Abstract
Robust, long-lasting immune responses are elicited by memory T cells that possess properties of stem cells, enabling them to persist long-term and to permanently replenish the effector pools. Thus, stem cell-like memory T (TSCM) cells are of key therapeutic value and efforts are underway to characterize TSCM cells and to identify means for their targeted induction.
Here, we show that inhibition of mechanistic/mammalian Target of Rapamycin (mTOR) complex 1 (mTORC1) by rapamycin or the Wnt-β-catenin signalling activator TWS119 in activated human naive T cells leads to the induction of TSCM cells. We show that these compounds switch T cell metabolism to fatty acid oxidation as favoured metabolic programme for TSCM cell generation. Of note, pharmacologically induced TSCM cells possess superior functional features as a long-term repopulation capacity after adoptive transfer. Furthermore, we provide insights into the transcriptome of TSCM cells.
Our data identify a mechanism of pharmacological mTORC1 inhibitors, allowing us to confer stemness to human naive T cells which may be significantly relevant for the design of innovative T cell-based cancer immunotherapies.
Keywords: Human T cells, T cell differentiation, Adoptive cell transfer therapy, Rapamycin
Highlights
-
•
Immunostimulatory effect of rapamycin on induction of TSCM cells
-
•
Previously unknown mTORC1 inhibiting drug effect of the Wnt activator TWS119
-
•
Insights into the metabolic regulation of human CD4 + TSCM cells and into their transcriptome
Stem cell-like memory T (TSCM) cells represent the newly identified memory T cell subset, which harbours key therapeutic value by its self-renewal capacity and ability to generate memory and effector T cells. However, the signalling pathways controlling TSCM cell formation remain incompletely understood. Here, we present the induction of CD4 + TSCM cells from highly purified naïve cells by pharmacological inhibition of mTORC1 by either rapamycin or TWS119. We carried out comprehensive transcriptome and metabolic analyses of induced and naturally occurring TSCM cells. Targeted induction of TSCM cells by pharmacological means is highly relevant for the design of novel immunotherapeutic approaches.
1. Introduction
Similar to solid organs, T cells have been suggested to harbour a self-renewing stem cell-like population which permanently replenishes the pools of further differentiated effectors. Since central memory T (TCM) cells have been shown to repopulate the effector memory T (TEM) cell and effector T (TEFF) cell pools in response to antigen stimulus (Graef et al., 2014, Wherry et al., 2003), they were thus far regarded as “memory stem cells”. However, further complexity was brought to this view by the recent discovery of an additional memory T cell subset, which was able to mediate a prolonged immune response in a mouse model of graft-versus-host disease (GVHD) (Zhang et al., 2005). This memory T cell subset, termed stem cell-like memory T (TSCM) cells, has been recently described in mice, non-human primates and in humans (Gattinoni et al., 2009, Gattinoni et al., 2011, Lugli et al., 2013). As least differentiated distinct memory T cell subset, TSCM cells have been put at the top of the hierarchy of all memory T cell subsets in a model of progressive T cell differentiation, leading from naive T (TN) cells over TSCM cells and TCM cells to TEM cells and TEFF cells. This position of TSCM cells between TN cells and memory T cells is phenotypically reflected by the expression of activation markers as the death receptor CD95, the β-chain of the IL-2 receptor (CD122) or the adhesion molecule CD58 on naive-appearing CCR7 +, CD45RA +, CD45RO − T cells (Gattinoni et al., 2011). After genetic modification into mesothelioma-specific CAR T cells, adoptively transferred TSCM cells were shown to mediate an improved anti-tumour immune response compared to TN cells, TCM cells and TEM cells in a humanized mouse model (Gattinoni et al., 2011), which seems to depend on a more efficient TSCM cell engraftment and long-term persistence in the host which enables them, while self-renewing, to constantly differentiate into TEFF cells and, thereby, to completely eradicate the tumour.
Because of these ideal characteristics there is a quest for the signalling pathways which mediate TSCM cell induction. Once identified, pharmacological interference with these signalling pathways could be used for their targeted induction in anti-tumour immunotherapy. In this regard, the in vitro activation of CD8 + TN cells in the presence of the Wnt-β-catenin (short: Wnt) signalling pathway activator TWS119, which inhibits glycogen synthase kinase-3β (GSK-3β) by phosphorylation, has been suggested to arrest TN cell differentiation and to generate TSCM cells (Gattinoni et al., 2011). However, the interpretability of these data remains inconclusive, since the starting pool of TN cells also contained TSCM cells so that an expansion effect of TWS119 on pre-existing TSCM cells or TSCM cell self-maintaining factors cannot be excluded. Moreover, increasing evidence suggests that T cell metabolism is an important determinant of T cell differentiation (Pearce et al., 2009), which raises the possibility that metabolic integrators like mechanistic/mammalian Target Of Rapamycin (mTOR) kinase might represent pharmacological targets for the enrichment of a desired differentiation-defined T cell population (Araki et al., 2009, Diken et al., 2013, Rao et al., 2010, Turner et al., 2011), thereby potentially favouring the induction of qualitatively improved memory T cells.
We, therefore, set out to investigate whether mTORC1 inhibitors like rapamycin would be relevant for the generation of human TSCM cells and whether a cross-talk between mTOR and Wnt signalling would exist. Moreover, since current knowledge on the generation and characterization of TSCM cells remains limited to CD8 + TSCM cells, apart from their phenotypic definition, CD4 + TSCM cells remain uninvestigated. The characterization of CD4 + TSCM cells seems to be of great importance all the more, as the role of CD4 + T cells as broad orchestrators of the immune response receives growing attention in anti-tumour immunotherapy (Kamphorst and Ahmed, 2013, Muranski and Restifo, 2009). In the present study, therefore, focus was put on the induction and characterization of CD4 + TSCM cells, nevertheless testing the relevance of our findings on TSCM cell induction also for CD8 + TSCM cells.
Here, we revealed the inhibition of mTORC1 with simultaneously active mTORC2 signalling as the molecular mechanism inducing TSCM cells and that TSCM cell induction takes place in complete independence from Wnt signalling. We furthermore present insights into the transcriptomes of naturally occurring and pharmacologically induced CD4 + TSCM cells, the in vivo survival and repopulation capacity of pharmacologically induced CD4 + TSCM cells and the metabolic regulation of CD4 + TSCM cell generation. Taken together, our findings are of direct relevance for the design of improved anti-tumour immunotherapies.
2. Materials & Methods
2.1. Human T Lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over a Ficoll-Paque gradient (Lymphoprep™) from buffy coats of healthy human female and male blood donors, obtained from the Vaud blood transfusion service. Experiments were performed in accordance to the guidelines of the Ethics Commission of the UNIL. Prior to sorting, PBMCs were purified with CD3, CD4 or CD8 Dynabeads® (Invitrogen™).
2.2. Animal Experiments
Animal experiments were performed in accordance to the guidelines of the Ethics Commission of the UNIL. In vitro experiments and assessment of TSCM cell frequencies were performed with female Raptor (CD4-Cre), β-/γ-catenin (Vav-Cre) KO mice and their corresponding WT forms. Adoptive T cell transfer was conducted with female NOD.Cg-PrkdcscidIl2rgtm1WjI/SzJ mice (NSG).
2.3. Cell Culture
T cells were cultured in RPMI-1640 supplemented with 8% heat inactivated, pooled human serum or 10% foetal calf serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, 4 mM l-glutamine, 1% (v/v) non-essential amino acids and 50 μM 2-mercaptoethanol. Sorted TN cells were primed with anti-CD3/CD28 beads (Invitrogen) or OKT3/anti-CD28 antibody (in house, derived from hybridoma cells) and IL-2 (Proleukin®, Roche Pharma AG). Pathway interfering drugs were TWS119 (Cayman Chemical), rapamycin (LC Laboratories), PP242 (Chemdea), KU-0063794 (Chemdea), Indirubin-3-monoxime (Sigma-Aldrich), SB216763 (Sigma-Aldrich) and recombinant human Wnt3A (R&D Systems).
2.4. Flow Cytometry
Flow cytometry acquisition was performed with a Gallios™ (Beckman Coulter) or a LSR II flow cytometer (BD Biosciences). Cell sorting was conducted with a FACS Aria (BD Biosciences) or a MoFlo® Astrios™ cell sorting instrument (Beckman Coulter). Flow cytometry analysis was performed with FlowJo software (Version 7.6.5, Treestar).
Antibodies and staining panels are listed in the Supplemental Experimental Procedures.
2.5. Phospho-specific Flow Cytometry
1,000,000 TN cells were sorted per condition. After activation with anti-CD3/CD28 beads (1:1 bead/cell ratio) in the presence of TWS119 (5 μM) or rapamycin (100 nM) for 4 h, TN cells were harvested, fixed and incubated with ice-cold 50% methanol for membrane permeabilization. Primary antibodies were pS6 ribosomal protein (Ser235/236), pGSK-3β (Ser9), pAKT (Ser473), p4EBP1 (Ser65), p4EBP1 (Thr37/46) (all from Cell Signaling). The secondary antibody was Alexa Fluor 647 goat anti rabbit IgG (Life Technologies).
2.6. Western Blot Analysis
For Western blot analysis activated (4 h in presence of indicated drugs) natural (n) CD4 + TN cells were washed in ice-cold PBS and lysed in RIPA buffer containing protease inhibitor and sodium orthovanadate (Santa Cruz Biotechnologies). Proteins were separated by 4% to 12% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore). Membranes were blocked with Odyssey® blocking buffer (LI-COR Biosciences) and immunoblotted with the primary antibodies pS6 ribosomal protein (Ser235/236), pAKT (Ser473), β-actin (all from Cell Signaling) followed by infrared secondary antibodies. Bands from immunoreactive proteins were visualized by an Odyssey® infrared imaging system (LI-COR Biosciences).
2.7. RNA Sequencing
RNA sequencing was conducted with nCD4 + TN, TSCM and TCM cells from 4 healthy human donors. Additionally, TWS119- and rapamycin-induced TSCM cells were resorted after 14 days of nCD4 + TN cell priming from the same donors. RNA was purified with Arcturus PicoPure RNA Isolation kit (Biosystems/Applied Life Technologies). An amount of 10 ng total RNA was amplified with the SMARTer Ultra Low RNA Kit for Illumina Sequencing (Clontech Laboratories, Inc.) and the Advantage 2 PCR Kit (Clontech Laboratories, Inc.). The cDNA from the amplification reactions was sheared with a Covaris ultrasonicator (Covaris, Inc.) and sequencing libraries were generated with a Truseq DNA kit (Illumina, Inc.). Libraries were sequenced at 100 nucleotides single read mode on an Illumina HiSeq 2000 instrument.
2.8. Adoptive T Cell Transfer
Adoptive T cell transfer was conducted by tail vein injection of 200,000 rapamycin-induced CD4 + TSCM cells and equal numbers of CD4 + TN-like and TCM-like cells. Control mice received an equal volume of culture medium. Lymphocytes from lung and liver were isolated by Percoll™ technique.
2.9. Cell Proliferation Assay
30,000 rapamycin-induced CD4 + TSCM cells and equal numbers of TN- and TCM-like cells were labelled for 6 days with carboxyfluorescein succinimidyl ester (CFSE, Life Technologies) (final concentration: 0.25 μM) and expanded in presence of IL-2 (50 IU/ml). Dilution of CFSE (488 nm) was assessed by flow cytometry. The proliferation index was calculated with ModFit LT software (Version 3.3.11, Verity Software House, Inc.).
2.10. MMP
Assessment of MMP was performed with TMRE — Mitochondrial Membrane Potential Assay Kit (Abcam®). 5,000,000 nCD4 + T cells and equal numbers of T cells derived from nCD4 + TN cells, which have been activated for 14 days in presence of rapamycin (100 nM), were used. T cells were incubated with 100 nM tetramethylrhodamine, ethyl ester (TMRE) for 30 min at 37 °C in the water bath. Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP, 100 μM) was added during acquisition.
2.11. 2-NBDG Uptake
2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG, Invitrogen) uptake was carried out with 5,000,000 nCD4 + T cells and equal numbers of T cells, derived from nCD4 + TN cells, which have been activated for 14 days in presence of rapamycin (100 nM). T cells were incubated for 15 min at 37 °C in glucose-free Krebs-Ringer Hepes buffer (Hepes 50 mM, NaCl 137 mM, KCl 4.7 mM, CaCl2 1.85 mM, MgSO4 1.3 mM, BSA 0.1% w/v, pH 7.4). T cells were pelleted, washed and incubated with 100 μM 2-NBDG at 37 °C in the water bath prior to measuring fluorescence by flow cytometry.
2.12. Assessment of ECAR and OCR
For extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) measurements we used an XF24 extracellular analyser (Seahorse™ Bioscience). TN (750,000 per well) and TSCM cells (200,000 per well) have been immobilized using CellTak™ agent (BD Biosciences). T cells were kept in non-buffered assay medium (KHB with 25 mM glucose, 1 mM sodium pyruvate, 2 mM glutamine for ECAR assessment or 2.5 mM glucose and 1.5 mM carnitine for OCR measurement) and incubated in a non-CO2 incubator for 60 min at 37 °C prior to analysis. Anti-CD3/CD28 beads, TWS119, rapamycin, oligomycin and palmitate were added prior to or during the analysis at the indicated time points and concentrations. ECAR and OCR were calculated using Seahorse™ Bioscience proprietary software.
2.13. Statistical Analysis
2.13.1. Flow Cytometry
Statistical analysis was performed with Prism software (Version 6, GraphPad), using a paired t-test.
2.13.2. RNA Sequencing
The raw sequencing reads were trimmed with Trim Galore! (http://www.bio-informatics.babra-ham.ac.uk/projects/trim_galore/) (Version 0.3.3), using cutadapt (Version 1.2.1) to remove low-quality bases (quality Phred score cut-off 15) and remaining adaptor sequences. Further information is provided in the Supplemental Experimental Procedures. For all analyses, a p-value (adj.) less than 0.05 was considered as statistically significant and labelled with *, less than 0.01 with **, less than 0.001 with *** and less than 0.0001 with ****.
3. Results
3.1. TWS119 and Rapamycin Induce Phenotypic TSCM Cells
To assess the influence of Wnt and mTOR on human TSCM cell generation, highly purified CD4 + and CD8 + TN cells (natural (n)) were sorted ex vivo (Fig. S1a) and activated with anti-CD3/CD28 beads (1:1 bead/cell ratio) and IL-2 (300 IU/ml) in the presence of the Wnt activator TWS119 (5 μM) or the mTOR inhibitor rapamycin (100 nM).
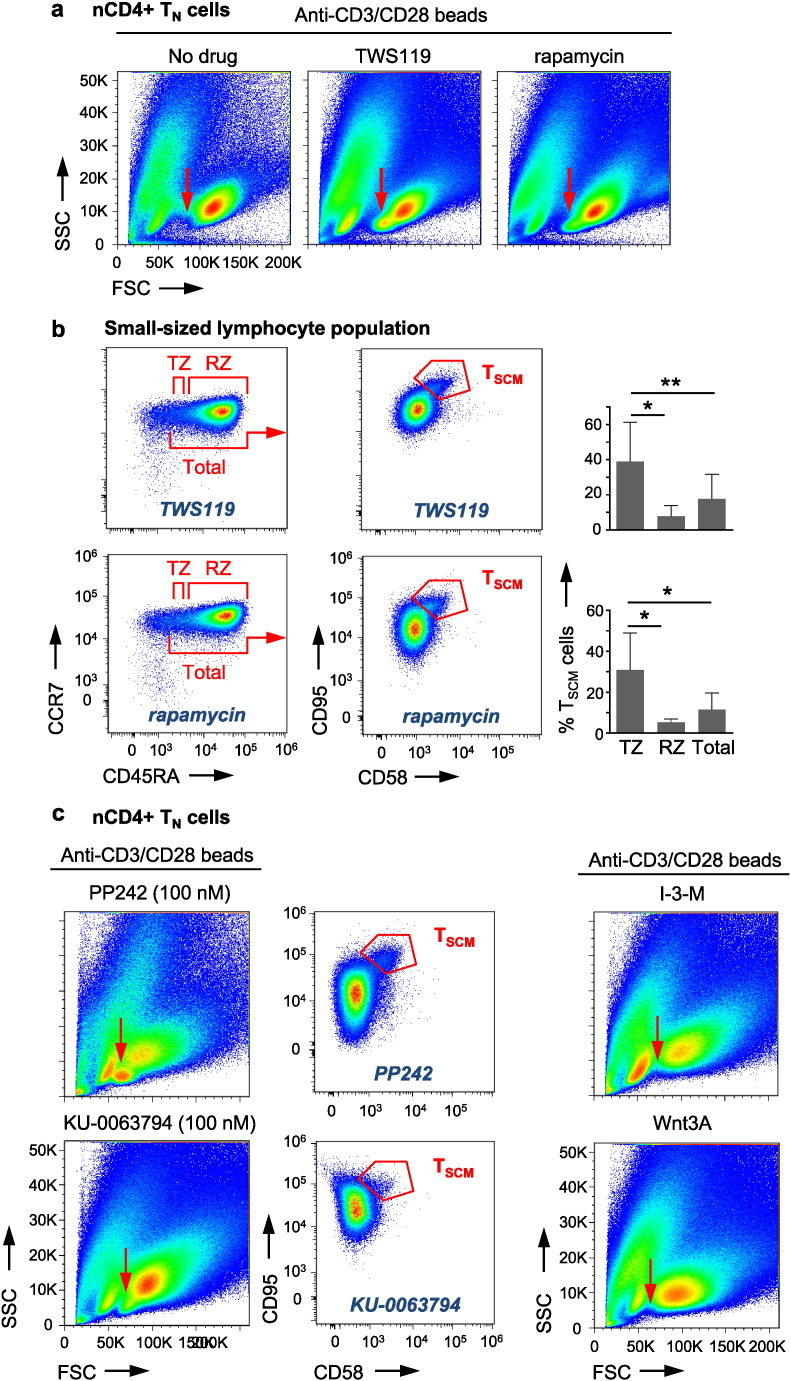
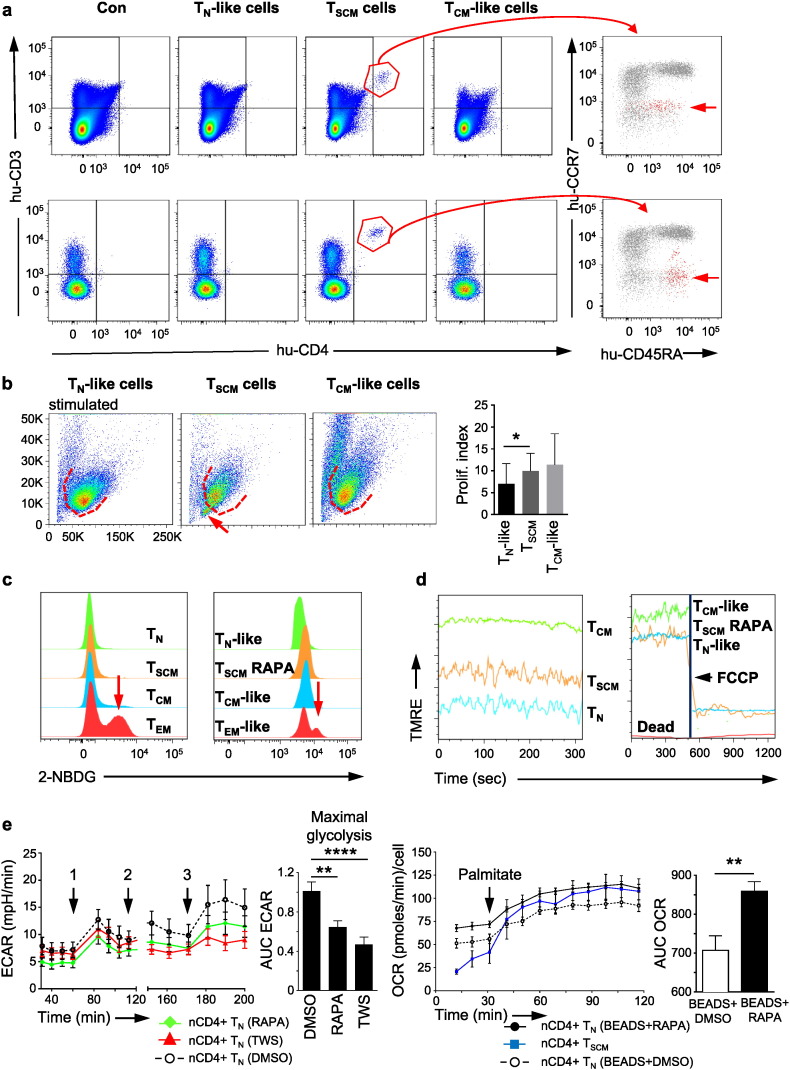
After 14 days, nCD4 + TN cells, primed in the presence of TWS119 or rapamycin, formed two lymphocyte populations, a small-sized and a large-sized one, based on forward scatter/side scatter (FSC/SSC) profiles. In contrast, nCD4 + TN cells which have been cultured in the absence of TWS119 or rapamycin did not generate the small-sized lymphocyte population (Fig. 1a). Phenotypic analysis revealed that the small-sized lymphocyte population mainly consists of T cells with a CCR7 +, CD45RA + TN-like phenotype and a small fraction of cells with a CCR7 +, CD45RA- TCM-like phenotype. Of note, the TN-like population displayed phenotypic CD95 +, CD58 + TSCM cells which were significantly increased in absolute cell numbers in comparison to TCM-like cells (Fig. 1b, Fig. S1b and Table 1), suggesting TWS119 and rapamycin as specific inducers of TSCM cells. These cells co-expressed high levels of the CD62L selectin (not shown). We then subdivided the TN-like population into a CCR7 +, CD45RAintermediate “transition zone” (TZ) and a CCR7 +, CD45RAhigh “rand zone” (RZ) to investigate whether TSCM cells would be preferentially located in a distinct region of the TN-like population. Interestingly, this was indeed the case, since the TZ contains higher frequencies of phenotypic TSCM cells compared to the RZ (Fig. 1B), in line with a model of progressive T cell differentiation, positioning TSCM cells in between TN cells and TCM cells. The large-sized lymphocyte population did not show significant differences in cellular composition upon drug treatment with or without TWS119 or rapamycin and exhibited a mixture of TN-like, TCM-like and TEM-like (CCR7 −, CD45RA −) cells (Table S1), suggesting the small-sized lymphocyte population as place of CD4 + TSCM cell induction.
Fig. 1.
TWS119 and rapamycin induce phenotypic TSCM cells. (a) Highly purified (TSCM cell-freed) human nCD4 + TN cells activated for 14 days in the presence of TWS119 (5 μM) or rapamycin (100 nM) form a small-sized lymphocyte population which is not induced in the absence of TWS119 or rapamycin (red arrows). n = 5. (b) The small-sized lymphocyte populations induced by TWS119 or rapamycin mainly consist of CCR7 +, CD45RA + TN-like cells (gated on live CD3 +, CD4 + T cells) and exhibit a fraction of phenotypic CD95 +, CD58 + TSCM cells with highest frequencies in the transition zone (TZ). The flow cytometry plot shows phenotypic TSCM cells in the total TN-like cell population. TSCM cell frequencies are depicted as percentages of the defined zones. Data are represented as mean ± SEM. FSC = forward scatter. SSC = side scatter. RZ = rand zone. TSCM = pharmacologically induced phenotypic TSCM cells. n = 5. (c) Activation of highly purified human nCD4 + TN cells for 14 days in the presence of PP242 (100 nM) or KU-0063794 (100 nM) also leads to the formation of the small-sized lymphocyte population (red arrows), containing phenotypic CD95 +, CD58 + TSCM cells (shown in the total TN-like cell population). In contrast, priming in the presence of the alternative Wnt activators Indirubin-3-monoxime (I-3-M, 4 μM) or Wnt3A (10 nM) does not lead to the formation of the small-sized lymphocyte population (red arrows). n = 4. TSCM = pharmacologically induced phenotypic TSCM cells.
Table 1.
Relative percentages and absolute cell numbers of live CD4 + TN, TSCM and TCM cells in the small-sized lymphocyte population. Numbers are presented as mean ± standard error of the mean (SEM). n = 5.
| Rapamycin |
TWS119 |
|||
|---|---|---|---|---|
| % | Absolute | % | Absolute | |
| TN | 86.37 ± 1.02 | 181.429 ± 19.055 | 72.36 ± 10.71 | 120.956 ± 22.647 |
| TSCM | 7.97 ± 0.89 | 16.434 ± 2.175 | 11.79 ± 2.03 | 18.312 ± 1.827 |
| TCM | 2.54 ± 0.48 | 5.215 ± 967 | 5.23 ± 2.46 | 7.741 ± 3.287 |
To further explore the mechanism of TSCM cell induction by TWS119 and rapamycin, we next tested the alternative Wnt activators Indirubin-3-monoxime (4 μM) and Wnt3A (10 nM) as well as the ATP-competitive mTOR inhibitors PP242 and KU-0063794. PP242 and KU-0063794 used at low concentrations (100 nM) inhibit mTORC1, while at higher concentrations (1 μM) they also block mTORC2 (data not shown). Supporting our finding with rapamycin, 14 days of nCD4 + TN cell priming in the presence of low-concentrations (100 nM) PP242 or KU-0063794 also triggered the formation of a small-sized lymphocyte population with phenotypic TSCM cells (Fig. 1c and Fig. S1d). However, surprisingly in contrast to TWS119, the alternative Wnt activators Indirubin-3-monoxime and Wnt3A did not generate a small-sized lymphocyte population (Fig. 1c), questioning an involvement of Wnt signalling in TSCM cell induction.
Testing these observations for CD8 + TSCM cell induction, priming of highly purified nCD8 + TN cells for 14 days in the presence of TWS119 or rapamycin, in comparison to drug absence or priming in the presence of Indirubin-3-monoxime (4 μM), resulted in increased frequencies of TN-like cells, displaying a population of phenotypic CD95 +, CD58 + TSCM cells (Fig. S1c), suggesting a common pharmacological mechanism of rapamycin and TWS119 also for CD8 + TSCM cell induction. Thus, taken together, inhibitors of mTOR induce phenotypic CD4 + and CD8 + TSCM cells, whereas, apart from TWS119, other Wnt activating drugs fail in doing so.
3.2. TWS119 Inhibits mTORC1
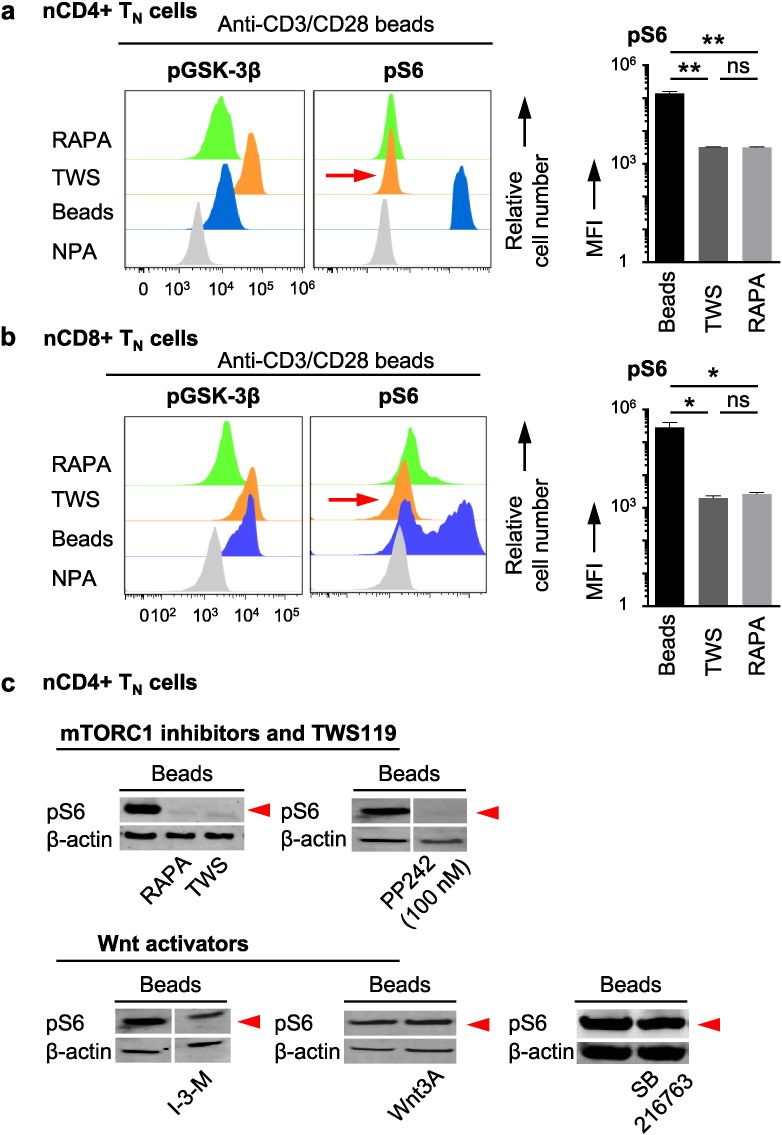
To test the hypothesis about a common pharmacological mechanism of TWS119 and rapamycin in TSCM cell induction, the phosphorylation of GSK-3β (pGSK-3β, read-out for Wnt activity) and the phosphorylation of S6 ribosomal protein (pS6, read-out for mTORC1 activity) were assessed by flow cytometry in highly purified human nCD4 + TN cells (Fig. 2a) and nCD8 + TN cells (Fig. 2b) as well as by Western blot analysis for pS6 in highly purified human nCD4 + TN cells (Fig. 2c) upon activation and drug interference.
Fig. 2.
TWS119 inhibits mTORC1. Assessment of phosphorylation of GSK-3β (Ser9) and of phosphorylation of S6 ribosomal protein (Ser235/236) by flow cytometry in (a) highly purified (TSCM cell-freed) nCD4 + TN cells and in (b) highly purified nCD8 + TN cells. n = 3. (c) Assessment of phosphorylation of S6 ribosomal protein (Ser235/236) by Western blot technique in highly purified nCD4 + TN cells. n = 3. Activation of TN cells in the presence of rapamycin (100 nM), PP242 (100 nM) and, of note, also of the Wnt activator TWS119 (5 μM) inhibits mTORC1 signalling. In contrast, the alternative Wnt activators Indirubin-3-monoxime (I-3-M, 4 μM), Wnt3A (10 nM) and SB216763 (4 μM) do not reduce phosphorylation of S6. NPA = no primary antibody. Beads = Anti-CD3/CD28 beads. TWS = TWS119. RAPA = rapamycin. MFI = median fluorescence intensity.
Surprisingly, the Wnt activator TWS119, which was able to induce phenotypic TSCM cells, also abolished the phosphorylation of S6 (Fig. 2a to c), suggesting an inhibitory effect of TWS119 on mTORC1. Interestingly, whereas the drugs which inhibited mTORC1 (decrease of S6 phosphorylation) were also capable to induce phenotypic TSCM cells, the drugs which activated Wnt (increase of pGSK-3β) did not induce phenotypic TSCM cells, suggesting that phenotypic TSCM cell induction is mediated by inhibition of mTORC1. In line with reports on differential effects on S6 and 4EBP1 (Choo et al., 2008), TWS119 and rapamycin had no significant effects on p4EBP1, another protein downstream of mTORC1, in nCD4 + TN cells (Fig. S2a) and nCD8 + TN cells (Fig. S2b). Moreover, our results suggest that both TWS119 and rapamycin are exerting a mild, suboptimal effect on mTORC1 inhibition (S6K is blocked but 4EBP1 is preserved, as is also mTORC2). Previous studies have demonstrated that S6K is a more sensitive target of mTOR blockade than 4EBP1 (Chresta et al., 2010, Feldman et al., 2009). Thus, inhibition of mTORC1 via decrease of S6 phosphorylation and independence from Wnt signalling emerge as the molecular mechanisms which underlie TSCM cell induction.
3.3. TWS119 Induces a Differentiation Arrest Independently From Wnt Signalling
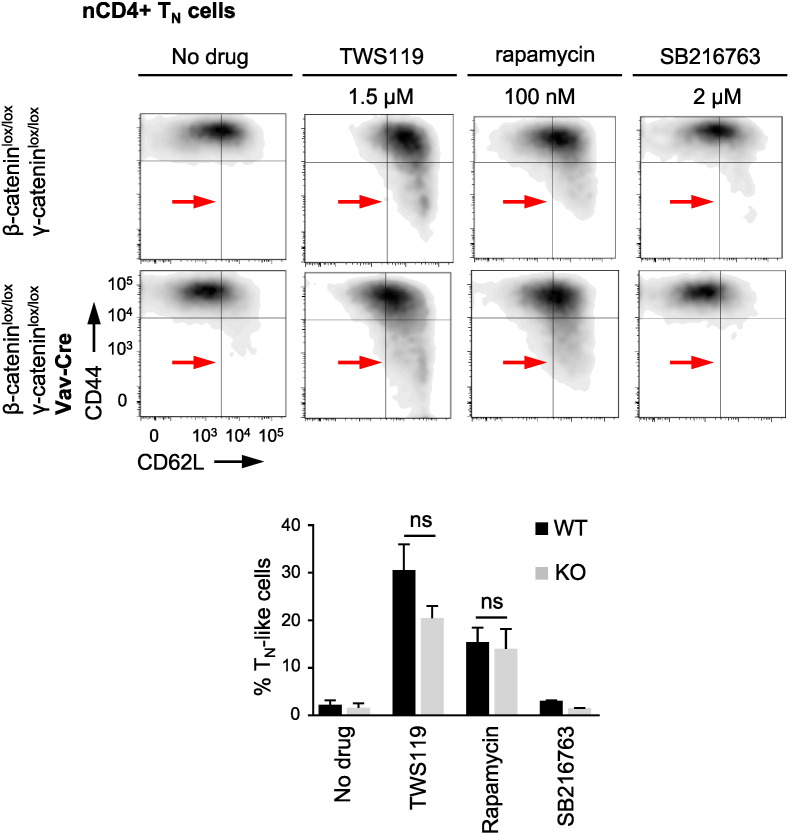
To confirm this conclusion on a genetic base, we took advantage of highly purified nCD4 + TN cells from mice with a haematopoietic deletion of β- and γ-catenin, resulting in abolished Wnt signalling. In mice, TSCM cells were phenotypically defined by the expression of Sca-1 (Ly6A/E) and CXC chemokine receptor 3 (CXCR3) on naive-appearing CD44 −, CD62L + T cells (Fig. S3a) (Gattinoni et al., 2009, Zhang et al., 2005). Since mouse nCD4 + TN cells did not tolerate long drug treatment phases, we needed to reduce priming periods to 4 days with 1.5 μM TWS119, an interval possibly too short to trigger an up-regulation of TSCM cell markers. However, to confirm its independence from Wnt signalling, we hypothesised that under these conditions TWS119 would at least arrest a fraction of activated β- and γ-catenin KO nCD4 + TN cells in a TN-like state, in analogy to the formation of the small-sized lymphocyte population observed in the in vitro experiments with human nCD4 + TN cells. Interestingly, as observed for the mTORC1 inhibitor rapamycin, this was also indeed the case for TWS119, whereas the alternative Wnt activator SB216763 (2 μM), which does not inhibit mTORC1, failed in doing so (Fig. 3 and Fig. 2c). Thus, these data further confirm that the mediation of a differentiation arrest, as prerequisite for TSCM cell induction, is independent from Wnt signalling, but dependent on mTORC1 inhibition. To further corroborate these findings, we then assessed TSCM cell levels in the spleens of β- and γ-catenin KO mice, using the above mentioned phenotype. Suggesting no impairment in TSCM cell generation in the absence of Wnt signalling, β- and γ-catenin KO mice exhibited naturally occurring TSCM cells in comparable frequencies as their WT counterparts (Fig. S3b). Furthermore, we took advantage of mice with a T cell-specific KO of the mTORC1 regulatory component Raptor, which leads to an abolishment of mTORC1 signalling. Interestingly, supporting our in vitro findings of rapamycin-mediated TSCM cell induction, we found significantly increased TSCM cell frequencies in these mice (Fig. S3c and Fig. S3d). Moreover, excluding an off-target effect of rapamycin, additional treatment of Raptor KO nCD4 + TN cells during 4-day priming with rapamycin (100 nM) did not result in an increased fraction of cells in a TN-like state (Fig. S3e). Altogether, these data present further evidence for the inhibition of mTORC1 as the molecular mechanism underlying TSCM cell induction.
Fig. 3.
TWS119 acts independently from Wnt. Both, TWS119 (1.5 μM) and rapamycin (100 nM), arrest a fraction of highly purified (TSCM cell-freed) wild-type (WT, β-cateninlox/lox γ-cateninlox/lox, top) and β- and γ-catenin knockout (KO, β-cateninlox/lox γ-cateninlox/loxvav-cre, bottom) nCD4 + TN cells after 4 days of in vitro activation with anti-CD3 (2 μg/ml), anti-CD28 antibody (2 μg/ml) and IL-2 (10 ng/ml). In contrast, SB216763 (2 μM) fails to mediate this differentiation arrest in activated WT and KO nCD4 + TN cells. TN-like cell frequencies are depicted as percentages of live CD3 +, CD4 + T cells. n = 3. Data are represented as mean ± SEM. ns = not significant.
3.4. Transcriptome Analysis of Naturally Occurring and Pharmacologically Induced TSCM Cells
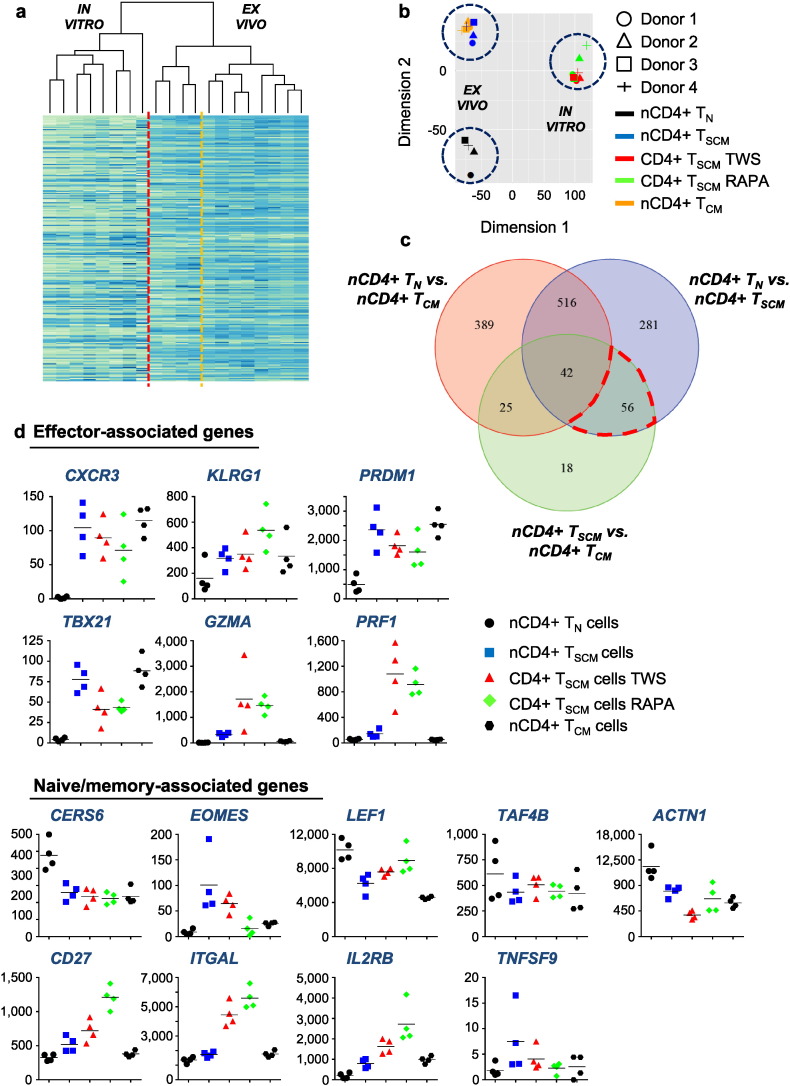
By transcriptome analysis, we next set out to assess the degree of relatedness between naturally occurring CD4 + TN, TSCM and TCM cells as well as TWS119- and rapamycin-induced CD4 + TSCM cells to gain insights into distinct profiles of gene expression in CD4 + TSCM cells. In naturally occurring T cell subsets, unsupervised analysis showed a very close relatedness between TSCM and TCM cells compared to TN cells (Fig. 4a and b), potentially indicating a continuous transition from TSCM to TCM cells during differentiation. Suggesting CD4 + T cell differentiation as process which may be strictly regulated by a core set of genes, only 895 genes were found to be significantly differentially expressed between TN and TSCM cells and 141 genes between TSCM and TCM cells by supervised analysis (adj. p < 0.05; | log2FC | > 1) (Fig. 4c, Table S2, Table S3). To identify further differences between TSCM and TCM cells with respect to the stem cell-like nature of TSCM cells, we carried out a gene set enrichment analysis for stem cell characteristic genes (view Supplemental Information) and could identify FGFR1, RB1 and NOTCH2 to be highly expressed in TSCM cells in comparison to TCM cells (adj. p = 0.07). Of further interest for the distinction of TSCM from TCM cells, 18 genes were found to be significantly differentially expressed between these otherwise closely related subsets and not shared by any other subset (Fig. 4c and Table 4). In addition, a set of 56 genes could be identified to be significantly differentially expressed between TN and TSCM cells and between TSCM and TCM cells, thus, showing a unique expression profile in TSCM cells (Fig. 4c and Table 2). Interestingly, from these 56 genes only 4 genes, SLC22A17, RAI2, SALL2 and LOC338651, were down-regulated in TSCM cells (Fig. S4a), whereas all the other genes were up-regulated. Moreover, with exception of TCF4, Wnt signalling transducers could be found to be highly expressed either in both, TN and TSCM, or significantly up-regulated in TN cells, further arguing against the theory that activation of the Wnt pathway in TN cells induces TSCM cells (Fig. S4b).
Fig. 4.
Fate-determining key factors in naturally occurring and pharmacologically induced TSCM cells. Heat map of gene expression among nCD4 + TN cells, TSCM cells and TCM cells as well as TWS119- and rapamycin-induced CD4 + TSCM cells. For interpretability, only the 1000 genes with the highest average normalized count levels across all samples were included in the heat map (Table S6). (b) Principal component analysis led to grouping of naturally occurring T cells on the one side and pharmacologically induced T cells on the other side by the first two principal components. In the first group, TSCM cells and TCM cells are most similar to each other. The first principle component captured a large fraction (35.5%) of the total variance in the data. (c) Venn diagram depicting overlaps among significantly differentially expressed genes found in pairwise comparisons between TN cells, TSCM cells and TCM cells. Numbers indicate genes. The set of 56 genes is circled in red. (d) Comparative graphic representation of expression levels of naive/memory- and effector-associated genes in TN cells, TSCM cells, TCM cells and TWS119- and rapamycin-induced TSCM cells. The y-axes show normalized counts. Data are based on transcriptome analysis in 4 healthy human individuals. D1–4 = Donor 1–4. TN = TN cells. TSCM = TSCM cells. TCM = TCM cells. TWS = TWS119-induced TSCM cells. RAPA = rapamycin-induced TSCM cells.
Table 2.
56 genes shared between the sets of genes found to be differentially expressed between the nCD4 + TN cell and nCD4 + TSCM cell groups as well as between the nCD4 + TSCM cell and nCD4 + TCM cell groups, but not between the nCD4 + TN cell and nCD4 + TCM cell groups.
| Gene name |
|---|
| 1. IKZF4 |
| 2. SWAP70 |
| 3. FAM49A |
| 4. HLA-DPB1 |
| 5. COBLL1 |
| 6. FCRL1 |
| 7. FCER1G |
| 8. HBA1 |
| 9. SELP |
| 10. HLA-DMB |
| 11. CCL4 |
| 12. IFI30 |
| 13. IGLL5 |
| 14. BTK |
| 15. LAYN |
| 16. HLA-DMA |
| 17. PTPN3 |
| 18. NKG7 |
| 19. WDFY4 |
| 20. PHACTR1 |
| 21. ANKRD33B |
| 22. CDK14 |
| 23. COL19A1 |
| 24. CCL3 |
| 25. FAM129C |
| 26. TLR5 |
| 27. SERPINA1 |
| 28. GFOD1 |
| 29. ARHGAP24 |
| 30. BANK1 |
| 31. ADAM12 |
| 32. GZMB |
| 33. ARAP3 |
| 34. SLC22A17 |
| 35. IGJ |
| 36. RAI2 |
| 37. TYROBP |
| 38. PRF1 |
| 39. CAV1 |
| 40. FHL3 |
| 41. CNR2 |
| 42. LOC100130357 |
| 43. SALL2 |
| 44. KIAA0226L |
| 45. CYBB |
| 46. CD22 |
| 47. MNDA |
| 48. MT1L |
| 49. ALAS2 |
| 50. LYN |
| 51. FCRL2 |
| 52. MS4A1 |
| 53. LOC338651 |
| 54. SNCA |
| 55. SETBP1 |
| 56. HBG2 |
Interestingly, TWS119- and rapamycin-induced TSCM cells showed a very close degree of relatedness. From 21,481 interrogated genes, only 565 genes were significantly differentially expressed between them (adj. p < 0.05; | log2FC | > 1), further supporting our finding of a common pharmacological mechanism of these drugs (Fig. 4a to c and Table S5). However, since TWS119- and rapamycin-induced TSCM cells have received strong activating stimuli over 14 days, it was likely that their transcriptome differed from the ones of naturally occurring TSCM cells, directly sorted ex vivo in their resting state. Nonetheless, we hypothesised that the set of well-known factors of human effector and memory T cell differentiation would show a comparable expression profile in naturally occurring and pharmacologically induced TSCM cells (Gattinoni et al., 2011). Indeed, similar expression levels of the regulators of effector differentiation CXCR3, KLRG1, PRDM1 and TBX21 could be found (Fig. 4d). Interestingly, in vitro induced TSCM cells exhibited higher expression levels of GZMA and PRF1 (Fig. 4d), probably equipping them with superior direct effector functions. In vitro induced and naturally occurring TSCM cells displayed a similar expression level of TNF, but, notably, in vitro induced TSCM cells exhibited low IFNG expression levels (Fig. S4c). This might be a result of IFNG down-regulation due to mTORC1 inhibition, a mechanism described for type I interferons in plasmacytoid dendritic cells mediated by interferon-regulatory factor (IRF) 7 (Cao et al., 2008). Interestingly, IRFs are involved in CD4 + T cell differentiation (Lohoff and Mak, 2005), and IRF7 was found to be up-regulated in in vitro induced TSCM cells (Fig. S4c). Furthermore, similar expression levels of the inhibitory factors for T cell activation and differentiation CERS6, EOMES, LEF1, TAF4B and ACTN1 as well as for the TSCM cell characteristic factors CD27, ITGAL, IL2RB and TNFSF9 could be identified (Fig. 4d). In addition, the expression levels of genes encoding distinct interleukins are shown in Fig. S4d. Finally, confirming the purity of the performed cell sorts, naturally occurring TN, TSCM and in vitro induced TSCM cells expressed similarly low amounts of HNRPLL, a key regulator of the alternative splicing of the CD45 pre-mRNA (Oberdoerffer et al., 2008). Additionally, FAS was among the most significantly differentially expressed genes between naturally occurring TN and TSCM cells (Fig. S4e). Altogether, these data present insights into the transcriptional regulation of TSCM cells and underline the pharmacological inhibition of mTORC1 as a molecular mechanism to confer stemness to a population of activated TN cells.
3.5. In Vitro Induced TSCM Cells Exhibit a Long-term Repopulation Capacity in Vivo
The distinct up-regulation of transcripts encoding telomerase, anti-apoptotic genes and positive cell cycle regulators and down-regulation of pro-apoptotic genes and CDK inhibitors (Fig. S5a) (Igney and Krammer, 2002, Vermeulen et al., 2003) suggested that in vitro induced CD4 + TSCM cells might exhibit a superior in vivo long-term persistence. We directly assessed this potential in vivo long-term persistence by adoptive transfer of 200,000 rapamycin-induced CD4 + TSCM cells, isolated to high purity by flow cytometry-based cell sorting, into NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. Ten weeks after adoptive transfer, peripheral blood, spleen, bone marrow, lung and liver were investigated by multicolour flow cytometry for TSCM cell persistence (Fig. 5a and Fig. S5b). Interestingly, in two of three experiments, the spleens of the mice, which have received TSCM cells, showed the presence of a live CD3 +, CD4 + T cell population with a CCR7 −, CD45RAintermediate phenotype (Fig. 5a), indicating both, the potential of in vitro induced CD4 + TSCM cells to persist long-term in vivo and their ability to repopulate the effector pools by giving rise to further differentiated progeny. In a third experiment, adoptively transferred TSCM cells yielded live CD3 +, CD4 + T cells in the peripheral blood ten weeks after adoptive transfer (Fig. 5a). In contrast, no T cells could be detected after adoptive transfer of same numbers of TN-like or TCM-like cells, re-sorted from the small-sized lymphocyte population from the same in vitro cell culture (Fig. S5b). Spleen size and weight did not exhibit any differences between the groups (Fig. S5b). Of note, compared to naturally occurring CD4 + TN cells, in vitro induced CD4 + TSCM cells showed an up-regulation of the haematopoietic stem cell engraftment genes HOXA1 and LPXN (Powers and Trobridge, 2013), which might have mediated their superior engraftment capacity (Fig. S5c). Together, these data suggest a potential advantage of pharmacologically induced CD4 + TSCM cells in mediating a prolonged immune response after adoptive transfer. Of note, we did not observe signs of xeno-GVHD in contrast to a report showing that rapamycin-treated T cells caused more xeno-GVHD (Amarnath et al., 2010). Further studies are warranted to assess the relative functional features of rapamycin-induced TSCM cells and their long-term repopulation capacity after adoptive transfer.
Fig. 5.
In vivo long-term repopulation capacity and cellular metabolism of CD4 + TSCM cells. (a) Live CD3 +, CD4 + T cells with a CCR7 −, CD45RA intermediate phenotype can be detected in the spleen and peripheral blood of NSG mice, ten weeks after adoptive transfer of 200,000 rapamycin-induced CD4 + TSCM cells. Con = control: injection of 200 μl culture medium. The grey underlay shows the distribution of CCR7 and CD45RA expression in live CD3 +, CD4 + T cells in a healthy donor control. n = 3. (b) Rapamycin-induced CD4 + TSCM cells show a proliferative profile similar to the one of TCM-like cells. In two of six experiments, activated rapamycin-induced CD4 + TSCM cells formed an additional small-sized lymphocyte population, which resisted to activating stimuli (red arrow). n = 6. (c) CD4 + TEM and TEM-like cells display a divergent glucose uptake capacity. In comparison, nCD4 + TN, TSCM and TCM cells as well as rapamycin-induced CD4 + TSCM, TN-like and TCM-like cells do not show high 2-NBDG incorporation. n = 3. (d) Increase of mitochondrial membrane potential (MMP), measured by TMRE uptake, goes along with progressive T cell differentiation. Administration of the oxidative decoupler FCCP leads to immediate MMP breakdown, proving the T cells` viability. n = 3. (e) Top: Activation of nCD4 + TN cells with anti-CD3/CD28 beads increases ECAR (1). Administration of TWS119 (5 μM) or rapamycin (100 nM) (2) hinders activated TN cells to fully develop glycolytic activity upon oligomycin (1 μg/ml) injection (3). n = 4. Bottom: Palmitate (500 μM) substitution increases the OCR in rapamycin-pre-treated, activated TN cells to similar levels as the ones of nCD4 + TSCM cells. n = 3. Data are represented as mean ± SEM. ECAR = extracellular acidification rate. OCR = oxygen consumption rate. AUC = area under the curve. TN = TN cells. TSCM = TSCM cells. TCM = TCM cells. TEM = TEM cells. TN-like = TN-like cells. TCM-like = TCM-like cells. TSCM RAPA = rapamycin-induced TSCM cells. RAPA = rapamycin. TWS = TWS119. BEADS = anti-CD3/CD28 beads.
Next, the proliferative capacity of 30,000 rapamycin-induced CD4 + TSCM cells in comparison to equal numbers of TN-like and TCM-like cells, re-sorted from the small-sized lymphocyte population from the same in vitro cell culture, was assessed by CFSE dilution assay. T cells were either left for 6 days in presence of IL-2 (50 IU/ml) or, additionally, stimulated with OKT3 (1 μg/ml) and anti-CD28 antibody (10 μg/ml) (Fig. 5b). Interestingly, in two of six experiments, stimulated in vitro induced TSCM cells formed a small-sized resting T cell population in addition to a large proliferating one, probably reflecting their stem cell nature, which is both, the capacity to self-renew and to differentiate (Fig. 5b).
3.6. Substrate Utilization and Cellular Metabolism of TSCM Cells
Increasing evidence suggests that T cell differentiation is controlled by fine-tuned modulations of glycolysis and fatty acid oxidation (FAO) (Cham et al., 2008, Fox et al., 2005, Zheng et al., 2009). To investigate the predominance of a distinct metabolic programme in TSCM cells, we next measured the uptake capacity of the fluorescent glucose analogue 2-NBDG in rapamycin-induced TSCM cells in comparison to TN-like, TCM-like and TEM-like cells and to the respective naturally occurring T cell subsets as reference point for glycolytic activity (Sukumar et al., 2013b). Interestingly, compared to the divergent glucose uptake of TEM cells and TEM-like cells, all other T cell subsets exhibited a limited glucose uptake, suggesting especially for TSCM cells an independence from glycolysis (Fig. 5c). In line, pharmacologically induced TSCM cells also showed low expression of the gene encoding the master regulator of glycolytic enzyme HIFA (Fig. S5d). Alternatively, as reference point for oxidative metabolism such as FAO, we measured the mitochondrial membrane potential (MMP) in the mentioned T cell subsets by assessment of their uptake of TMRE, a dye, which accumulates in active mitochondria of short-lived effectors (Sukumar et al., 2013a). Accordingly, we could find an increase of TMRE uptake going along with progressive cellular differentiation (Fig. 5d).
Since these observations suggest that TSCM cells rather gain their energy from an oxidative metabolism, we hypothesised that FAO might also be the relevant metabolic programme in the induction of TSCM cells. We, therefore, investigated the impact of the pharmacological TSCM cell inducers TWS119 and rapamycin on the metabolism of activated nCD4 + TN cells by assessment of ECAR, which is an indicator of glycolytic activity, and OCR, which is an indicator of mitochondrial respiration, by Seahorse analysis (Fig. 5e left). Interestingly, administration of TWS119 (5 μM) or rapamycin (100 nM) prevented the development of full cellular glycolytic activity in response to oligomycin (1 μg/ml), a drug, enforcing maximal glycolysis. Thus, we hypothesised that TWS119 and rapamycin initiate a metabolic programme for TSCM cell induction which is alternative to glycolysis. To disclose whether this metabolic programme would be FAO, we triggered mitochondrial respiration in rapamycin-pretreated (100 nM, 2 h) and activated nCD4 + TN cells by administration of palmitate (500 μM), the ester of retinol and palmitic acid. Interestingly, the presence of palmitate increased mitochondrial respiration to the levels measured in nCD4 + TSCM cells (Fig. 5e right). Thus, these data present evidence for FAO as metabolic programme characteristic of TSCM cells and needed for TSCM cell induction.
4. Discussion
The identification of the signalling pathways, underlying TSCM cell formation, allows their targeted induction and paves the way for the design of novel immunotherapeutic approaches. Here, we show the emergence of a T cell population with phenotypic, transcriptional, functional and metabolic hallmarks of naturally occurring TSCM cells upon in vitro inhibition of mTORC1 during priming of human TN cells. These findings emphasize the potential relevance of the signalling network of mTOR kinase in immunotherapy and of mTOR modulating pharmacological agents.
Interestingly, we show that mTORC1 inhibition with drugs like rapamycin mediates an immunostimulatory effect by the induction of TSCM cells, although these drugs are generally used because of their immunosuppressive function (Cobbold, 2013, Ferrer et al., 2011). Thus, these observations indicate that there are distinct conditions which trigger either a preferential immunostimulatory or an immunosuppressive rapamycin effect. Among a variety of different molecular mechanisms, rapamycin has been suggested to fulfil its immunosuppressive function by prevention of full T cell activation (Loewith et al., 2002, Thomson et al., 2009). This effect can be circumvented by strong stimulation of the TCR and co-stimulatory receptors (Slavik et al., 2004). Similarly, in the in vitro experiments the high degree of activation of TN cells by anti-CD3/CD28 beads in a 1:1 bead/cell ratio and 300 IU/ml IL-2 might have favoured an immunostimulatory rapamycin effect. Furthermore, rapamycin has been shown to increase the antigen-specific T cell response to a pathogen (short-term persistence of the antigen), but to fail in doing so in response to a graft (long-term persistence of the antigen) (Ferrer et al., 2010). These findings strongly suggest that the period of antigen persistence also regulates the immunological outcome of rapamycin. Thus, the rather short periods of TN cell activation (14 days and 4 days) in our in vitro experiments might have tipped the balance towards an immunostimulatory rapamycin effect. The used concentration of rapamycin also emerges as an important factor for mediating either an immunostimulatory or an immunosuppressive drug effect. For the in vitro induction of TSCM cells, rapamycin was used in 100 nM (90 ng/ml), since a rather high concentration of 40–100 ng/ml rapamycin, administered during the contraction phase, has been shown to qualitatively improve antigen-specific memory T cells in a mouse model of CD8 + T cell response to acute viral infection (Araki et al., 2009). In contrast, 8–12 ng/ml rapamycin blood levels are intended to induce immunosuppression after transplantation (Baan et al., 2005). Together, this suggests that a low rapamycin concentration preferentially results in an immunosuppressive effect, whereas a high rapamycin concentration triggers an immunostimulatory one. Also the immunomodulatory actions of rapamycin might be regulated by the interplay between the two mTOR complexes. Whereas TSCM cell induction, as shown here, follows mTORC1 inhibition without additional inhibition of mTORC2, formation of immunosuppressive regulatory T cells is favoured in additional absence of mTORC2 signalling (Chi, 2012). Thus, immunomodulation by rapamycin appears to be a fine-tuned, highly multidimensional process.
Furthermore, we show that the induction of TSCM cells is completely independent from the Wnt signalling pathway. In line with this, the role of Wnt in memory T cell formation has already been called into question by reports about memory T cell formation in CD8 + T cells, in which β-catenin was conditionally knocked out (Driessens et al., 2010, Prlic and Bevan, 2011). Nevertheless, these reports have to be seen with caution, since, in contrast to our study, specifically, TSCM cell formation was not investigated and mice with KO of only β-catenin, which might have favoured a bypassed activity of Wnt signalling by γ-catenin, were used. Moreover, our data offer an unexpected answer to the paradox finding that the Wnt activator TWS119 induces TSCM cells, whereas none of alternative Wnt activators was able to do so, by the discovery of an mTORC1 inhibiting effect of TWS119. Interestingly, this effect finds further confirmation by a recent report, confirming in mouse T cells that TWS119 inhibits mTORC1 (Xiao et al., 2013). At present, it is unclear what the scope of TWS119 off-target effects on other kinases is or whether its inhibition of the mTORC1 kinase requires, as rapamycin, FKBP12. Future biochemical and pharmacological studies will have to address the precise molecular mechanism of mTORC1 inhibition by TWS119.
We also showed that mTORC1 inhibition switched the metabolic programme of activated nCD4 + TN cells to an oxidative metabolism dependent on FAO. However, prominently, in the in vitro experiments only a fraction of activated TN cells was arrested in a TN-like state by TWS119 or rapamycin, suggesting that not all TN cells from the phenotypically homogenous CCR7 +, CD45RA + starting population react to mTORC1 inhibition in the same way. Future studies will have to address whether TN cell intrinsic factors can be identified which predispose certain cells to stop differentiation upon mTORC1 inhibition. One such factor might be Krüppel-like-factor 2 (KLF2) which has been shown to maintain the expression of CCR7 and CD62L and has been suggested to be up-regulated upon mTORC1 inhibition (Chi, 2012, van der Windt et al., 2012). Interestingly, only a small fraction of the CCR7 +, CD45RA +, nCD4 + TN cell starting pool exhibited a high KLF2 expression, whereas the vast majority showed a low expression of KLF2 (Fig. S5e), suggesting KLF2 as possible discriminator to delineate TN cells with TSCM cell precursor potential.
We sought to compare the transcriptome of CD4 + TSCM cells induced by either TWS119 or rapamycin. Indeed, we observed a very highly overlapping gene expression signature shared by rapamycin- and TWS119-induced CD4 + TSCM cells. Among 21,481 interrogated genes, only 565 genes were significantly differentially expressed between them (adj. p < 0.05; | log2FC | > 1), further supporting a common pharmacological mechanism of these drugs. It is very interesting that several of the up-regulated genes in the rapamycin treatment group were related with cell metabolism (Supplemental Table 5). Of note, the most highly up-regulated gene with rapamycin induction is NAD(P)H:quinone oxidoreductase (NQO1), which protects cells against oxidative stress and toxic quinones. In line with this, TXNRD1 (encoding the thioredoxin reductase 1) was also up-regulated in rapamycin-induced TSCM cells. This protein could reduce thioredoxins and plays an important role in protection against oxidative stress. High expression of NQO1 and TXNRD1 might be closely related with the increased oxidative phosphorylation and fatty acid oxidation upon rapamycin induction of TSCM, which definitely needs to be addressed further. On the other hand, the most up-regulated gene in TWS119-induced TSCM cells is LAMP3 (CD63). CD63 is barely expressed in naïve T cells but induced upon T cell activation. Crosslinking of CD63 has been shown to deliver a potent co-stimulatory signal to T cells. To our surprise, we noticed a striking induction of interferon responsive gene expression pattern in TWS119-induced TSCM cells (for example, interferon-induced protein with tetratricopeptide repeats 2, IFIT2; Interferon-Induced Protein with Tetratricopeptide Repeats 3, IFIT3; interferon alpha-inducible proteins 6, IFI6; interferon alpha-inducible proteins 27, IFI27). Many of these genes have been shown to be important for antiviral innate immunity. Some of them emerge to play important roles in regulating T cell activation and immune response. For instance, ISG15 protease UBP43 (USP18) regulates T cell activation. USP18 deficient T cells exhibit hyperactivation of NF-κB and NFAT upon TCR triggering and are defective in Th17 differentiation. The roles of many of those genes in regulating T cells immunity remain to be determined in the near future.
From a translational standpoint, TSCM cells emerge as most promising population for immunotherapy. In this regard, recent reports which indicate that the efficacy of CAR T cells might be based on their acquisition of a TSCM cell phenotype are highly encouraging (Yang et al., 2014); however, previous work showed that also other T cell populations have the capacity to persist long-term in vivo (Berger et al., 2008, Markley and Sadelain, 2010). Our data suggest the use of rapamycin for efficient in vitro TSCM cell induction or in vivo application to enrich for antigen-specific TSCM cells, which should be performed over a short period and by high drug concentration. However, the latter approach will need clinical studies titrating rapamycin doses and assessing different application time-points. Also our insights into TSCM cell metabolism could be used for clinical purposes, since it seems to be rational to provide glucose in treatment phases in which a strong immune attack by TEFF cells is desired. In contrast, in periods of long-term tumour control, in which T cells should enter low differentiation states, substrates allowing FAO should be unrestrictedly provided. In addition, future studies will have to assess the characteristics of and interplay between naturally occurring CD8 + TSCM cells and CD4 + TSCM cells as well as their rapamycin- and TWS119-induced counterparts in preclinical and clinical in vivo settings.
Thus, cellular signalling and metabolism emerge as most promising targets to influence TSCM cell differentiation for the design of innovative immunotherapies.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
G.S. designed the study, performed the experiments and wrote the manuscript. C.J. designed the study, performed in vitro experiments and wrote the manuscript. L.Z. performed in vivo experiments and wrote the manuscript. C.G. performed experiments with β-/γ-catenin KO mice. IC.L-M. carried out metabolic analysis. C.S. performed statistical data analysis. M.D. provided critical input in statistical data analysis. W.H. provided critical input in experimental design. L.F. provided critical input in the assessment of cellular metabolism. O.D. designed the study. P.R. designed and supervised the study, wrote the manuscript, provided critical input, set up collaborations and secured material funding.
Acknowledgements
This study was supported by a grant from the German Research Foundation: Scholz, G (2012): The role of Wnt and mTOR in human memory T cell differentiation. The authors sincerely thank Danny Labes (Flow Cytometry Facility, UNIL) for his input in technical issues and assistance in data acquisition and analysis. The authors also thank Keith Harshman and his team (Genomic Technologies Facility, Center for Integrative Genomics, UNIL) for the professional collaboration in RNA sequencing. The authors also sincerely thank Glenn L. Radice (Department of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania, USA) and Eliane J. Müller (Vetsuisse Faculty, University of Bern, Bern, Switzerland) for providing conditional γ-catenin KO mice. C.J. was funded in part by a Marie Heim-Vögtlin grant from the Swiss National Science Foundation (SNSF). W.H. was funded in part by a grant from the SNSF. L.Z. and P.R. were funded in part by a SNSF grant Sinergia (CRSII3_141879).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.01.019.
Appendix A. Supplementary Data
Supplementary material.
References
- Amarnath S., Flomerfelt F.A., Costanzo C.M., Foley J.E., Mariotti J., Konecki D.M., Gangopadhyay A., Eckhaus M., Wong S., Levine B.L. Rapamycin generates anti-apoptotic human Th1/Tc1 cells via autophagy for induction of xenogeneic GVHD. Autophagy. 2010;6:523–541. doi: 10.4161/auto.6.4.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F., Larsen C.P., Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan C.C., van der Mast B.J., Klepper M., Mol W.M., Peeters A.M.a., Korevaar S.S., Balk A.H.M.M., Weimar W. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80:110–117. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- Berger C., Jensen M.C., Lansdorp P.M., Gough M., Elliott C., Riddell S.R. Adoptive transfer of effector CD8 + T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Manicassamy S., Tang H., Kasturi S.P., Pirani A., Murthy N., Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham C.M., Driessens G., O'Keefe J.P., Gajewski T.F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8 + T cells. Eur. J. Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo A.Y., Yoon S.-O., Kim S.G., Roux P.P., Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chresta C.M., Davies B.R., Hickson I., Harding T., Cosulich S., Critchlow S.E., Vincent J.P., Ellston R., Jones D., Sini P. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- Cobbold S.P. The mTOR pathway and integrating immune regulation. Immunology. 2013;391-398 doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diken M., Kreiter S., Vascotto F., Selmi A., Attig S., Diekmann J., Huber C., Türeci Ö., Sahin U. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer immunol. res. 2013;1:386–392. doi: 10.1158/2326-6066.CIR-13-0046. [DOI] [PubMed] [Google Scholar]
- Driessens G., Zheng Y., Gajewski T.F. Beta-catenin does not regulate memory T cell phenotype. Nat. Med. 2010;16:513–514. doi: 10.1038/nm0510-513. author reply 514-515. [DOI] [PubMed] [Google Scholar]
- Feldman M.E., Apsel B., Uotila A., Loewith R., Knight Z.A., Ruggero D., Shokat K.M. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I.R., Wagener M.E., Robertson J.M., Turner A.P., Araki K., Ahmed R., Kirk A.D., Larsen C.P., Ford M.L. Cutting edge: rapamycin augments pathogen-specific but not graft-reactive CD8 + T cell responses. J. Immunol. 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I.R., Araki K., Ford M.L. Paradoxical aspects of rapamycin immunobiology in transplantation. Am. J. Transplant. 2011;11:654–659. doi: 10.1111/j.1600-6143.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.J., Hammerman P.S., Thompson C.B. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Zhong X.S., Palmer D.C., Ji Y., Hinrichs C.S., Yu Z., Wrzesinski C., Boni A., Cassard L., Garvin L.M. Wnt signaling arrests effector T cell differentiation and generates CD8 + memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Lugli E., Ji Y., Pos Z., Paulos C.M., Quigley M.F., Almeida J.R., Gostick E., Yu Z., Carpenito C. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef P., Buchholz V.R., Stemberger C., Flossdorf M., Henkel L., Schiemann M., Drexler I., Höfer T., Riddell S.R., Busch D.H. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8 + central memory T cells. Immunity. 2014;41:116–126. doi: 10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Igney F.H., Krammer P.H. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- Kamphorst A.O., Ahmed R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy. 2013;5:975–987. doi: 10.2217/imt.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Mak T.W. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat. Rev. Immunol. 2005;5:125–135. doi: 10.1038/nri1552. [DOI] [PubMed] [Google Scholar]
- Lugli E., Gattinoni L., Roberto A., Mavilio D., Price D.A., Restifo N.P., Roederer M. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat. Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley J.C., Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Restifo N.P. Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer S., Moita L.F., Neems D., Freitas R.P., Hacohen N., Rao A. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science. 2008;321:686–691. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J.M., Trobridge G.D. Identification of hematopoietic stem cell engraftment genes in gene therapy studies. J.Stem Cell Res. Ther. 2013;2013:1–15. doi: 10.4172/2157-7633.S3-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M., Bevan M.J. Cutting edge: β-catenin is dispensable for T cell effector differentiation, memory formation, and recall responses. J. Immunol. 2011;187:1542–1546. doi: 10.4049/jimmunol.1100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., Shrikant P.A. The mTOR kinase determines effector versus memory CD8 + T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik J.M., Lim D.-g., Burakoff S.J., Hafler D.A. Rapamycin-resistant proliferation of CD8 + T cells correlates with p27kip1 down-regulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J. Biol. Chem. 2004;279:910–919. doi: 10.1074/jbc.M209733200. [DOI] [PubMed] [Google Scholar]
- Sukumar M., Liu J., Crompton J., Rao M., Ji Y., Finkel T., Gattinoni L., Restifo N. Mitochondrial activity regulates T cell memory, self renewal and anti tumor function in CD8 + T cells. J. ImmunoTherapy of Cancer. 2013;1:O11-O11. [Google Scholar]
- Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D. Inhibiting glycolytic metabolism enhances CD8 + T cell memory and antitumor function. J. Clin. Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A.W., Turnquist H.R., Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.P., Shaffer V.O., Araki K., Martens C., Turner P.L., Gangappa S., Ford M.L., Ahmed R., Kirk A.D., Larsen C.P. Sirolimus enhances the magnitude and quality of viral-specific CD8 + T-cell responses to vaccinia virus vaccination in rhesus macaques. Am. J. Transplant. 2011;11:613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt G.J.W., Everts B., Chang C.-H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., Pearce E.L. Mitochondrial respiratory capacity is a critical regulator of CD8 + T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen K., Van Bockstaele D.R., Berneman Z.N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Teichgräber V., Becker T.C., Masopust D., Kaech S.M., Antia R., von Andrian U.H., Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Sun Z., Smyth K., Li L. Wnt signaling inhibits CTL memory programming. Mol. Immunol. 2013;56:423–433. doi: 10.1016/j.molimm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Zhang, M., Ramos, C., Durett, A., Liu, E., Dakhova, O., Liu, H., Creighton, C.J., Gee, A.P., Heslop, H.E., et al. (2014). Closely-related T-memory stem cells correlate with in-vivo expansion of CAR.CD19-T cells in patients and are preserved by IL-7 and IL-15. Blood. [DOI] [PMC free article] [PubMed]
- Zhang Y., Joe G., Hexner E., Zhu J., Emerson S.G. Host-reactive CD8 + memory stem cells in graft-versus-host disease. Nat. Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Delgoffe G.M., Meyer C.F., Chan W., Powell J.D. Anergic T cells are metabolically anergic. J. Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.