
Rivaroxaban has a half-life of 5–9 h, however the daily dose for patients with CrCl > 50 ml/min is 20 mg OD. Pharmacokinetic data from 870 patients with acute DVT in phase II studies found that for the same total daily doses, the peak plasma concentration was approximately 20% higher and the trough concentration was approximately 60% lower with the use of once-daily compared with twice-daily regimen (Bauersachs et al., 2010). However because the concentration parameters substantially overlapped with the two regimens, once-daily dosing should not expose patients to a greater risk of bleeding at the peak plasma concentration, or a higher risk of thrombogenesis at the trough plasma concentration compared with the twice-daily dosing (Mueck et al., 2011).
Given the short half-lives of the NOACs, patients should be counselled that missing even a single dose might compromise thromboprophylaxis, and NOACs should not be used in patients with poor adherence to medication. In addition, NOACs have not been evaluated in patients with severe renal impairment [i.e., creatinine clearance (CrCl) < 15 mL/min], in patients with valvular AF or malignancy (Heidbuchel et al., 2015). The CrCl should be assessed before initiating anticoagulation and at least annually thereafter and at least every 6 months in patients with an eGFR < 60 mL/min (Hart et al., 2013).
6. Cost Effectiveness of NOACs
Cost effectiveness of the NOACs has been assessed in various studies. Despite the higher drug costs, rivaroxaban and dabigatran reduce overall medical costs compared to warfarin, unless the TTR for warfarin is above 65% and 70%, respectively. Apixaban has been estimated to reduce medical costs compared to warfarin throughout a TTR range of 30–90%) (Amin et al., 2014).
In the United Kingdom, NICE has concluded that the NOACs are all cost effective and be available to National Health Service (NHS) patients within their licenced indications. Patients who might particularly benefit from NOACs include those who cannot take VKAs, have persistently poor TTR (e.g., < 65%) and those taking aspirin for stroke prevention for whom a NOAC may be an option (Supporting local implementation of NICE guidance on use of the novel (non-Vitamin K antagonist) oral anticoagulants in non-valvular atrial fibrillation, 2015). A large reduction in medical cost was mainly driven by reductions in the risks of major bleedings with the NOACs (Krejczy et al., 2015).
7. Net Clinical Benefit of Oral Anticoagulants
A decision to advise OAC should be based upon the individual risks for stroke and other thromboembolic events and bleeding, with estimation of resultant net clinical benefit (NCB) of treatment. Whilst there are various ways to define NCB, it is most commonly estimated by balancing the risk of ischemic stroke without OAC against the risk of intracranial haemorrhage with OAC (Lip et al., 2015).
NCB is clearly in favour of OAC in patients with CHADS2 score ≥ 1 or CHA2DS2-VASc score ≥ 2. NCB is positive or neutral for VKA therapy in patients with ≥ 1 stroke risk factors (i.e. CHA2DS2-VASc score ≥ 1). Regardless of HAS-BLED score, there is negative NCB for OAC in patients with ‘truly low risk’ (i.e., CHA2DS2-VASc score 0) (Olesen et al., 2011b).
NCB analyses from recent randomised trials as well as modelling analyses applying the clinical trial data to patient cohorts all show a positive NCB for the NOACs vs warfarin, aspirin or no treatment. Indeed, one analysis estimated that the use of NOACs has lowered the threshold for starting OAC is an ischaemic stroke rate of 0.9%/year (Eckman et al., 2011). In one modelling analysis with CHA2DS2-VASc = 1, apixaban and both doses of dabigatran (110 mg and 150 mg bid) have a positive NCB. In patients with CHA2DS2-VASc ≥ 2, all three analysed NOACs (dabigatran, rivaroxaban and apixaban) had NCB superior to warfarin, regardless of risk of bleeding as determined by HAS-BLED score (Banerjee et al., 2012b, Blann et al., 2015).
8. Practical Considerations for Oral Anticoagulants
When choosing the most appropriate OAC for a given patient, physician should be fully aware of the drug pharmacology, the trial results, stroke and bleeding risk profile and the patient's preferences (Potpara and Lip, 2013). Common clinical and demographic factors can influence the quality of oral anticoagulation, making it feasible to identify patients who are less likely to keep within the target INR range. These factors are incorporated into the simple SAMe-TT2R2 score (Table 5) [sex female, age < 60 years, medical history (more than two comorbidities), treatment (interacting drugs, e.g. amiodarone for rhythm control), tobacco use (doubled), race non-Caucasian (doubled)]. The SAMe-TT2R2 score is predictive of patients likely to have poor INR control (as reflected by poor TTR) and could aid decision making in management of AF by identifying patients who would do well on VKAs (SAMe-TT2R2 score 0–2) or, conversely, those who are likely to require NOAC or some intervention(s) to help them achieve acceptable anticoagulation control (ie, SAMe-TT2R2 score > 2) (Apostolakis et al., 2013d).
A number or retrospective cohorts have independently validated the SAMe-TT2R2 score (Abumuaileq et al., 2015, Roldán et al., 2015, Gallego et al., 2014, Poli et al., 2014). In one prospective multicentre study performed in Spain on the nationwide sample of patients with non-valvular AF receiving VKA the SAMe-TT2R2 score had a significant, although moderate, ability to identify patients with a good anticoagulation control as well as modest values of sensitivity and specificity (Ruiz-Ortiz et al., 2015). In a large French cohort, a high SAMe-TT2R2 score translates into labile INRs, which in turn is associated with a greater risk of thromboembolism, mortality and severe bleeding (Lip et al., 2014a).
Thus, SAMe-TT2R2 is an acceptable predictor of the quality of anticoagulation control in patients with non-valvular AF on VKAs, and its predictive ability can be improved if integrated with the physician's judgement, which accounts for the overall circumstances of the patient.
9. Ethnicity and Thromboprophylaxis in Atrial Fibrillation
Intracerebral or subarachnoid haemorrhage (ICH) accounts for 15–20% of strokes in white subjects (Thrift et al., 2001) and ICH rates in Asians are substantially higher (20–30%) among people of black (Broderick et al., 1992), Hispanic (Sacco et al., 1998) or Asian origin (Klatsky et al., 2005, Hu et al., 1992). Non-white patients, especially those of Asian origin, experienced substantially higher risk for warfarin-related ICH compared with whites (Shen et al., 2007). An important consideration is whether all races/ethnicities require the same anticoagulation intensity with VKA. NOACs have overcome that issue and fared even better in Asian patients compare with non-Asians. A recent meta-analysis (Wang et al., 2015) of the large phase III NOAC trials found that standard doses of NOACs were more effective than warfarin in the reduction of systemic embolism and haemorrhagic stroke in Asians; also, the absolute risk reduction in major bleeding by standard-dose NOACs was generally greater in Asians than in non-Asian patients.
10. Management of Anticoagulation Related Bleeding
Mild bleedings (such as epistaxis and ecchymosis) can be managed conservatively with the application of local haemostatic measures. In patients experiencing a moderate to severe bleeding, the type and location of bleeding should be considered. For example, gastrointestinal bleedings, which occur into an open cavity are rarely fatal. In case of a severe/life-threatening bleeding, such as bleeding into a critical organ (particularly intracranial haemorrhage) or bleeding that causes haemodynamic instability, all antithrombotic therapy should be stopped immediately. Patients should be managed with maximum supportive measures and early referral for procedural or surgical intervention if appropriate (Weitz and Pollack, 2015).
For VKAs, replacement is necessary to correct the low levels of factors II, VII, IX and X. This can be achieved by using prothrombin complex concentrate (PCC), preferably, or fresh frozen plasma (FFP) (Tran et al., 2013). Administration of vitamin K, whilst a specific antidote for the VKA drugs, takes > 12 h to work, and supportive measures are still needed in the interim.
The NOACs have a moderate half-life (ranging from 5 to 9 h for rivaroxaban to 12–17 h for dabigatran) and in the event of a NOAC overdose supportive measures should be initiated and continued until the drug concentrations drop. In addition, non-specific reversal agents (such as PCC or recombinant factor VIIa) can be considered, but this is based on limited clinical evidence (Weitz and Pollack, 2015). Dabigatran can be partially removed by dialysis in the case of life-threatening bleeding or renal failure. However, dialysis may be time consuming to be practical in a life-threatening situation and can only be performed on haemodynamically stable patients. The direct factor Xa inhibitors are highly bound to plasma proteins; therefore, dialysis is not expected to reduce their plasma levels.
11. Specific Antidotes to NOACs
Idarucizumab, a monoclonal antibody fragment, binds dabigatran with an affinity that is 350 times higher than with thrombin and neutralises dabigatran activity. The prospective RE-VERSE AD study cohort assessed the efficacy and safety of idarucizumab for reversal of the anticoagulant effects of dabigatran in patients who presented with serious bleeding or who required urgent surgery or intervention (Pollack et al., 2015a). Idarucizumab rapidly and completely reversed the anticoagulant effect of dabigatran in 98% of the patients. Only 1 of the 90 patients (1%) had a thrombotic event within 72 h after idarucizumab administration, and antithrombotic therapy had not been reinitiated in that patient (Pollack et al., 2015b). Idarucizumab received its first approval for clinical practise in the USA in October 2015 for use in adult patients treated with dabigatran etexilate when rapid reversal of its anticoagulant effects is required for emergency surgery/urgent procedures or in life-threatening or uncontrolled bleeding.
Andexanet alpha is a modified recombinant FXa lacking enzymatic activity which is currently in an earlier stage of development for neutralisation of direct and indirect FXa inhibitors. A phase 3 study to evaluate the effect of andexanet in bleeding patients receiving FXa-inhibitors started in 2015. In addition, a small molecule ciraparantag has entered phase I clinical trials. Ciraparantag binds non-covalently to anticoagulants, inhibiting the anticoagulant effects of low molecular weight heparins, fondaparinux, oral FXa inhibitors and dabigatran (Greinacher et al., 2015).
12. Restarting Oral Anticoagulation After Ischaemic or Haemorrhagic Stroke and Major Gastrointestinal Bleeding
The observed incidence of early (within 14 days) recurrent ischaemic stroke is 8% (Berge, 2000). Early initiation of anticoagulation may thus be effective in preventing early recurrence. This potential benefit must, however be balanced with the potential risk for ICH.
Observational data suggest that independent predictors for symptomatic haemorrhagic transformation of ischaemic stroke are large infarct, previous haemorrhagic stroke and low platelet count (Lee et al., 2010). For patients without high-risk features, the risk for symptomatic ICH whilst undergoing anticoagulation therapy is 1.5% within 14 days (Lee et al., 2010). In the European Atrial Fibrillation Trial (EAFT), which enrolled patients with TIA or minor stroke, oral anticoagulation was found to be effective in a protocol that initiated warfarin within 14 days of symptom onset in approximately half of the patients ([European atrial fibrillation trial (EAFT), 2015). In most trials of NOACs, the study drug could not be started within 7 to 14 days of a stroke event (Connolly et al., 2009, Granger et al., 2011, Patel et al., 2011). However, the RE-LY trial delayed eligibility for 6 months after a severe stroke (Diener et al., 2010). Recent guidelines from the American Heart Association/American Stroke Association advise that for most patients with a stroke or TIA in the setting of AF, it is reasonable to initiate OAC within 14 days after the onset of neurological symptoms; however, in the presence of high risk for haemorrhagic conversion, it is reasonable to delay initiation of OAC beyond 14 days (Kernan et al., 2014).
ICH occurs in up to 1% of patients on OAC per year and it is the most feared and devastating complication of this treatment (Lansberg et al., 2012). AF patients who experience an ICH are at very high risk of ischemic stroke and mortality if they are not on antithrombotic therapy (Brønnum Nielsen et al., 2015). Factors contributing to recurrent ICH include increasing age, concomitant use of aspirin or nonsteroidal anti-inflammatory drugs, uncontrolled hypertension, spontaneous ICH, lobar bleed and cerebral amyloid angiopathy (Nielsen et al., 2015, Qureshi et al., 2001). Patients with lobar haemorrhage, microbleeds or cerebral amyloid angiopathy remain at higher risk for anticoagulant-related ICH recurrence than thromboembolic events and may be best managed without anticoagulants. Patients with deep hemispheric ICH and a baseline risk of ischemic stroke > 6.5% per year, that corresponds to CHA2DS2-VASc ≥ 5, may have net benefit from restarting anticoagulation. To date, a reasonable recommendation regarding time from stroke to resumption of anticoagulation therapy would be 8–10 weeks (Lansberg et al., 2012, Paciaroni and Agnelli, 2014).
Reported rates of major bleeding among individuals with AF taking oral VKAs vary widely ranging from 1.3% to 7.2% per year. This variability of the values reflects the differences in the studies patient populations and the methodology employed (Lip et al., 2011). Most bleeding events are gastrointestinal bleedings (Hansen et al., 2010).
The largest published cohort study was contacted in Denmark and included 4602 patients with AF discharged from hospital after gastrointestinal bleeding while receiving antithrombotic treatment. Among patients surviving the first 90 days after gastrointestinal bleeding, restarting single treatment with oral anticoagulation was associated with the lowest risk of all-cause mortality and thromboembolism. In one medium size retrospective cohort study analysis of patients who developed major gastrointestinal bleedings whilst taking warfarin and later had evidence of the bleeding resolution, restarting warfarin was independently associated with decreased mortality (adjusted hazard ratio 0.66, 95% CI 0.55–0.80, p < 0.0001) (Qureshi et al., 2014). In a smaller observational cohort study resuming OAC after hospitalisation for gastrointestinal bleedings was associated with a significantly decreased adjusted risk of major thromboembolic events over 90 days, without a significantly increased risk of recurrent gastrointestinal bleedings (Sengupta et al., 2015). These data support the recommendation that OAC should be continued after an episode of gastrointestinal bleedings whenever possible.
13. Antiplatelet Therapy
Antiplatelet therapy is inferior to adjusted dose warfarin for prevention of stroke by approximately 40%. Aspirin does not lower the absolute risk of stroke, particularly in patients who are more than 75 years old (J. Stroke Cerebrovasc. Dis., 1993). Different aspirin doses appear to have similar effect in AF (Hart et al., 2007) and the risk of major bleeding or intracranial haemorrhage with aspirin is similar to that of OAC, especially in the elderly (Camm et al., 2012b). Based on meta-analysis of four trials aspirin does not significantly reduce all-cause mortality in AF (Hart et al., 1999).
For the majority of patients with AF, aspirin has limited role in stroke prevention. Nonetheless, aspirin may be considered in AF patients in the early stages following acute coronary syndrome, and after angioplasty, in combination with an oral anticoagulant and clopidogrel, as appropriate. Clopidogrel monotherapy is probably inferior to warfarin monotherapy in the prevention of ischemic stroke and bears a risk of bleeding similar to that of aspirin monotherapy (Hansen et al., 2010). Notably, aspirin combined with clopidogrel shows only modest benefit in stroke prevention compared with aspirin monotherapy in patients with AF who refuse oral anticoagulant drugs (Lip, 2011). The use of antiplatelet therapy for stroke prevention in AF should be limited to the minority of patients who refuse any form of OAC (Camm et al., 2012b).
14. Oral Anticoagulation in Severe Renal Impairment (Creatinine Clearance < 15 ml/min)
The overall prevalence of AF is approximately 15% among haemodialysis patients (Hart et al., 2013). In a large cohort study, among patients with AF, non-end stage chronic kidney disease and disease requiring renal replacement therapy were both associated with increased risks of stroke or systemic embolism and bleeding. Warfarin therapy was associated with a significant reduction in the risk of stroke or systemic embolism among patients with chronic kidney disease but the risk of bleeding among such patients was significantly increased (Hart et al., 2013). Thus, the net clinical effect of warfarin treatment in chronic kidney disease requires careful assessment on individual basis (Olesen et al., 2012).
Another large cohort study showed that haemodialysis was associated with a significantly higher risk of AF than peritoneal dialysis. Progressive cardiac enlargement was more frequent in haemodialysis patients despite adequate blood pressure control thus predisposing to AF occurrence and persistence (Abbott et al., 2003).
Patients with severe renal impairment were excluded from all major NOACs trials (Camm et al., 2012a). All NOACs are partly or predominantly excreted by the kidneys, but only dabigatran is removed by dialysis. There is uncertainty regarding appropriate NOAC dosing in patients with end stage renal disease on dialysis. In a recent study that was conducted in US, dabigatran and rivaroxaban in patients on hemodialysis was associated with a higher risk of hospitalisation or death from bleeding when compared with warfarin (Chan et al., 2015).
15. Oral Anticoagulation and Percutaneous Coronary Intervention
In patients on OAC undergoing PCI, peri-procedural anticoagulation should be managed depending on the setting in which PCI is performed (i.e. elective vs ACS). In general, radial artery vascular access is preferable to reduce risk of bleeding (Rubboli et al., 2014). In patients with stable CAD and AF undergoing PCI at low bleeding risk (HAS-BLED 0-2), triple antithrombotic therapy (OAC, aspirin 75–100 mg daily and clopidogrel 75 mg daily) should be given for a minimum of 4 weeks (and no longer than 6 months) after PCI, following by dual therapy with OAC (either a NOAC or a VKA) and clopidogrel 75 mg/day (or alternatively, aspirin 75–100 mg/day) for up to 12 months. In patients with myocardial infarction and AF at low risk of bleeding (HAS-BLED 0–2), the initial use of triple therapy (OAC, aspirin, and clopidogrel) should be considered for 6 months following PCI irrespective of stent type; this should be followed by long-term therapy (up to 12 months) with OAC and clopidogrel 75 mg/day. In patients with ACS and AF at high risk of bleeding (HAS-BLED ≥ 3), the initial use of triple therapy (OAC, aspirin, and clopidogrel) should be considered for 4 weeks following PCI irrespective of stent type; this should be followed by long-term therapy (up to 12 months) with OAC and a single antiplatelet drug (preferably clopidogrel 75 mg/day, or as an alternative, aspirin 75–100 mg/day). Long-term antithrombotic therapy (beyond 12 months) should be with OAC alone (VKA or a NOAC).
The open-label, randomised, controlled WOEST trial (Dewilde et al., 2013) suggested that patients undergoing PCI and are on treatment with warfarin (only two-thirds had AF) should be managed by a combination of warfarin and clopidogrel for 1 year post PCI and then with warfarin indefinitely. This was based on the finding that the use of clopidogrel without aspirin was associated with a significant reduction in bleeding complications and no increase in the rate of thrombotic events. However, the HAS-BLED score and the CHA2DS2-VASc score were not used for decision making in the trial. Also, a placebo was not used instead of aspirin in the double-therapy group. Other limitations of this trial have been discussed (PubMed — NCBI, 2015a).
In patients receiving VKA, the TTR should be maintained above 70% to avoid excess in stroke or haemorrhage. The lower tested dose of the NOACs for stroke prevention is advisable, when used in combination with antiplatelet therapy. The combination of OACs with prasugrel or ticagrelor should be avoided (Lip et al., 2014b).
16. Oral Anticoagulation and Cardioversion
Based on guidelines, in patients with AF of duration more 48 h (or AF of unknown duration) oral anticoagulation should be given for at least 3 weeks prior to cardioversion, or transoesophageal echocardiography should be performed to rule out left atrial thrombi (Camm et al., 2010).
Observational data from the RE-LY trial showed a comparatively low stroke rate related to cardioversion in patients treated with either dabigatran and VKAs (Nagarakanti et al., 2011). Analysis of data from the ARISTOTLE trial showed that patients undergoing cardioversions being managed with either apixaban or warfarin had no thromboembolic events within the first 90 days (Flaker et al., 2014). Likewise, there was no difference in the ROCKET-AF trial in the number of strokes or systemic embolisms between warfarin and rivaroxaban following electrical or pharmacological cardioversion (Piccini et al., 2013).
The pre-procedural time before cardioversion is usually not delayed with NOACs, as is common with VKAs due to non-therapeutic INRs (unless there is a compliance issue). It is mandatory to explicitly ask the patient about adherence over the previous 3 weeks and to document the answer in the history notes. If compliance with NOAC has been confirmed, cardioversion seems acceptably safe. Transoesophageal echocardiogram should be considered if there is doubt about compliance. In patients with stroke risk factors or at high risk of recurrence, OAC should be continued long-term, whether with a VKA or a NOAC (Heidbuchel et al., 2015, Lip and Lane, 2015b).
17. Catheter Ablation and Anticoagulation in Atrial Fibrillation
Catheter ablation should be reserved for patients with AF who remain symptomatic despite optimal medical therapy (Camm et al., 2010). A medium size randomised study showed that performing catheter ablation of AF without warfarin discontinuation and with a therapeutic INR in patients at high risk for stroke significantly reduces the occurrence of periprocedural stroke/TIA and minor bleeding complications (Di Biase et al., 2014). The VENTURE-AF is the first randomised prospective comparative trial of an uninterrupted NOAC (Rivaroxaban) vs. VKA therapy in patients with AF undergoing catheter ablation. In patients undergoing catheter ablation for AF, the use of uninterrupted oral rivaroxaban was feasible and event rates were similar to those for uninterrupted VKA therapy (Cappato et al., 2015). More trials are underway to define the role of dabigatran [RE-CIRCUIT, DAPPAR-AF (NCT01468155) and ODIn-AF (PubMed — NCBI, 2015b)] around the time of ablation procedures.
18. Should Bridging Therapy Be Used or Not?
Bridging anticoagulation is defined as administration of a short-acting anticoagulant (low molecular weight heparin or unfractionated heparin) during interruption of VKA therapy when the INR is not within a therapeutic range (Douketis et al., 2012). Use of bridging anticoagulation was associated with significantly higher overall rates of bleeding and the data do not support routine use of bridging in anticoagulated patients with non-valvular AF (Steinberg et al., 2015, Douketis et al., 2015).
Post-procedural heparin use should be reserved for patients with the highest thromboembolic risk (e.g., patients with mitral or multiple mechanical heart valves or prior stroke) (Schulman et al., 2015). Given the rapid onset and offset of action of NOACs, no bridging therapy is required for the majority of interventions, although this is dependent upon balancing the risks of stroke/thromboembolism vs. bleeding in individual cases and the HAS-BLED score can to assist the decision. Following surgery, NOACs can be restarted as soon as effective haemostasis has been achieved, and the anticoagulant effect will be evident within a few hours after the first dose (Camm et al., 2012b).
19. Left Atrial Appendage Occlusion
The left atrial appendage (LAA) is considered the main (but not the only) site of thrombi formation in the heart in AF. Minimally invasive epicardial and interventional trans-septal techniques have been developed for occlusion of the LAA orifice to reduce the stroke risk (Camm et al., 2012b). The two trials assessed the LAA occlusion devices (PROTECT-AF, PREVAIL) and showed that the devices were non-inferior to warfarin for ischemic stroke prevention or systemic embolism during 7 days post-procedure, although non-inferiority was not achieved for overall efficacy (Table 6) (Holmes et al., 2009, Holmes et al., 2014). Current consensus is that LAA occlusion may be considered in patients with a high stroke risk and contraindications for long-term OAC (Atrial fibrillation: management | Guidance and guidelines | NICE, 2015).
Table 6.
Comparison of the PREVAIL and PROTECT studies.
| Study |
PREVAIL (Holmes et al., 2014) |
PROTECT (Holmes et al., 2009) |
||
|---|---|---|---|---|
| Group | Interventiona | Controlb | Interventiona | Controlb |
| Number of patients | 269 | 138 | 463 | 244 |
| CHADS2 score mean ± standard deviation (range) | 2.6 ± 1.0 (1.0, 6.0) | 2.2 ± 1.2 (1.0, 6.0) | ||
| Age, years mean ± standard deviation (range) | 74.0 ± 7.4 (50.0, 94.0) | 71.7 ± 8.8 (46.0, 95.0) | ||
| Implant success | 95.1% | 90.9% | ||
| All 7-day procedural complications | 4.5% | 8.7% | ||
| Ischemic stroke | 1.9% 18-month |
0.7% 18-month |
15/694 · 6 Events/ patient-years |
6/372 · 3 Events/ patient-years |
| All-cause mortality | 2.6% 18-month |
2.2% 18-month |
21/708 · 4 Events/ patient-years |
18/374 · 9 Events/ patient-years |
| Primary efficacy event rate (after the first 7 days from randomisation) for prevention of stroke and Systemic embolism | Non-inferior to warfarin | Non-inferior to warfarin | ||
Percutaneous closure of the LAA by use of WATCHMAN device.
Warfarin for the duration of the study (target international normalised ratio [INR] between 2 · 0 to 3 · 0).
20. Thrombolysis in AF Patients Receiving Oral Anticoagulation
Patients with AF who experience acute ischaemic stroke should be considered for urgent thrombolytic therapy to restore perfusion and function of the ischemic brain. However, effective anticoagulation present at the time of reperfusion is a contraindication for thrombolysis because of the possibility of increased risk of symptomatic haemorrhage (De Keyser et al., 2007). Therefore, current guidelines recommend against using the intravenous recombinant tissue-type plasminogen activator alteplase in patients with acute ischemic stroke who have an international normalised ratio (INR) > 1.7 (Xian et al., 2012). The possible risk of thrombolysis in patients given NOACs remains unclear.
Point-of-care coagulation tests are urgently warranted for management of acute stroke, especially in patients pre-treated with NOAC and who are eligible for intravenous thrombolysis. A patient taking one of the NOACs who experiences an acute ischemic stroke should not be considered a candidate for thrombolysis unless his/her clinical history and the results of laboratory tests reliably indicate the absence of an anticoagulant effect, or until at least two half-lives have elapsed since the most recent dose in patients with normal renal function and coagulation tests are normal (Diener et al., 2013). In a patient taking dabigatran, a quick and simple test could be an activated partial thromboplastin test (aPTT), and for rivaroxaban, a calibrated dilute prothrombin time (Heidbuchel et al., 2015).
21. Conclusion
Oral anticoagulation is the cornerstone for stroke prevention in AF. Recent advances in AF related stroke and bleeding risk stratification facilitate an essential shift in clinical decision-making regarding the use of oral anticoagulant therapy in non-valvular AF. However there are still clinical scenarios, like valvular AF and end stage renal failure associated with AF, where NOACs have a limited role and the use of VKAs is needed. There is insufficient evidence to recommend one NOAC over another, although some patient characteristics, drug compliance, tolerability and cost may be important considerations in the choice of agent. The development of specific NOAC antidotes marks a step forward in addressing concerns about safety of these drugs. Availability of specific reversal agents for the NOACs would improve the confidence of clinicians and patients in the NOACs and promote adequate anticoagulation in these patients.
22. Outstanding Questions
Many questions still need to be answered regarding the use and the safety of the NOACs. Should patients with stable TTR be switched to a NOAC? Will poor adherence have a greater negative impact with the NOACs than with VKAs? What is the optimal management of patients with fluctuating renal function? Will individualised NOAC dose adjustment lead to greater efficacy and safety? Will poor adherence have a greater negative impact with the NOACs than with VKAs?
To optimise the effectiveness of NOACs in routine practise across a wide spectrum of patients, further data are needed to answer these questions and improve confidence of both physicians and patients when using these drugs.
23. Search Strategy
Data for this review were identified by searches of PubMed using the search terms: atrial fibrillation, thromboembolism, stroke risk, risk stratification, oral anticoagulation, warfarin, aspirin, non-Vitamin K oral anticoagulant, net clinical benefit and thromboprophylaxis. Our search was conducted for articles up to 31 October 2015 and we focused on primary published research articles and systematic reviews, as well as on clinical trials complemented by large observational cohorts.
Author Contributions
Christos Voukalis: literature review, drafting of the manuscript, critical revision.
Gregory YH Lip: manuscript design, critical revision.
Eduard Shantsila: drafting and critical revision.
References
- [European atrial fibrillation trial (EAFT) Secondary prevention with anticoagulants and aspirin in patients with non-valvular atrial fibrillation. — PubMed — NCBI. 2015. http://www.ncbi.nlm.nih.gov/pubmed/2006364 (Accessed January 6, 2016)
- Abbott K.C., Trespalacios F.C., Taylor A.J., Agodoa L.Y. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol. 2003;4(1):1. doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumuaileq R.R.-Y., Abu-Assi E., Raposeiras-Roubin S. Evaluation of SAMe-TT2R2 risk score for predicting the quality of anticoagulation control in a real-world cohort of patients with non-valvular atrial fibrillation on vitamin-K antagonists. Europace. 2015;17(5):711–717. doi: 10.1093/europace/euu353. [DOI] [PubMed] [Google Scholar]
- Agarwal M., Apostolakis S., Lane D.A., Lip G.Y.H. The impact of heart failure and Left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin. Ther. 2014;36(9):1135–1144. doi: 10.1016/j.clinthera.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Amin A., Deitelzweig S., Jing Y. Estimation of the impact of warfarin’s time-in-therapeutic range on stroke and major bleeding rates and its influence on the medical cost avoidance associated with novel oral anticoagulant use-learnings from ARISTOTLE, ROCKET-AF, and RE-LY trials. J. Thromb. Thrombolysis. 2014;38(2):150–159. doi: 10.1007/s11239-013-1048-z. [DOI] [PubMed] [Google Scholar]
- Anandasundaram B., Lane D.A., Apostolakis S., Lip G.Y.H. The impact of atherosclerotic vascular disease in predicting a stroke, thromboembolism and mortality in atrial fibrillation patients: a systematic review. J. Thromb. Haemost. 2013;11(November 2012):975–987. doi: 10.1111/jth.12177. [DOI] [PubMed] [Google Scholar]
- Apostolakis S., Lane D.A., Buller H., Lip G.Y.H. Comparison of the CHADS2, CHA2DS2-VASc and HAS-BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb. Haemost. 2013;110(5):1074–1079. doi: 10.1160/TH13-07-0552. [DOI] [PubMed] [Google Scholar]
- Apostolakis S., Lane D.A., Guo Y., Buller H., Lip G.Y.H. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J. Am. Coll. Cardiol. 2013;61(3):386–387. doi: 10.1016/j.jacc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Apostolakis S., Lane D.A., Guo Y., Buller H., Lip G.Y.H. Performance of the HEMORR 2 HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J. Am. Coll. Cardiol. 2013;61(3):386–387. doi: 10.1016/j.jacc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Apostolakis S., Sullivan R.M., Olshansky B., Lip G.Y.H. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT₂R₂ score. Chest. 2013;144(5):1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- Atrial fibrillation: management | Guidance and guidelines | NICE 2015. https://www.nice.org.uk/guidance/cg180 (Accessed November 5, 2015)
- Ball J., Carrington M.J., McMurray J.J.V., Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 2013;167(5):1807–1824. doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Taillandier S., Olesen J.B. Ejection fraction and outcomes in patients with atrial fibrillation and heart failure: the Loire Valley Atrial Fibrillation Project. Eur. J. Heart Fail. 2012;14(3):295–301. doi: 10.1093/eurjhf/hfs005. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Lane D.A., Torp-Pedersen C., Lip G.Y.H. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a “real world” atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb. Haemost. 2012;107(3):584–589. doi: 10.1160/TH11-11-0784. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Taillandier S., Olesen J.B. Pattern of atrial fibrillation and risk of outcomes: the Loire Valley Atrial Fibrillation Project. Int. J. Cardiol. 2013;167(6):2682–2687. doi: 10.1016/j.ijcard.2012.06.118. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Clementy N., Haguenoer K., Fauchier L., Lip G.Y.H. Prior history of falls and risk of outcomes in atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Am. J. Med. 2014;127(10):972–978. doi: 10.1016/j.amjmed.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Bauersachs R., Berkowitz S.D., Brenner B. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- Berge E. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. Lancet. 2000;355(9211):1205–1210. doi: 10.1016/s0140-6736(00)02085-7. ( http://www.thelancet.com/newlancet/sub/issues/vol355no9211/menu_NOD999.html) [DOI] [PubMed] [Google Scholar]
- Beyth R.J., Quinn L.M., Landefeld C.S. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am. J. Med. 1998;105(2):91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- Blann A.D., Banerjee A., Lane D.A., Torp-Pedersen C., Lip G.Y.H. Net clinical benefit of edoxaban versus no treatment in a “real world” atrial fibrillation population: A modelling analysis based on a nationwide cohort study. Int. J. Cardiol. 2015;201:693–698. doi: 10.1016/j.ijcard.2015.08.074. [DOI] [PubMed] [Google Scholar]
- Broderick J.P., Brott T., Tomsick T., Huster G., Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N. Engl. J. Med. 1992;326(11):733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- Brønnum Nielsen P., Larsen T.B., Gorst-Rasmussen A., Skjøth F., Rasmussen L.H., Lip G.Y.H. Intracranial hemorrhage and subsequent ischemic stroke in patients with atrial fibrillation: a nationwide cohort study. Chest. 2015;147(6):1651–1658. doi: 10.1378/chest.14-2099. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Kirchhof P., Lip G.Y.H. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur. Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Lip G.Y.H., De Caterina R. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation * Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Lip G.Y.H., De Caterina R. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–1413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- Cappato R., Marchlinski F.E., Hohnloser S.H. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur. Heart J. 2015;36(28):1805–1811. doi: 10.1093/eurheartj/ehv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.E., Edelman E.R., Wenger J.B., Thadhani R.I., Maddux F.W. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–979. doi: 10.1161/CIRCULATIONAHA.114.014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Lip G.Y.H. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol. Haemost. Thromb. 2004;33(5–6):282–289. doi: 10.1159/000083815. (doi:83815) [DOI] [PubMed] [Google Scholar]
- Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- Connolly S.J., Eikelboom J., Joyner C. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- De Caterina R., Husted S., Wallentin L. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC working group on thrombosis—task force on anticoagulants in heart disease. Thromb. Haemost. 2013;110(6):1087–1107. doi: 10.1160/TH13-06-0443. [DOI] [PubMed] [Google Scholar]
- De Keyser J., Gdovinová Z., Uyttenboogaart M., Vroomen P.C., Luijckx G.J. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007;38(9):2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- Dewilde W.J.M., Oirbans T., Verheugt F.W.A. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet (London, England) 2013;381(9872):1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- Di Biase L., Burkhardt J.D., Santangeli P. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patient. Circulation. 2014;129(25):2638–2644. doi: 10.1161/CIRCULATIONAHA.113.006426. [DOI] [PubMed] [Google Scholar]
- Diener H.C., Connolly S.J., Ezekowitz M.D. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9(12):1157–1163. doi: 10.1016/S1474-4422(10)70274-X. [DOI] [PubMed] [Google Scholar]
- Diener H.-C., Foerch C., Riess H., Röther J., Schroth G., Weber R. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol. 2013;12(7):677–688. doi: 10.1016/S1474-4422(13)70101-7. [DOI] [PubMed] [Google Scholar]
- Donzé J., Clair C., Hug B. Risk of falls and major bleeds in patients on oral anticoagulation therapy. Am. J. Med. 2012;125(8):773–778. doi: 10.1016/j.amjmed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Douketis J.D., Spyropoulos A.C., Spencer F.A. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl) doi: 10.1378/chest.11-2298. (e326S - 50S) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douketis J.D., Spyropoulos A.C., Kaatz S. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N. Engl. J. Med. 2015;373(9) doi: 10.1056/NEJMoa1501035. (150622051516008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman M.H., Singer D.E., Rosand J., Greenberg S.M. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ. Cardiovasc. Qual. Outcomes. 2011;4(1):14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M.C., Go A.S., Chang Y. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibrillation A., Group W. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69(6):546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- Flaker G., Lopes R.D., Al-Khatib S.M. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) J. Am. Coll. Cardiol. 2014;63(11):1082–1087. doi: 10.1016/j.jacc.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Friberg L., Benson L., Rosenqvist M., Lip G.Y.H. Assessment of female sex as a risk Factor in Atrial Fibrillation in Sweden: Nationwide Retrospective Cohort Study. BMJ. 2012;344 doi: 10.1136/bmj.e3522. ( http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3365143&tool=pmcentrez&rendertype=abstract. Accessed November 25, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg L., Rosenqvist M., Lip G.Y.H. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation Cohort Study. Eur. Heart J. 2012;33(12):1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- Friberg L., Rosenqvist M., Lip G.Y.H. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125(19):2298–2307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J. Am. Med. Assoc. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. ( http://www.ncbi.nlm.nih.gov/pubmed/11401607. Accessed October 10, 2015) [DOI] [PubMed] [Google Scholar]
- Gage B.F., Van Walraven C., Pearce L. Selecting patients with atrial fibrillation for anticoagulation: Stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- Gage B.F., Yan Y., Milligan P.E. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am. Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gallego P., Roldan V., Marín F. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb. Haemost. 2013;110(6):1189–1198. doi: 10.1160/TH13-07-0556. [DOI] [PubMed] [Google Scholar]
- Gallego P., Roldán V., Marin F. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am. J. Med. 2014;127(11):1083–1088. doi: 10.1016/j.amjmed.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Giugliano R.P., Ruff C.T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- Granger C.B., Alexander J.H., McMurray J.J.V. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- Greinacher A., Thiele T., Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb. Haemost. 2015;113(5):931–942. doi: 10.1160/TH14-11-0982. [DOI] [PubMed] [Google Scholar]
- Hankey G.J. Unanswered questions and research priorities to optimise stroke prevention in atrial fibrillation with the new oral anticoagulants. Thromb. Haemost. 2014;111(5):808–816. doi: 10.1160/TH13-09-0741. [DOI] [PubMed] [Google Scholar]
- Hansen M.L., Sørensen R., Clausen M.T. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch. Intern. Med. 2010;170(16):1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- Hart R.G., Benavente O., McBride R., Pearce L.A. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 1999;131(7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. ( http://www.ncbi.nlm.nih.gov/pubmed/10507957. Accessed September 15, 2015) [DOI] [PubMed] [Google Scholar]
- Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. ( http://www.ncbi.nlm.nih.gov/pubmed/17577005. Accessed May 30, 2015) [DOI] [PubMed] [Google Scholar]
- Hart R.G., Eikelboom J.W., Brimble K.S., McMurtry M.S., Ingram A.J. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can. J. Cardiol. 2013;29(7 Suppl):S71–S78. doi: 10.1016/j.cjca.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Heidbuchel H., Verhamme P., Alings M. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10) doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- Hijazi Z., Oldgren J., Andersson U. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: A Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) Substudy. Circulation. 2012;125(13):1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C., Brindle P. Derivation and validation of QStroke score for predicting risk of ischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study. Bmj. 2013;346(may02 1):f2573. doi: 10.1136/bmj.f2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.R., Reddy V.Y., Turi Z.G. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet (London, England) 2009;374(9689):534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- Holmes D.R., Kar S., Price M.J. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J. Am. Coll. Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Supporting local implementation of NICE guidance on use of the novel (non-Vitamin K antagonist) oral anticoagulants in non-valvular atrial fibrillation. 2015. https://www.nice.org.uk/resource/CG180/pdf/c/cg180-atrial-fibrillation-nic-consensus-statement-on-the-use-of-noacs?id=gvyb3hjdqrcjtn6ytpwx3ydb64 (Accessed November 17, 2015)
- Hu H.H., Sheng W.Y., Chu F.L., Lan C.F., Chiang B.N. Incidence of stroke in Taiwan. Stroke. 1992;23(9):1237–1241. doi: 10.1161/01.str.23.9.1237. ( http://www.ncbi.nlm.nih.gov/pubmed/1519277. Accessed January 2, 2016) [DOI] [PubMed] [Google Scholar]
- A differential effect of aspirin on prevention of stroke in atrial fibrillation. J. Stroke Cerebrovasc. Dis. 1993;3(3):181–188. doi: 10.1016/S1052-3057(10)80159-4. [DOI] [PubMed] [Google Scholar]
- January C.T., Wann F.L.S., Joseph F. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J. Am. Coll. Cardiol. 2014;64(21):2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Kernan W.N., Ovbiagele B., Black H.R. vol 45. 2014. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. [DOI] [PubMed] [Google Scholar]
- Kirchhof P., Lip G.Y.H., Van Gelder I.C. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options. Executive summary of the report from the 3rd AFNET/EHRA Consensus Conference. Thromb. Haemost. 2011;106(6):1012–1019. doi: 10.1160/TH11-07-0517. [DOI] [PubMed] [Google Scholar]
- Klatsky A.L., Friedman G.D., Sidney S., Kipp H., Kubo A., Armstrong M.A. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology. 2005;25(1):26–31. doi: 10.1159/000085310. [DOI] [PubMed] [Google Scholar]
- Krejczy M., Harenberg J., Wehling M., Obermann K., Lip G.Y.H. Cost-Effectiveness of Anticoagulation in Patients with Nonvalvular Atrial Fibrillation with Edoxaban Compared to Warfarin in Germany. Biomed. Res. Int. 2015;2015:876923. doi: 10.1155/2015/876923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg M.G., O'Donnell M.J., Khatri P. nineth ed. 141(2 Suppl) American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis. (Chest). (e601S - 36S) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Park K.-Y., Shin J.-H. Symptomatic hemorrhagic transformation and its predictors in acute ischemic stroke with atrial fibrillation. Eur. Neurol. 2010;64(4):193–200. doi: 10.1159/000319048. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. CHEST J. 2010;137(2):263. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H. The role of aspirin for stroke prevention in atrial fibrillation. Nat. Rev. Cardiol. 2011;8(10):602–606. doi: 10.1038/nrcardio.2011.112. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H. The CHA2DS2-VASc score for stroke risk stratification in patients with atrial fibrillation: a brief history. Eur. Heart J. 2015 doi: 10.1093/eurheartj/ehv431. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Lane D.A. Assessing bleeding risk in atrial fibrillation with the HAS-BLED and ORBIT scores: clinical application requires focus on the reversible bleeding risk factors. Eur. Heart J. 2015 September doi: 10.1093/eurheartj/ehv415. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Lane D.A. Stroke Prevention in Atrial Fibrillation. J. Am. Med. Assoc. 2015;313(19):1950. doi: 10.1001/jama.2015.4369. [DOI] [PubMed] [Google Scholar]
- Lip G.Y., Lip P.L., Zarifis J. Fibrin D-dimer and beta-thromboglobulin as markers of thrombogenesis and platelet activation in atrial fibrillation. effects of introducing ultra-low-dose warfarin and aspirin. Circulation. 1996;94(3):425–431. doi: 10.1161/01.cir.94.3.425. ( http://www.ncbi.nlm.nih.gov/pubmed/8759084. Accessed November 26, 2015) [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Andreotti F., Fauchier L. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13(5):723–746. doi: 10.1093/europace/eur126. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Haguenoer K., Saint-Etienne C., Fauchier L. Relationship of the SAMe-TT₂R₂ score to poor-quality anticoagulation, stroke, clinically relevant bleeding, and mortality in patients with atrial fibrillation. Chest. 2014;146(3):719–726. doi: 10.1378/chest.13-2976. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Windecker S., Huber K. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on. Eur. Heart J. 2014;35(45):3155–3179. doi: 10.1093/eurheartj/ehu298. [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Skjøth F., Nielsen P.B., Larsen T.B. Non-valvular atrial fibrillation patients with none or one additional risk factor of the CHA2DS2-VASc score. A comprehensive net clinical benefit analysis for warfarin, aspirin, or no therapy. Thromb. Haemost. 2015;114(4):826–834. doi: 10.1160/TH15-07-0565. [DOI] [PubMed] [Google Scholar]
- Man-Son-Hing M., Nichol G., Lau A., Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch. Intern. Med. 1999;159(7):677–685. doi: 10.1001/archinte.159.7.677. ( http://www.ncbi.nlm.nih.gov/pubmed/10218746. Accessed November 25, 2015) [DOI] [PubMed] [Google Scholar]
- Mearns E.S., White C.M., Kohn C.G. Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta-analysis and meta-regression. Thromb. J. 2014;12:14. doi: 10.1186/1477-9560-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgaard L., Rasmussen L.H., Skjøth F., Lip G.Y.H., Larsen T.B. Age dependence of risk factors for stroke and death in young patients with atrial fibrillation: a nationwide study. Stroke. 2014;45(5):1331–1337. doi: 10.1161/STROKEAHA.114.004903. [DOI] [PubMed] [Google Scholar]
- Mikkelsen A., Lindhardsen J., Lip G., Gislason G.H., Torp-Pedersen C., Olesen J.B. Female sex asa risk factor for stroke in atrial fibrillation: a nationwide cohort study. J. Thromb. Haemost. 2012:1745–1751. doi: 10.1111/j.1538-7836.2012.04853.x. (July) [DOI] [PubMed] [Google Scholar]
- Mueck W., Lensing A.W.A., Agnelli G., Decousus H., Prandoni P., Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 2011;50(10):675–686. doi: 10.2165/11595320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nagarakanti R., Ezekowitz M.D., Oldgren J. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131–136. doi: 10.1161/CIRCULATIONAHA.110.977546. [DOI] [PubMed] [Google Scholar]
- Nielsen P.B., Larsen T.B., Skjøth F., Gorst-Rasmussen A., Rasmussen L.H., Lip G.Y.H. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding. CLINICAL PERSPECTIVE. Circulation. 2015;132(6):517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- O'Brien E.C., Simon D.N., Thomas L.E. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur. Heart J. 2015 September doi: 10.1093/eurheartj/ehv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J.B., Lip G.Y.H., Hansen M.L. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide Cohort Study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. ( http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3031123&tool=pmcentrez&rendertype=abstract. Accessed September 27, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J.B., Lip G.Y.H., Lindhardsen J. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: A net clinical benefit analysis using a “real world” Nationwide Cohort Study. Thromb. Haemost. 2011;106(4):739–749. doi: 10.1160/TH11-05-0364. [DOI] [PubMed] [Google Scholar]
- Olesen J.B., Lip G.Y.H., Kamper A.-L. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012;367(7):625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- Omran H., Bauersachs R., Rübenacker S., Goss F., Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation: Results from the national multicentre BNK Online Bridging Registry (BORDER) Thromb. Haemost. 2012;108(1):65–73. doi: 10.1160/TH11-12-0827. [DOI] [PubMed] [Google Scholar]
- Paciaroni M., Agnelli G. Should oral anticoagulants be restarted after warfarin-associated cerebral haemorrhage in patients with atrial fibrillation? Thromb. Haemost. 2014;111(1):14–18. doi: 10.1160/TH13-08-0667. [DOI] [PubMed] [Google Scholar]
- Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- Piccini J.P., Stevens S.R., Lokhnygina Y. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF Trial. J. Am. Coll. Cardiol. 2013;61(19):1998–2006. doi: 10.1016/j.jacc.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest. 2010;1093-1100 doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- Pisters R., Lane D.A., Marin F., Camm a.J., Lip G.Y.H. Stroke and Thromboembolism in Atrial Fibrillation. Circ. J. 2012;76(10):2289–2304. doi: 10.1253/circj.cj-12-1036. [DOI] [PubMed] [Google Scholar]
- Poli D., Antonucci E., Testa S., Lip G.Y.H. A prospective validation of the SAME-TT2R2 score: how to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Intern. Emerg. Med. 2014;9(4):443–447. doi: 10.1007/s11739-014-1065-8. [DOI] [PubMed] [Google Scholar]
- Pollack C.V., Reilly P.A., Bernstein R. Design and rationale for RE-VERSE AD: A Phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb. Haemost. 2015;114(1):198–205. doi: 10.1160/TH15-03-0192. [DOI] [PubMed] [Google Scholar]
- Pollack C.V., Reilly P.A., Eikelboom J. Idarucizumab for Dabigatran Reversal. N. Engl. J. Med. 2015;373(6):511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- Potpara T.S., Lip G.Y.H. Novel oral anticoagulants in non-valvular atrial fibrillation. Best Pract. Res. Clin. Haematol. 2013;26(2):115–129. doi: 10.1016/j.beha.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Predictors of thromboembolism in atrial fibrillation II. Echocardiographic features of patients at risk. The stroke prevention in atrial fibrillation investigators. Ann. Intern. Med. 1992;116(1):6–12. doi: 10.7326/0003-4819-116-1-6. ( http://www.ncbi.nlm.nih.gov/pubmed/1727097. Accessed November 8, 2015) [DOI] [PubMed] [Google Scholar]
- PubMed – NCBI Dual or single antiplatelet therapy with anticoagulation? — PubMed — NCBI. 2015. http://www.ncbi.nlm.nih.gov/pubmed/23415014 (Accessed January 6, 2016)
- PubMed – NCBI Rationale and design of the ODIn-AF Trial: randomized evaluation of the prevention of silent cerebral thromboembolism by oral anticoagulation with. — PubMed — NCBI. 2015. http://www.ncbi.nlm.nih.gov/pubmed/26514352 (Accessed January 6, 2016) [DOI] [PubMed]
- Qureshi A.I., Tuhrim S., Broderick J.P., Batjer H.H., Hondo H., Hanley D.F. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Qureshi W., Mittal C., Patsias I. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am. J. Cardiol. 2014;113(4):662–668. doi: 10.1016/j.amjcard.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Roldán V., Marín F., Manzano-Fernández S. The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J. Am. Coll. Cardiol. 2013;62(23):2199–2204. doi: 10.1016/j.jacc.2013.08.1623. [DOI] [PubMed] [Google Scholar]
- Roldán V., Cancio S., Gálvez J. The SAMe-TT2R2 score predicts poor anticoagulation control in AF patients: a prospective “real-world” Inception Cohort Study. Am. J. Med. 2015;128(11):1237–1243. doi: 10.1016/j.amjmed.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Rubboli A., Faxon D.P., Juhani Airaksinen K.E. The optimal management of patients on oral anticoagulation undergoing coronary artery stenting. The 10th Anniversary Overview. Thromb. Haemost. 2014;112(6):1080–1087. doi: 10.1160/TH14-08-0681. [DOI] [PubMed] [Google Scholar]
- Ruff C.T., Giugliano R.P., Braunwald E. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortiz M., Bertomeu V., Cequier Á., Marín F., Anguita M. Validation of the SAMe-TT2R2 score in a nationwide population of nonvalvular atrial fibrillation patients on vitamin K antagonists. Thromb. Haemost. 2015;114(4):695–701. doi: 10.1160/TH15-02-0169. [DOI] [PubMed] [Google Scholar]
- Sacco R.L., Boden-Albala B., Gan R. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am. J. Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. ( http://www.ncbi.nlm.nih.gov/pubmed/9482500. Accessed January 2, 2016) [DOI] [PubMed] [Google Scholar]
- Schulman J.M., Majeed A., Mattsson E., Schulman S., Holmström M., Ågren A. Strategies and outcomes of periprocedural bridging therapy with low-molecular-weight heparin in patients with mechanical heart valves. J. Thromb. Thrombolysis. 2015;40(4):430–436. doi: 10.1007/s11239-015-1216-4. [DOI] [PubMed] [Google Scholar]
- Sengupta N., Feuerstein J.D., Patwardhan V.R. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am. J. Gastroenterol. 2015;110(2):328–335. doi: 10.1038/ajg.2014.398. [DOI] [PubMed] [Google Scholar]
- Shen A.Y.-J., Yao J.F., Brar S.S., Jorgensen M.B., Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007;50(4):309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- Shields A.M., Lip G.Y.H. Choosing the right drug to fit the patient when selecting oral anticoagulation for stroke prevention in atrial fibrillation. J. Intern. Med. 2015;278(1):1–18. doi: 10.1111/joim.12360. [DOI] [PubMed] [Google Scholar]
- Singer D.E., Chang Y., Borowsky L.H. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: The ATRIA study stroke risk score. J. Am. Heart Assoc. 2013;2(3) doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjøth F., Larsen T.B., Rasmussen L.H., Lip G.Y.H. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb. Haemost. 2014;111(5):981–988. doi: 10.1160/TH14-02-0118. [DOI] [PubMed] [Google Scholar]
- Smith J.G., Wieloch M., Koul S. Triple antithrombotic therapy following an acute coronary syndrome: prevalence, outcomes and prognostic utility of the HAS-BLED score. EuroIntervention J. Eur. Collab. Work Gr. Interv. Cardiol. Eur. Soc. Cardiol. 2012;8(6):672–678. doi: 10.4244/EIJV8I6A105. [DOI] [PubMed] [Google Scholar]
- Steinberg B.A., Peterson E.D., Kim S. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Circulation. 2015;131(5):488–494. doi: 10.1161/CIRCULATIONAHA.114.011777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Stroke Prevention in Atrial Fibrillation Investigators Predictors of Thromboembolism in Atrial Fibrillation: II. Echocardiographic Features of Patients at Risk. Ann. Intern. Med. 1992;116(1):6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- Thrift A.G., Dewey H.M., Macdonell R.A., McNeil J.J., Donnan G.A. Incidence of the major stroke subtypes: initial findings from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2001;32(8):1732–1738. doi: 10.1161/01.str.32.8.1732. ( http://www.ncbi.nlm.nih.gov/pubmed/11486098. Accessed January 2, 2016) [DOI] [PubMed] [Google Scholar]
- Tran H.A., Chunilal S.D., Harper P.L., Tran H., Wood E.M., Gallus A.S. An update of consensus guidelines for warfarin reversal. Med. J. Aust. 2013;198(4):198–199. doi: 10.5694/mja12.10614. ( http://www.ncbi.nlm.nih.gov/pubmed/23451962. Accessed November 21, 2015) [DOI] [PubMed] [Google Scholar]
- Verma A., Cairns J.A., Mitchell L.B. 2014 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2014;30(10):1114–1130. doi: 10.1016/j.cjca.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Wagstaff A.J., Overvad T.F., Lip G.Y.H., Lane D.A. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM. 2014;107(12):955–967. doi: 10.1093/qjmed/hcu054. [DOI] [PubMed] [Google Scholar]
- Wan Y., Heneghan C., Perera R. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ. Cardiovasc. Qual. Outcomes. 2008;1(2):84–91. doi: 10.1161/CIRCOUTCOMES.108.796185. [DOI] [PubMed] [Google Scholar]
- Wang K.-L., Lip G.Y.H., Lin S.-J., Chiang C.-E. Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention in Asian Patients With Nonvalvular Atrial Fibrillation: Meta-Analysis. Stroke. 2015;46(9):2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee S.G., Whincup P.H., Lennon L., Rumley A., Lowe G.D. Fibrin D-dimer, tissue-type plasminogen activator, von Willebrand factor, and risk of incident stroke in older men. Stroke. 2012;43(5):1206–1211. doi: 10.1161/STROKEAHA.111.636373. [DOI] [PubMed] [Google Scholar]
- Weitz J.I., Pollack C.V. Practical management of bleeding in patients receiving non-vitamin K antagonist oral anticoagulants. Thromb. Haemost. 2015:114(5). doi: 10.1160/TH15-03-0222. [DOI] [PubMed] [Google Scholar]
- Xian Y., Liang L., Smith E.E. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. J. Am. Med. Assoc. 2012;307(24):2600–2608. doi: 10.1001/jama.2012.6756. [DOI] [PubMed] [Google Scholar]
- You J.J., Singer D.E., Howard P.A. nineth ed. 141(2 Suppl) American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis. (Chest). (e531S - 75S) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalgoitia M., Halperin J.L., Pearce L.A., Blackshear J.L., Asinger R.W., Hart R.G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators. J. Am. Coll. Cardiol. 1998;31(7):1622–1626. doi: 10.1016/s0735-1097(98)00146-6. ( http://www.ncbi.nlm.nih.gov/pubmed/9626843. Accessed November 25, 2015) [DOI] [PubMed] [Google Scholar]