Abstract
To determine the immunological profile most important for IRIS prediction, we evaluated 20 baseline plasma biomarkers in Acquired Immunodeficiency Syndrome (AIDS) patients initiating antiretroviral therapy (ART). Patients were enrolled in a randomized, placebo-controlled ART initiation trial in South Africa and Mexico to test whether maraviroc could prevent IRIS. Participants were classified prospectively as having IRIS within 6 months of ART initiation. Twenty plasma biomarkers were measured at study enrollment for 267 participants. Biomarkers were tested for predicting IRIS with adjustment for covariates chosen through forward stepwise selection. Sixty-two participants developed IRIS and of these 19 were tuberculosis (TB)-IRIS. Baseline levels of vitamin D and higher d-dimer, interferon gamma (IFNγ), and sCD14 were independently associated with risk of IRIS in multivariate analyses. TB-IRIS cases exhibited a distinct biosignature from IRIS related to other pathogens, with increased levels of C-reactive protein (CRP), sCD14, IFNγ, and lower levels of Hb that could be captured by a composite risk score. Elevated markers of Type 1 T helper (Th1) response, monocyte activation, coagulation and low vitamin D were independently associated with IRIS risk. Interventions that decrease immune activation and increase vitamin D levels warrant further study.
Keywords: IRIS, HIV, Biomarker, Vitamin D, d-Dimer, Inflammatory cytokine
Highlights
-
•
We compared immunologic patterns in 267 AIDS patients in South Africa and Mexico based on IRIS development after starting ART
-
•
Prior to starting ART, elevated markers of Th1 response, monocyte activation, coagulation and low vitamin D predicted IRIS
-
•
Our results support future IRIS prevention studies that aim to decrease immune activation and increase vitamin D levels
Over 36 million people are now infected with HIV, and almost all reside in developing countries. HIV antiretroviral treatment saves lives by promoting immune system recovery. Unfortunately, treatment can cause a serious condition called immune reconstitution inflammatory syndrome (IRIS) in up to one-third of patients. Experts think IRIS results from unbalanced recovery of the immune system and it is difficult to predict which patients will develop it. We found that prior to starting treatment, low vitamin D and a unique immunological pattern predicted risk. Our results suggest that IRIS prevention studies should focus on decreasing inflammation and increasing vitamin D levels.
1. Introduction
Immune reconstitution inflammatory syndrome manifests as paradoxical worsening or uncovering of infection or malignancy following ART initiation, despite successful suppression of HIV replication and effective microbiologic control of underlying infection in cases of paradoxical IRIS. Among patients with HIV infection in resource-limited settings, IRIS usually occurs within the first few weeks and up to six months after start of therapy; in these settings, resource utilization and mortality can be high (Hoyo-Ulloa et al., 2011, Muller et al., 2010). Despite a substantial global disease burden, diagnostic criteria are ill defined, molecular mechanisms accounting for pathogenesis are unknown, and effective therapies to mitigate risk are needed (Sereti et al., 2010).
In an earlier retrospective study increased baseline plasma levels of CRP, d-dimer, interleukin-6 (IL-6), and hyaluronic acid (HA) predicted IRIS/death within the first year of ART (Boulware et al., 2011). It is uncertain whether the same markers would have clinical utility when applied prospectively to a population at higher risk due to lower CD4 count at ART initiation and higher prevalence of TB (Boulware et al., 2011).
Recent attention has focused on the role of vitamin D in infectious (de Haan et al., 2014) and autoimmune disease, including tuberculosis (Yang et al., 2013). In resource-limited settings, which have the largest burden of advanced HIV disease, mycobacteria are the most common pathogen involved in the development of IRIS (Conesa-Botella et al., 2009). Vitamin D deficiency is also prevalent and associated with AIDS progression (Van Den Bout-Van Den Beukel et al., 2008). A recent randomized, placebo-controlled trial of vitamin D supplementation in patients with pulmonary tuberculosis demonstrated more rapid clinical recovery than was seen in placebo recipients, although, further investigation of vitamin D for the prevention or reactivation of tuberculosis infection is needed (Salahuddin et al., 2013). Indeed, mounting evidence indicates a strong role for vitamin D in the regulation of the human immune response (Modlin, 2007) and resolution of TB-induced inflammation (Coussens et al., 2012). Multiple in vitro studies have shown that vitamin D suppresses the stimulation of cell-mediated immunity (Coussens et al., 2012). Furthermore, a prominent role for monocyte activation in paradoxical TB-IRIS was highlighted recently (Andrade et al., 2014). Biomarkers that indicate monocyte and myeloid cell activation may improve prediction of IRIS and suggest new pathways of exploration for preventive and therapeutic strategies.
As an adjunctive study to a large randomized controlled trial of antiretroviral treatment (ART) plus maraviroc or ART alone in treatment-naïve individuals in South Africa and Mexico, we tested the hypothesis that pro-inflammatory cytokine levels, myeloid cell activation, coagulation and fibrosis markers were associated with IRIS risk prior to starting ART. We further speculated that high levels of vitamin D might protect against IRIS. Our findings suggest that T-cell and monocyte activation, inflammation and low vitamin D levels are independently associated with IRIS risk.
2. Methods
2.1. Study Outline
Between 2009 and 2012, the C-C Chemokine Receptor 5 (CCR5) Antagonist to Decrease the Occurrence of Immune Reconstitution Inflammatory Syndrome in HIV-Infection (CADIRIS) trial randomized and followed 276 ART-naïve HIV-infected patients for six months to test the utility of the CCR5 antagonist maraviroc as an adjuvant to a standard ART regimen to reduce the occurrence of IRIS (Sierra-Madero et al., 2014, Mendonca et al., 2013). Participants received maraviroc 600 mg twice daily or placebo added to an ART regimen that included tenofovir, emtricitabine, and efavirenz for 48 weeks. The primary endpoint was an IRIS diagnosis within 6 months of ART initiation. Clinical data were prospectively collected by health care providers at the clinical sites. The study was sponsored by the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran. The main clinical trial was sponsored by Pfizer Inc. This study was approved by the Ministry of Health and Federal Commission for Sanitary Risks Protection of Mexico, and the Medicines Control Council and Human Research Ethics Committee of South Africa. The ClinicalTrials.gov registration number is NCT00988780. Results of the main clinical trial were published in the Lancet HIV (Sierra-Madero et al., 2014).
2.2. Study Participants
Eligible subjects in the CADIRIS Trial were HIV-infected, at least 18 years-old, had a CD4 cell count < 100 μL and had not received steroids within two weeks of randomization. We first evaluated IRIS and mortality according to baseline levels of selected biomarkers in the entire CADIRIS population. IRIS cases were reviewed by a central adjudication committee and identified as those who had an event during the first 24 weeks of ART (Sierra-Madero et al., 2014). IRIS events were pre-defined as symptoms consistent with an infectious or inflammatory condition, temporally related to ART initiation and associated with an increase in CD4 count, a decrease in viral load, or both, not explained by a new infection, the expected clinical course of a previously diagnosed infection, or side effects of ART according to the ACTG IRIS criteria. On-site clinicians utilized the above criteria to make a preliminary diagnosis of IRIS, and documented criteria in an electronic data management system. To capture all possible IRIS cases, the study coordination center in both countries actively monitored case report forms and electronic data management system of all patient visits. The central adjudication committee of four experts not involved in study execution or data collection reviewed all preliminary cases and ultimately determined the classification of IRIS events by consensus.
Opportunistic infections occurring in IRIS and non-IRIS cases are described in the original publication of the related CADIRIS Trial in electronic Table 3, Table 4.
Table 3.
Biomarker measurements at initiation of ART: paradoxical vs. unmasking IRIS.
| Biomarker | Paradoxical IRIS (n = 20) Median (IQR) |
Unmasking IRIS (n = 42) Median (IQR) |
p-Value |
|---|---|---|---|
| IFNγ (pg/mL) | 9.4 (3.2–22.0) | 5.4 (1.8–8.2) | 0.03a |
| IL-6 (pg/mL) | 3.2 (1.6–4.4) | 2.4 (1.5–4.0) | 0.80 |
| IL-8 (pg/mL) | 13.2 (9.2–17.6) | 9.4 (6.1–14.9) | 0.13 |
| IL-10 (pg/mL) | 13.1 (9.0–18.3) | 14.5 (10.6–20.5) | 0.97 |
| IL-12p70 (pg/mL) | 1.8 (0.9–5.4) | 1.3 (0.8–2.9) | 0.53 |
| IL-17 (pg/mL) | 0.4 (0.2–0.7) | 0.3 (0.2–0.6) | 0.85 |
| TNFα (pg/mL) | 22.0 (14.6–29.8) | 18.8 (15.9–26.8) | 0.23 |
| CRP (mg/L) | 6.0 (3.1–24.5) | 3.9 (1.7–11.4) | 0.22 |
| SAA (mg/L) | 10.3 (3.5–34.8) | 6.2 (2.6–24.4) | 0.57 |
| P-selectin (ng/mL) | 62.2 (34.9–88.3) | 55.2 (42.8–67.4) | 0.58 |
| IP-10 (pg/mL) | 2917 (1690–4134) | 2587 (1517–3843) | 0.36 |
| sCD14 (μg/mL) | 3.01 (2.33–3.76) | 2.18 (1.78–2.78) | 0.007a |
| sCD163 (ng/mL) | 686.5 (458.4–914.7) | 607.4 (369.5–832.0) | 0.87 |
| sCD40L (pg/mL) | 1093 (603.8–1695) | 779.9 (471.1–1197) | 0.14 |
| Fibrinogen (mg/dL) | 749.9 (537.3–2428) | 843.7 (575.0–1768) | 0.99 |
| Protein C (%) | 3516 (3152–4325) | 3810 (3077–4237) | 0.91 |
| Protein S (%) | 3494 (2813–3937) | 3848 (3169–4927) | 0.06 |
| HA (pg/mL) | 89.2 (45.0–141) | 63.1 (39.9–91.5) | 0.11 |
| d-Dimer (mg/L) | 1.6 (1.2–2.9) | 1.1 (0.8–2.1) | 0.08 |
| Vitamin D (ng/mL) | 9.3 (5.7–13.8) | 7.5 (4.5–13.3) | 0.22 |
Data reported are medians and interquartile ranges unless otherwise noted. Biomarkers with non-parametric distributions were log-transformed for statistical analyses. Log-transformed p-values are reported with the exceptions of sCD14, sCD163, sCD41L, P-selectin, Proteins C & S.
Significant in univariate analysis.
Table 4.
Biomarker measurements in all patients: baseline AIDS-defining Illness at ART Initiation.
| Biomarker | AIDS-defining illness (n = 108) Median (IQR) |
No AIDS-defining illness (n = 159) Median (IQR) |
p-Value |
|---|---|---|---|
| IFNγ (pg/mL) | 4.5 (2.4–8.8) | 1.8 (1.1–3.6) | <.0001a |
| IL-6 (pg/mL) | 2.2 (1.5–3.6) | 1.4 (1.1–3.6) | 0.02a |
| IL-8 (pg/mL) | 10.2 (6.7–17.5) | 5.9 (3.9–9.0) | <.0001a |
| IL-10 (pg/mL) | 12.2 (8.6–18.6) | 9.3 (7.1–12.9) | <.0001a |
| IL-12p70 (pg/mL) | 1.1 (0.5–2.8) | 1.3 (0.8–3.8) | 0.98 |
| IL-17 (pg/mL) | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) | 0.94 |
| TNFα (pg/mL) | 18.9 (14.1–25.9) | 14.9 (11.0–19.0) | 0.04a |
| CRP (mg/L) | 4.7 (1.7–11.3) | 2.1 (1.1–5.6) | 0.03a |
| SAA (mg/L) | 6.5 (2.3–19.0) | 4.4 (1.5–10.1) | 0.21 |
| P-selectin (ng/mL) | 52.4 (39.2–74.8) | 59.4 (47.1–77.5) | 0.28 |
| IP-10 (pg/mL) | 2669 (1708–3895) | 1567 (991.5–2230) | <.0001a |
| sCD14 (μg/mL) | 2.31 (1.94–2.96) | 1.83 (1.52–2.18) | <.0001a |
| sCD163 (ng/mL) | 690.8 (444.9–951.4) | 630.0 (407.1–925.8) | 0.59 |
| sCD40L (pg/mL) | 887.0 (337.6–1333) | 1062 (707.4–1574) | 0.20 |
| Fibrinogen (mg/dL) | 931.9 (554.8–1551) | 959.0 (581.2–1871) | 0.46 |
| Protein C (%) | 3749 (3177–4378) | 3788 (3241–4319) | 0.25 |
| Protein S (%) | 3789 (3177–4510) | 3982 (3277–4927) | 0.07 |
| HA (pg/mL) | 66.5 (41.8–120) | 36.9 (13.8–76.0) | <.0001a |
| d-Dimer (mg/L) | 1.2 (0.6–1.8) | 0.8 (0.5–1.3) | 0.04a |
| Vitamin D (ng/mL) | 9.5 (5.5–15.9) | 9.2 (5.1–15.8) | 0.94 |
Data reported are medians and interquartile ranges unless otherwise noted· Biomarkers with non-parametric distributions were log-transformed for statistical analyses· Log-transformed p-values are reported with the exceptions of sCD14, sCD163, sCD41L, P-selectin, Proteins C &S.
Significant in univariate analysis.
2.3. Biomarker Measurement
All plasma samples were obtained at study enrollment, prior to ART initiation, and were stored at -80°. Biomarkers were measured in duplicate after a single freeze–thaw cycle in batched assays. Coagulation markers were measured in plasma collected in citrate tubes and the remaining biomarkers were measured in plasma collected in Ethylenediaminetetraacetic acid (EDTA).
Interferon-γ, interleukin (IL)-1b, IL-6, IL-8, IL-10, IL-12p70, IL-17 and tumor necrosis factor-α (TNFα), CRP, serum amyloid A (SAA), P-selectin, interferon-inducible protein (IP)-10 were measured by electrochemiluminescence (Mesoscale Discovery, Rockville MD). Leukotriene B4 (LTB4), soluble (s) CD14, sCD40 ligand, sCD163, Von Willebrand Factor (vWF) activity, fibrinogen levels, proteins C and S, and HA were assessed with the use of standardized enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, AdipoBioscience, Zymutest, and Corgenix). d-Dimer was measured with the use of an enzyme-linked fluorescence assay on a VIDAS instrument (Biomerieux). 25 hydroxyvitamin D is the most abundant of all circulating vitamin D metabolites and is generally accepted as the best indicator of vitamin D supply (Aloia et al., 2008). Therefore, the plasma concentration of 25 hydroxyvitamin D was measured by a standard ELISA assay (ALPCO).
2.4. Statistical Analysis
In this study, all participants who developed IRIS were included as IRIS cases and participants who did not develop IRIS were controls. Descriptive statistics were used to compare baseline demographics, laboratory test results, and biomarker measurements between the groups. Results of laboratory tests were analyzed as continuous variables and variables not normally distributed were log10-transformed prior to comparisons. We used Fisher's Exact test to evaluate the association between categorical variables and IRIS.
We used logistic regression to examine the association between biomarker levels and IRIS. We first performed univariate analyses to assess the potential impact of baseline variables, selecting those with a two-sided p value of < 0.10 for inclusion in a forward stepwise regression analysis to determine which were independently associated with the development of IRIS. Separate analyses were performed for all-cause IRIS, TB-IRIS and viral IRIS. Next, we performed univariate analyses of the biomarkers, adjusting for the covariates above, and selected those significantly associated with all-cause IRIS (p < 0.10) for inclusion in a forward stepwise regression analysis to identify those markers appearing independently associated with all-cause IRIS, TB-IRIS, and viral IRIS.
Further sub-analyses were performed by IRIS type (no-IRIS, TB-IRIS and other IRIS) utilizing Kruskal–Wallis tests with Dunn's multiple comparisons post-test because most variables in the clinical subgroups were not normally distributed even after logarithmic transformation.
The inferential networks (described here as host interactome) were generated from Spearman correlation matrices containing values of each biomarker measured in the plasma samples, as described before (Andrade et al., 2014, Mendonca et al., 2013, Mendonca et al., 2015). The values were inputted in JMP 11.0 software (SAS, Cary, NC, USA). Each mediator was selected as a target, and the software performed a search within the other mediators for those that were correlated, with the target calculating a correlation matrix using Spearman rank tests. As a result, the features related to the selected target are linked. Thus, the links shown in the networks represent statistically significant Spearman rank correlations (p < 0.05). Graphics for the network analysis were customized using the Ingenuity Systems Pathway Analysis software (Ingenuity Systems, Redwood City, CA, USA) and Adobe Illustrator (Adobe Systems Inc.).
A composite score was created using the variables shown to be statistically different in TB-IRIS group compared to the other groups (CRP, sCD14, IFNγ and Hb). A score of one (+ 1) was attributed whenever CRP, sCD14 or IFNγ values were above the 75th percentile and Hb levels below the 25th percentile of the entire study population. The rationale for the use of the 75th and 25th percentiles respectively was to identify individuals with the highest values of CRP, sCD14 and IFNγ and with the lowest values of Hb in the study population. This composite score could then range between zero and 4. The values obtained between IRIS groups were compared using the Kruskal–Wallis test with Dunn's multiple comparisons post-test. Receiver Operator Characteristics (ROC) curves were employed to test the performance of the composite score to distinguish TB-IRIS cases from other types of IRIS or individuals who did not develop IRIS.
All analyses were pre-specified. Two-sided p values of < 0.05, unadjusted for multiple testing, were considered statistically significant. Data were collected and stored at a central data repository. Statistical analyses were done using STATA (version 13; StataCorp., College Town, TX, USA) and JMP 11.0 software.
3. Role of Funding Sources
The main clinical trial and this substudy were investigator-initiated. Pfizer Inc. had no role in the design and conduct of the study; in the collection, analysis, or interpretation of data; in the decision to publish this study or main clinical trial; in the preparation, review, or approval of this manuscript. All substudy samples were processed and analyzed by NIAID. All authors had full access to all of the data in the study and the final responsibility for the decision to submit for publication.
4. Results
4.1. Clinical Characteristics and IRIS
Three hundred sixty-two patients were screened for eligibility and 276 were enrolled. Of these, 267 had blood banked at enrollment and were included in this study. Patients in the maraviroc plus ART and ART alone treatment arms had similar baseline demographics, clinical characteristics and no difference in risk of IRIS after 48 weeks of follow-up, as previously reported (Sierra-Madero et al., 2014). Eleven participants died; five had IRIS and six did not.
Characteristics of the 267 participants at the initiation of ART are shown in Table 1. Participants had a median age of 36 years [Interquartile range (IQR), 30–43], median CD4 count of 33 cells per μL (IQR 18–59), and median HIV-RNA 5.4 log10 copies/mL (IQR, 5.0–5.7 log10 copies/mL). Sixty-two patients (23%) developed IRIS within 6 months of ART initiation while 204 patients did not. Nineteen cases (31% of IRIS events) were TB-IRIS. Four additional cases were non-mycobacterial IRIS. There were a total of 69 IRIS events with seven patients having multiple IRIS presentations. IRIS cases by type and pathogen are described in Table 2. IRIS events were more common in men (odds ratio (OR) for male compared to female participants, 2.4 [95% confidence interval (CI), 1.2 to 4.6]). Patients who experienced IRIS were more likely to be from Mexico, to have anemia, and to present with an AIDS defining illness. There were no significant differences between study arms with respect to CD4 count or HIV RNA level at baseline. Other laboratory values analyzed were albumin and CD8 count, which did not differ significantly between groups.
Table 1.
Characteristics of study participants at initiation of ART.
| Characteristic | All patients (n = 267) |
IRIS diagnosed (n = 62) |
IRIS not diagnosed (n = 205) |
p-Value |
|---|---|---|---|---|
| Age, years | 36.0 (30.0–43.0) | 35.5 (30.0–41.0) | 36.0 (31.0–43.0) | 0.76 |
| Female sex, no. (%) | 92 (34.4%) | 13 (21.0%) | 79 (38.5%) | 0.01 |
| Maraviroc treatment group, no. (%) | 135 (50.6%) | 32 (51.6%) | 103 (50.2%) | 0.88 |
| Country, no. (%) | 0.008 | |||
| South Africa | 143 (53.6%) | 24 (38.7%) | 119 (58.0%) | |
| Mexico | 124 (46.4%) | 38 (61.3%) | 86 (42.0%) | |
| Death, no. (%) | 11 (4%) | 5 (8%) | 6 (3%) | 0.135 |
| AIDS-defining illness, no. (%) | 159 (59.5%) | 49 (79.0%) | 110 (53.7%) | 0.001 |
| Tuberculosis | 53 (20%) | 16 (26%) | 37 (18%) | 0.179 |
| CD4 + cell count, per mm3 | 33.0 (18.0–59.0) | 30.5 (11.0–59.0) | 35·0 (19.0–59.0) | 0.24 |
| CD8 + cell count, per mm3 | 476.5 (341.5–743.0) | 431 (313.5–620.0) | 497.5 (348.5–758.5) | 0.192 |
| Hemoglobin, g/dL | 12.1 (10.8–13.4) | 11.8 (10.7–13.0) | 12.3 (11.0–13.7) | 0.08 |
| Plasma HIV RNA, log copies/mL | 5.4 (5.0–5.7) | 5.4 (5.0–5.7) | 5.4 (5.0–5.7) | 0.39 |
Data reported are medians and interquartile ranges unless otherwise noted.
Table 2.
IRIS events by pathogen.
| Pathogen | IRIS event n = 69 |
Paradoxical no. (%) |
Unmasking no. (%) |
Steroid treatment no. (%) |
Median days to IRIS (IQR) |
|---|---|---|---|---|---|
| Tuberculosis | 19 | 14 (70.0%) | 5 (30.0%) | 5 (26.3%) | 13 (11–19) |
| Varicella zoster | 17 | 0 | 17 (100%) | 0 | 46 (29–99) |
| Herpes simplex | 13 | 0 | 13 (100%) | 1 (7.7%) | 18 (13–26) |
| Non-tuberculosis mycobacteria | 4 | 1 (33.3%) | 3 (66.6%) | 0 | 10 (7–22) |
| Human papilloma virus | 3 | 0 | 3 (100%) | 0 | 69 (42–125) |
| Histoplasmosis | 3 | 1 (33.3%) | 2 (66.6%) | 1 (33.3%) | 126 (75–157) |
| Eosinophilic folliculitisa | 3 | 1 (33.3%) | 2 (66.6%) | 0 | 15 (12–50) |
| Kaposi's sarcoma | 2 | 1 (50.0%) | 1 (50.0%) | 2 (100%) | 82.5 (49–116) |
| Cryptosporidium | 1 | 1 (100%) | 0 | 1 (100%) | 14 |
| Toxoplasmosis | 1 | 1 (100%) | 0 | 0 | 8 |
| Molluscum contagiousum | 1 | 1 (100%) | 0 | 0 | 18 |
| Cytomegalovirus | 1 | 0 | 1 (100%) | 0 | 33 |
| Pruritic eruptiona | 1 | 1 (100%) | 0 | 0 | 25 |
| Any IRIS | 69 | 22 (31.9%) | 47 (68.1%) | 9 (13.0%) | 25 (13–50) |
Seven patients had more than one IRIS event. These participants were classified by the first event. Patients who received steroids within two weeks of enrollment were excluded from the clinical trial and this substudy. All biomarkers were measured prior to IRIS diagnoses. Severe IRIS cases were treated with systemic steroids, consistent with the standard of care at the clinical trial center.
IRIS cases in which the pathogen was not identified.
4.2. Biomarker Measurement and Paradoxical vs. Unmasking IRIS
Among all IRIS cases, participants who developed paradoxical IRIS had significantly higher levels of IFNγ and sCD14 (Table 3). There was a trend for higher levels of d-dimer and hyaluronic acid in parodixal IRIS.
4.3. Biomarker Measurement and AIDS-defining Illness
Participants with a prior AIDS-defining illness at the time of study enrollment had higher measurements of IFNγ, IL-6, IL-8, IL-10, TNFα, CRP, IP-10, sCD14, Hyaluronic acid, and d-dimer (Table 4).
4.4. Inflammation and IRIS
Levels of d-dimer and CRP were significantly higher in IRIS (Table 5). d-Dimer levels remained significantly associated with risk of IRIS when the analysis was adjusted for gender, AIDS-defining illness, and Hb level (OR per unit increase in log d-dimer value, 3.85; 95% CI, 1.43–10.3) while CRP did not.
Table 5.
Biomarker measurements at initiation of ART.
| Biomarker | IRIS diagnosed Median (IQR) |
IRIS not diagnosed Median (IQR) |
p-Value |
|
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| IFNγ (pg/mL) | 5.6 (2.2–10.6) | 2.7 (1.4–5.2) | <.001 | 0.01a |
| IL-6 (pg/mL) | 2.5 (1.5–4.0) | 1.7 (1.2–3.1) | 0.048 | 0.48 |
| IL-8 (pg/mL) | 10.5 (6.6–15.4) | 7.6 (4.8–13.8) | 0.04 | 0.79 |
| IL-10 (pg/mL) | 14.0 (9.4–20.3) | 10.4 (7.6–15.7) | 0.04 | 0.19 |
| IL-12p70 (pg/mL) | 1.3 (0.9–4.8) | 1.1 (0.7–3.2) | 0.48 | 0.45 |
| IL-17 (pg/mL) | 0.4 (0.2–0.6) | 0.3 (0.2–0.6) | 0.74 | 0.79 |
| TNFα (pg/mL) | 19.9 (15.4–28.1) | 15.5 (12.1–21.9) | 0.04 | 0.23 |
| CRP (mg/L) | 4.5 (1.8–13.7) | 2.5 (1.3–9.0) | 0.03 | 0.28 |
| SAA (mg/L) | 6.3 (2.6–25.2) | 5.3 (1.6–12.6) | 0.15 | 0.54 |
| P-selectin (ng/mL) | 55.4 (39.9–69.9) | 56.1 (41.7–76.5) | 0.86 | 0.98 |
| Log10IP-10 (pg/mL) | 3.4 (3.2–3.6) | 3.3 (3.1–3.5) | 0.005 | 0.16 |
| sCD14 (μg/mL) | 2.4 (2.0–3.2) | 2.0 (1.6–2.5) | <.0001 | 0.01a |
| sCD163 (ng/mL) | 607.6 (391.6–893.5) | 677.0 (442.9–979.9) | 0.23 | 0.13 |
| sCD40L (pg/mL) | 912.7 (489.1–1318) | 963.7 (449.0–1532) | 0.35 | 0.49 |
| Fibrinogen (mg/dL) | 829.6 (562.9–1977) | 990.1 (575.0–1596) | 0.99 | 0.76 |
| Protein C (%) | 3660 (3108–4270) | 3780 (3220–4388) | 0.34 | 0.66 |
| Protein S (%) | 3711 (2912–4439) | 3891 (3264–4764) | 0.21 | 0.47 |
| HA (pg/mL) | 70.7 (43.7–102.4) | 51.3 (26.1–93.0) | 0.10 | 0.95 |
| d-Dimer (mg/L) | 1.3 (0.8–2.3) | 0.8 (0.5–1.5) | 0.0008 | 0.007a |
| Vitamin D (ng/mL) | 8.2 (5.0–13.8) | 9.6 (5.8–17.9) | 0.04 | 0.04a |
Data reported are medians and interquartile ranges unless otherwise noted. Biomarkers with non-parametric distributions were log-transformed for statistical analyses. Log-transformed p-values are reported with the exceptions of sCD14, sCD163, sCD41L, Proteins C &S.
Significant after adjustment for gender, AIDS-defining condition, and Hb level.
In univariate unadjusted analyses, elevated levels of inflammatory cytokines and chemokines (IL-6, IL-8, TNFα, IFNγ and IP-10) were significantly associated with IRIS. Following adjustment for clinical confounders, IFNγ remained significant (OR per unit increase in log10-transformed IFNγ value, 2.9; 95% CI, 1.3–6.4). To assess the role of myeloid cell activation in IRIS pathogenesis, sCD14, sCD40L, and sCD163 were compared between cases and the remaining cohort and of these, only sCD14 was significantly associated in multivariate analyses.
4.5. Vitamin D Levels and IRIS
Participants had very low vitamin D levels with a median level of 9.4 ng per mL (IQR 5.4–15.9) compared to a commonly accepted cut-off for clinical deficiency of < 30 ng/mL (LeBlanc et al., 2014). Higher vitamin D levels were associated with protection against development of IRIS events. The multivariate-adjusted odds ratio of IRIS per unit increase in the log vitamin D value was 0.37 (95% CI, 0.14–0.95).
4.6. TB-IRIS
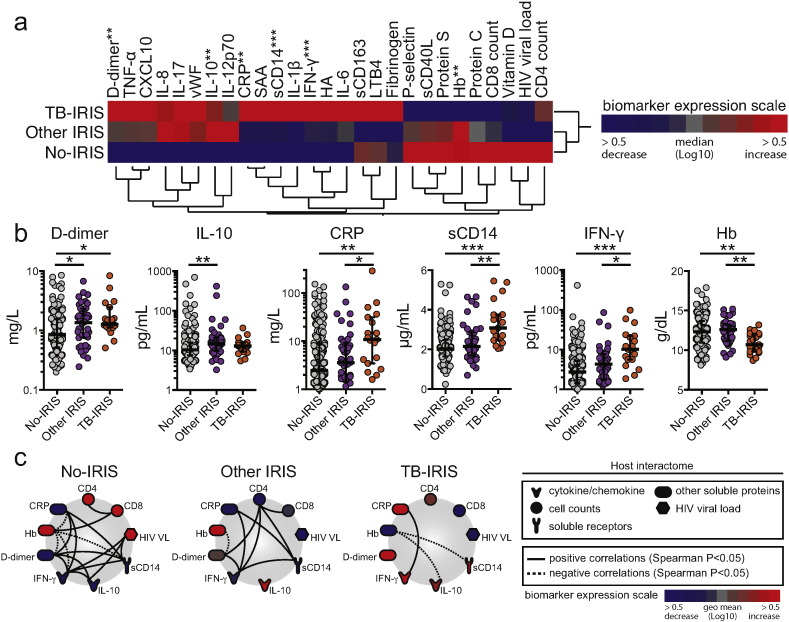
Differences between TB-IRIS cases and the remaining cohort are shown in Table 6. TB-IRIS cases were more frequently male, from Mexico, and had lower levels of hemoglobin. In univariate analyses, higher concentrations of IL-10, CRP, sCD14, IFNγ, and lower Hb, were present in TB-IRIS (Fig. 1a; Table 7). Of these markers, values of CRP, sCD14, IFNγ and Hb were different in TB-IRIS cases compared to the no-IRIS and other IRIS groups (Fig. 1b). An exploratory analysis using networks from Spearman correlation matrices demonstrated distinct networking profiles (Fig. 1c and Supplemental File 1), and that, in general, Hb levels exhibited statistically significant negative correlations with markers of inflammation and coagulation within each study group.
Table 6.
Characteristics of study participants at initiation of ART based on TB versus other IRIS event.
| Characteristic | TB-IRIS (n = 19) |
Other IRIS (n = 43) |
IRIS not diagnosed (n = 205) |
p-Value |
|---|---|---|---|---|
| Age, years | 38.0 (29.0–42.0) | 35.0 (31.0–41.0) | 36.0 (30.0–43.0) | 0.87 |
| Female sex, no. (%) | 4 (19.0%) | 9 (21.5%) | 79 (38.5%) | 0.04 |
| Treatment group, no. (%) | 9 (42.9%) | 23 (56.1%) | 103 (50.2%) | 0.60 |
| Country, no. (%) | 0.03 | |||
| Mexico | 13 (61.9%) | 25 (61.0%) | 86 (42.0%) | |
| Death, no. (%) | 2 (9.5%) | 3 (7.3%) | 6 (3.0%) | 0.19 |
| CD4 + cell count, per mm3 | 31.0 (17.5–56.5) | 28.0 (19.0–59.0) | 35.0 (19.0–60.0) | 0.47 |
| CD8 + cell count, per mm3 | 430.0 (278.5–639.0) | 431.0 (313.5–597.0) | 502.0 (356.0–761.5) | 0.26 |
| Hemoglobin, g/dL | 10.7 (9.8–12.0) | 12.6 (11.1–13.2) | 12.3 (11.0–13.7) | 0.002 |
| Plasma HIV RNA, log copies/mL | 5.5 (5.0–5.7) | 5.4 (5.0–5.7) | 5.4 (5.0–5.7) | 0.81 |
Data reported are medians and interquartile ranges unless otherwise noted.
Fig. 1.
Using pre-ART plasma levels of inflammatory biomarkers to predict TB-IRIS. Pre-ART levels of selected plasma markers were compared among individuals who developed TB-IRIS (n = 19), viral or other kinds of IRIS (n = 43) within 6 months of ART initiation, and those who did not develop IRIS (n = 205). (a) A heat map was designed to depict the overall expression pattern of plasma cytokines, chemokines, and inflammatory markers in the study population. A two-way hierarchical cluster analysis (Ward's method) of circulating biomarkers by clinical group was performed. Expression scale for each biomarker represents log10 fold-change from the median values of the entire study population. Markers in bold identify those that were statistically different between the groups using the Kruskal–Wallis test (**p < 0.01; ***p < 0.001). (b) Scatter plots of six biomarkers that displayed significant differences assessed by Kruskal–Wallis tests are shown, and groups were compared using Dunn's multiple comparisons post-test (*p < 0.05; **p < 0.01; ***p < 0.001). (c) The network analysis (interactome) shows statistically significant correlations (p < 0.05). Data were analyzed using Spearman rank tests. See Supplemental File 1 for additional details on the strength (r value) and level of significance (p-value) of each individual correlation. In (b), lines represent median values and interquartile ranges.
Table 7.
Biomarker measurements at initiation of ART: TB-IRIS.
| Biomarker | TB-IRIS diagnosed Median (IQR) |
TB-IRIS not diagnosed Median (IQR) |
p-Value |
|
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| IFNγ (pg/mL) | 10.2 (5.6–22.0) | 2.9 (1.5–5.8) | <.0001 | 0.003a |
| IL-6 (pg/mL) | 3.5 (1.6–5.0) | 1.8 (1.3–3.2) | 0.07 | 0.53 |
| IL-8 (pg/mL) | 11.8 (7.7–14.5) | 8.0 (5.0–14.0) | 0.04 | 0.54 |
| IL-10 (pg/mL) | 12.9 (9.2–15.8) | 10.9 (7.8–17.4) | 0.32 | 0.58 |
| IL-12p70 (pg/mL) | 1.5 (0.6–5.4) | 1.2 (0.7–3.2) | 0.95 | 0.73 |
| IL-17 (pg/mL) | 0.3 (0.20–0.50) | 0.3 (0.2–0.6) | 0.85 | 0.78 |
| TNFα (pg/mL) | 22.0 (16.0–29.2) | 16.1 (12.4–22.3) | 0.12 | 0.55 |
| CRP (mg/L) | 10.8 (3.5–31.4) | 2.7 (1.3–9.0) | 0.002 | 0.04 |
| SAA (mg/L) | 15.6 (4.4–70.5) | 5.1 (1.7–13.7) | 0.03 | 0.13 |
| P-selectin (ng/mL) | 55.4 (34.9–87.2) | 56.1 (42.0–75.9) | 0.76 | 0.86 |
| IP-10 (pg/mL) | 2917 (2227–3993) | 2080 (1342–3185) | 0.04 | 0.47 |
| sCD14 (μg/mL) | 3.08 (2.46–3.76) | 2.05 (1.66–2.52) | <.0001 | 0.003a |
| sCD163 (ng/mL) | 695.1 (373.3–949.9) | 652.0 (432.9–952.9) | 0.90 | 0.62 |
| sCD40L (pg/mL) | 656.3 (413.5–1140) | 963.7 (489.1–1474) | 0.77 | 0.74 |
| Fibrinogen (mg/dL) | 857.8 (537.3–3607) | 951.6 (575.0–1554) | 0.40 | 0.54 |
| Protein C (%) | 3516 (3014–4247) | 3781 (3208–4366) | 0.38 | 0.68 |
| Protein S (%) | 3516 (2813–4269) | 3886 (3246–4765) | 0.08 | 0.12 |
| HA (pg/mL) | 86.2 (48.4–140.8) | 54.3 (27.1–93.0) | 0.06 | 0.93 |
| d-Dimer (mg/L) | 1.2 (1.1–2.5) | 0.9 (0.5–1.6) | 0.16 | 0.16 |
| Vitamin D (ng/mL) | 9.7 (5.2–13.3) | 9.4 (5.4–15.9) | 0.04 | 0.25 |
Data reported are medians and interquartile ranges unless otherwise noted. Biomarkers with non-parametric distributions were log-transformed for statistical analyses. Log-transformed p-values are reported with the exceptions of sCD14, sCD163, sCD41L, Proteins C &S.
Significant after adjustment for AIDS-defining condition and Hb level.
We next constructed an inflammatory score, which compiled variables significantly linked to TB-IRIS, and compared it to the non-IRIS and other-IRIS groups (Fig. 2a). TB-IRIS cases accumulated a higher score than either the non-IRIS or other IRIS groups (Fig. 2b). Increases above 1 point in the composite score were associated with TB-IRIS after adjustments for age, gender and country (unadjusted OR: 7.46, 95% CI: 2.86–19.4, p < 0.0001; adjusted OR: 5.67, 95% CI: 1.92–15.63, p < 0.0001). ROC curves confirmed that the composite score had potential to identify TB-IRIS cases in this cohort (TB-IRIS vs. non-IRIS AUC: 0.82, sensitivity: 71.4%, specificity: 73.2%, p < 0.0001; TB-IRIS vs. other IRIS AUC: 0.85, sensitivity: 71.4%, specificity: 80.0%, p < 0.0001; Fig. 2b).
Fig. 2.
A composite score of inflammatory markers to predict TB-IRIS. (a) A composite score was created using the variables shown to be statistically different in TB-IRIS group compared to the other groups (CRP, sCD14, IFNγ, and Hb). A score of one (+ 1) was attributed whenever CRP, sCD14 or IFNγ values were above the 75th percentile and Hb levels below the percentile 25th of the entire study population (percentile values were: CRP = 10.44 mg/L, sCD14 = 2.65 μg/mL, IFNγ = 16.5 pg/mL, Hb = 10.8 g/dL). This composite score could then range between zero and four; values obtained between the study groups were compared using the Kruskal–Wallis test with Dunn's multiple comparisons post-test (***p < 0.001). (b) Receiver Operator Characteristic (ROC) curves were employed to test the performance of the composite score to distinguish TB-IRIS cases from Other IRIS or non-IRIS individuals. In (a), data represent median and interquartile range.
4.7. Viral IRIS
Baseline characteristics for participants who developed viral IRIS compared to participants who did not are described in Table 8. Participants who developed viral IRIS were more immunosuppressed and otherwise, similar to the remaining participants. Biomarkers measured prior to ART were compared between participants with viral IRIS to those without (Table 9). Only IL-10 was significantly associated with disease. In our multivariate analysis, following adjustment for CD4 count, IL-10 remained the only significant association, although we observed trends for higher levels of d-dimer and TNFα in viral IRIS.
Table 8.
Characteristics of study participants at ART initiation by viral IRIS.
| Characteristic | Viral IRIS (n = 36) |
No viral IRIS (n = 231) |
p-Value |
|---|---|---|---|
| Age, years | 37.0 (32.5–42.5) | 36.0 (30.0–43.0) | 0.48 |
| Female sex, no. (%) | 8 (22.2%) | 84 (36.4%) | 0.10 |
| Treatment group, no. (%) | 19 (52.8%) | 116 (50.2%) | 0.77 |
| Country, no. (%) | 0.24 | ||
| Mexico | 20 (55.6%) | 104 (45.0%) | |
| Death, no. (%) | 1 (2.8%) | 10 (4.3%) | 0.67 |
| CD4 + cell count, per mm3 | 26.5 (6.5–53.0) | 34.0 (19.0–61.0) | 0.03 |
| CD8 + cell count, per mm3 | 416.5 (302.0–597.0) | 493.0 (351.0–757.0) | 0.23 |
| Hemoglobin, g/dL | 12.5 (11.2–13.2) | 12.1 (10.8–13.6) | 0.77 |
| Plasma HIV RNA, log copies/mL | 5.4 (5.0–5.8) | 5.4 (5.0–5.7) | 0.57 |
Data reported are medians and interquartile ranges unless otherwise noted.
Table 9.
Biomarker measurements at initiation of ART: Viral IRIS.
| Biomarker | Viral IRIS diagnosed Median (IQR) |
Viral IRIS not diagnosed Median (IQR) |
p-Value |
|
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| IFNγ (pg/mL) | 4.0 (1.6–7.5) | 2.9 (1.6–6.2) | 0.71 | 0.61 |
| IL-6 (pg/mL) | 2.3 (1.4–3.9) | 1.8 (1.3–3.2) | 0.65 | 0.61 |
| IL-8 (pg/mL) | 10.5 (6.3–15.4) | 8.0 (5.0–13.8) | 0.16 | 0.27 |
| IL-10 (pg/mL) | 16.0 (10.6–24.9) | 10.7 (7.8–15.6) | 0.005 | 0.005a |
| IL-12p70 (pg/mL) | 1.2 (0.9–5.2) | 1.2 (0.7–3.2) | 0.26 | 0.18 |
| IL-17 (pg/mL) | 0.4 (0.2–0.7) | 0.3 (0.2–0.6) | 0.39 | 0·25 |
| TNFα (pg/mL) | 19.7 (15.4–27.0) | 16.0 (12.3–22.2) | 0.21 | 0.08 |
| CRP (mg/L) | 3.3 (1.6–8.1) | 3.1 (1.4–11.0) | 0.78 | 0.71 |
| SAA (mg/L) | 4.7 (2.2–15.3) | 5.5 (1.7–15.6) | 0.76 | 0.78 |
| P-selectin (ng/mL) | 56.5 (39.9–73.4) | 56.0 (41.7–76.3) | 0.86 | 0.63 |
| IP-10 (pg/mL) | 2197 (1486–3824) | 2119 (1342–3153) | 0.22 | 0.18 |
| sCD14 (μg/mL) | 2.10 (1.67–3.23) | 2.10 (1.73–2.63) | 0.48 | 0.48 |
| sCD163 (ng/mL) | 582.1 (391.6–935.4) | 678.7 (436.5–952.9) | 0.60 | 0.60 |
| sCD40L (pg/mL) | 943.5 (497.8–1355) | 962.9 (446.9–1501) | 0.49 | 0.68 |
| Fibrinogen (mg/dL) | 857.8 (575.0–1559) | 967.3 (564.4–173) | 0.97 | 0.89 |
| Protein C (%) | 3854 (3132–4308) | 3841 (3208–4378) | 0.94 | 0.99 |
| Protein S (%) | 3806 (2912–4773) | 3885 (3234–4686) | 0.81 | 0.84 |
| HA (pg/mL) | 63.4 (41.7–92.2) | 54.5 (27.3–95.0) | 0.54 | 0.72 |
| d-Dimer (mg/L) | 1.2 (0·8–2.3) | 1.0 (0.5–1.6) | 0.13 | 0.06 |
| Vitamin D (ng/mL) | 8.9 (5.0–14.6) | 9.4 (5.4–17.1) | 0.43 | 0.55 |
Data reported are medians and interquartile ranges unless otherwise noted. Biomarkers with non-parametric distributions were log-transformed for statistical analyses. Log-transformed p-values are reported with the exceptions of sCD14, sCD163, sCD41L, P-selectin, Proteins C &S.
Significant after adjustment for CD4 count.
5. Discussion
In this multicenter, prospective study, we identified biomarkers that were associated with increased IRIS risk when measured immediately prior to ART initiation. Our findings suggest that vitamin D, d-dimer and markers of T cell and monocyte activation (IFNγ, sCD14), may help identify patients at highest risk. Notably, d-dimer and vitamin D were not associated with TB-specific IRIS in a multivariate sub-analysis. This study was not powered for this sub-analysis and may best explain the observed discrepancy. Taken together, our results support a potential role for vitamin D in IRIS pathogenesis and thus, a potential target for intervention.
To our knowledge, this is the largest, comprehensive prospective study of baseline biomarker measurement and IRIS risk to date in patients without a pre-specified infection at baseline. Distinctively, our study participants represent an important HIV patient group particularly susceptible to IRIS in settings endemic for TB and poor immune status at therapy initiation. Further, our results were derived from a study performed on two different continents, increasing their generalizability. Moreover, plasma samples were obtained prior to ART initiation and thus prior to the onset of IRIS symptoms.
The exploratory nature of our study allowed us to screen twenty biomarkers and evaluate their association with IRIS development. Plasma specimens were drawn prior to ART initiation, and therefore prior to symptom onset, thereby permitting our measurements to capture differences in cytokine expression and other inflammatory biomarkers prior to immune reconstitution with ART. Study monitoring was also rigorous.
There are limitations to our study. Firstly, patients with severe laboratory abnormalities, mental status changes and CNS infections were not eligible for participation, thus these results may not be generalizable to critically ill patients. Additionally, baseline biomarker measurement allowed for the assessment of IRIS risk prediction but did not allow us to evaluate temporal changes in biomarker levels; an approach that might have improved our understanding of the pathophysiology of IRIS. It is also important to note that IRIS incidence was higher in patients in Mexico compared to those in South Africa. This is likely explained by a higher prevalence of AIDS-defining illnesses among patients in Mexico as reported in our published clinical trial results (79.0% vs. 42.8%; Sierra-Madero et al., 2014).
The active form of vitamin D has anti-inflammatory properties and higher vitamin D levels are associated with lower risks of immune-mediated disorders, multiple sclerosis and graft versus host disease (Salzer et al., 2012, von Bahr et al., 2015). In a randomized clinical trial of vitamin D supplementation in patients with TB, a subset of patients with a polymorphism in the vitamin D receptor showed improved infection clearance (Martineau et al., 2011). Most patients with HIV infection in low-resource settings have demonstrable vitamin D deficiency and this deficiency is directly related to the degree of immunosuppression (Aziz et al., 2013). A recent clinical trial of vitamin D supplementation in HIV infection showed a reduction in immune activation, suggesting an anti-inflammatory role (Fabre-Mersseman et al., 2014). Consistent with those results, our study revealed an association between lower vitamin D levels and IRIS risk. In contrast, a recent nested case–control study of TB-IRIS in HIV linked severe vitamin D deficiency with underlying inflammation, irrespective of IRIS status, suggesting that low vitamin D levels represent markers of inflammation, rather than of IRIS itself (Conesa-Botella et al., 2012). This hypothesis may best explain why a trend for significance between TB-IRIS risk and vitamin D was not upheld after multivariate adjustment. However in the same study, while corticosteroid use improved inflammatory cytokine expression, it did not influence vitamin D levels, suggesting that in this setting vitamin D deficiency may not be driven by inflammation but rather may drive it or alternatively, may play an independent role in IRIS pathogenesis (Conesa-Botella et al., 2012).
d-Dimer may be the biomarker most strongly associated with adverse events in HIV infection — from all-cause mortality in the SMART trial to venous thromboembolism and cardiovascular disease in prior published work (Kuller et al., 2008, Musselwhite et al., 2011, Nordell et al., 2014). Recent studies using peripheral blood monocyte populations have identified a unique relationship between d-dimer and monocyte activation — specifically that activated monocyte phenotypes are preferentially expanded in HIV infection and express tissue factor, promoting activation of the extrinsic coagulation cascade resulting in clot formation and elevation of d-dimer levels (Funderburg et al., 2012). These findings support our observations, which link higher d-dimer levels and sCD14 to IRIS risk.
Consistent with our findings of elevated IFNγ and sCD14 in IRIS, other studies have suggested that an exaggerated Th1 response may characterize the pathogenesis of IRIS (Grant et al., 2012, Vignesh et al., 2013, Ravimohan et al., 2015). The presence of a pathogen or malignancy triggers Th1 cytokine production, thereby converting myeloid cell precursors into potent, activated monocytes resulting in downstream inflammatory cytokine signaling.
Of note, higher levels of proinflammatory biomarkers TNFα and CRP were significantly associated with IRIS risk in our univariate analysis, while after adjustment, this statistical relationship tempered. It may be that these biomarkers better reflect a concomitant opportunistic infection, rather than IRIS risk. Indeed the relationship between IRIS and d-dimer, IFNγ, sCD14, and vitamin D were upheld following multivariate adjustment and may shed better light on the true pathophysiology of IRIS itself — independent of a pre-existing AIDS-defining illness.
In the present study, we identified a unique biosignature in participants who developed TB-IRIS compared to other IRIS causes. The biomarkers composing this signature indicate systemic inflammation (elevated CRP concentrations) monocyte activation (heightened sCD14 levels), increased Th1 responses (elevated IFNγ levels) and anemia (low Hb levels) in TB-IRIS, which when considered together in a composite score could indicate heightened risk. In addition, findings from our network analysis using Spearman matrices shows a negative correlations between Hb and sCD14 — another independently associated biomarker, highlighting the interplay between immune-mediated inflammation and anemia in these patients. We identified similar findings in TB-IRIS patients in prospective cohorts from India and South Africa (Narendran et al., 2013). Together, our results suggest that monocyte activation and the Th1 responses are robustly related to TB-IRIS.
A post-hoc, exploratory viral IRIS sub-analysis revealed a significant, independent relationship with IL-10. We recently reported a parallel observation in HIV-positive persons co-infected with hepatitis B or C who developed hepatitis flares after ART (Andrade et al., 2013). Further, higher hepatitis B and C viral loads were associated with hepatitis flares, suggesting that pathogen burden itself may play a role in IL-10 elevation and pathogenesis. Unfortunately, measurement of viral antigen and replication was beyond the scope of this study. Interleukin-10 is elevated in HIV-uninfected patients with hepatitis B flares (Tan et al., 2010), CMV reactivation (Frantzeskaki et al., 2015), and Herpes Zoster lesions (Zhang et al., 2011). Combined, these data highlight a replicable relationship between IL-10 concentration and viral infections commonly implicated in IRIS. In IRIS patients, we need to next determine whether it is pre-ART pathogen load itself or other host factors responsible for enhanced IL-10 concentration. In doing so, we may identify the best intervention target.
If our findings are reproducible in similar high-risk groups of patients, future research may be warranted to measure specific biomarkers to identify patients for whom vitamin D supplementation, anti-inflammatory or anti-thrombotic drugs may be of clinical benefit.
Acknowledgments
Author Contributions
IS and LWM designed the study, contributed to the analysis, and interpretation of the data and in writing the manuscript. AR performed laboratory studies, analyzed data, and reviewed manuscript. AT and SE performed all statistical analyses with the exception of the Interactome and composite score prediction analyses, which were performed by BBA. BBA assisted with the study design and interpretation of statistical analyses. JGSM, PFB, ML, and IS contributed to the collection of data, interpretation of results, and revised the manuscript. All authors have reviewed and approved the final version of the manuscript for publication.
Declaration of Interests
Dr. Ellenberg, Dr. Lederman, and Ms. Tierney report grants from Pfizer, Inc. to support the conduct of this study. Dr. Sierra-Madero reports grants from BMS, grants from Pfizer, Inc. grants from MSD, and grants from Stendhal, outside the submitted work. Dr. Sereti, Dr. Musselwhite, Dr. Andrade, Dr. Sanne, Dr. Belaunzaran-Zamudio, and Mr. Rupert have nothing to disclose.
Funding
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the National Cancer Institute through Contract No. HHSN261200800001E, and the work of Dr Musselwhite was also supported by the Foundation of the American Medical Association. The main clinical trial was supported by Pfizer Inc., New York, NY, USA. We acknowledge the Penn CFAR P30 AI 045008) for support in study planning.
Support
We thank all the CADIRIS participants. We also thank all study team members, including clinical staff at domestic and international study sites, for their contributions to patient care, data collection, and laboratory testing.
Footnotes
Preliminary results of this study were presented at the 22nd Conference on Retroviruses and Opportunistic Infections (CROI 2015) in Seattle, February 23–26, 2015. Abstract 308.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.01.016.
Appendix A. Supplementary data
Spearman correlations used to build the interactome graphs.
References
- Aloia J.F., Patel M., Dimaano R. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am. J. Clin. Nutr. 2008;87(6):1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- Andrade B.B., Hullsiek K.H., Boulware D.R. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J. Infect. Dis. 2013;207(9):1379–1388. doi: 10.1093/infdis/jit033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade B.B., Singh A., Narendran G. Mycobacterial antigen driven activation of CD14 ++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog. 2014;10(10):e1004433. doi: 10.1371/journal.ppat.1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Livak B., Burke-Miller J. Vitamin D insufficiency may impair CD4 recovery among Women's Interagency HIV Study participants with advanced disease on HAART. AIDS. 2013;27(4):573–578. doi: 10.1097/QAD.0b013e32835b9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D.R., Hullsiek K.H., Puronen C.E. Higher levels of CRP, d-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 2011;203(11):1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa-Botella A., Mathieu C., Colebunders R. Is vitamin D deficiency involved in the immune reconstitution inflammatory syndrome? AIDS Res. Ther. 2009;6:4. doi: 10.1186/1742-6405-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa-Botella A., Meintjes G., Coussens A.K. Corticosteroid therapy, vitamin d status, and inflammatory cytokine profile in the hiv-tuberculosis immune reconstitution inflammatory syndrome. Clin. Infect. Dis. 2012;55(7):1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens A.K., Wilkinson R.J., Hanifa Y. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. U. S. A. 2012;109(38):15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan K., Groeneveld A.B., de Geus H.R., Egal M., Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit. Care. 2014;18(6):660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Mersseman V., Tubiana R., Papagno L. Vitamin D supplementation is associated with reduced immune activation levels in HIV-1-infected patients on suppressive antiretroviral therapy. AIDS. 2014;28(18):2677–2682. doi: 10.1097/QAD.0000000000000472. [DOI] [PubMed] [Google Scholar]
- Frantzeskaki F.G., Karampi E.S., Kottaridi C. Cytomegalovirus reactivation in a general, nonimmunosuppressed intensive care unit population: incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. J. Crit. Care. 2015;30(2):276–281. doi: 10.1016/j.jcrc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Funderburg N.T., Zidar D.A., Shive C. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120(23):4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.M., Komarow L., Lederman M.M. Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. J. Infect. Dis. 2012;206(11):1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo-Ulloa I., Belaunzaran-Zamudio P.F., Crabtree-Ramirez B., Galindo-Fraga A., Perez-Aguinaga M.E., Sierra-Madero J.G. Impact of the immune reconstitution inflammatory syndrome (IRIS) on mortality and morbidity in HIV-infected patients in Mexico. Int. J. Infect. Dis. 2011;15(6):e408–e414. doi: 10.1016/j.ijid.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller L.H., Tracy R., Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc E., Chou R., Zakher B., Daeges M., Pappas M. Agency for Healthcare Research and Quality (US); Rockville (MD): 2014. Screening for Vitamin D Deficiency: Systematic Review for the US Preventive Services Task Force Recommendation. [PubMed] [Google Scholar]
- Martineau A.R., Timms P.M., Bothamley G.H. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca V.R., Queiroz A.T., Lopes F.M., Andrade B.B., Barral-Netto M. Networking the host immune response in Plasmodium vivax malaria. Malar. J. 2013;12:69. doi: 10.1186/1475-2875-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca V.R., Andrade B.B., Souza L.C. Unravelling the patterns of host immune responses in Plasmodium vivax malaria and dengue co-infection. Malar. J. 2015;14:315. doi: 10.1186/s12936-015-0835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. The Vitamin D receptor and its role in inflammation and host defence: interview with Dr Robert Modlin by Emma Quigley. Expert Opin. Ther. Targets. 2007;11(4):431–433. doi: 10.1517/14728222.11.4.431. [DOI] [PubMed] [Google Scholar]
- Muller M., Wandel S., Colebunders R. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselwhite L.W., Sheikh V., Norton T.D. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. 2011;25(6):787–795. doi: 10.1097/QAD.0b013e3283453fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran G., Andrade B.B., Porter B.O. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8(5):e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell A.D., McKenna M., Borges A.H. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J. Am. Heart Assoc. 2014;3(3):e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan S., Tamuhla N., Steenhoff A.P. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect. Dis. 2015;15(4):429–438. doi: 10.1016/S1473-3099(15)70008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [supplementary cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect. Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J., Hallmans G., Nystrom M., Stenlund H., Wadell G., Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79(21):2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- Sereti I., Rodger A.J., French M.A. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Curr. Opin. HIV AIDS. 2010;5(6):504–510. doi: 10.1097/COH.0b013e32833ed774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Madero J.G., E. S., Rassool M.S., Tierney A., Belaunzarán-Zamudio P.F., López-Martínez A., Piñeirúa-Menéndez A., Montaner L.J., Azzoni L., Benítez C.R., Sereti I., Andrade-Villanueva J., Mosqueda-Gómez J.L., Rodriguez B., Sanne I., Lederman M.M., CADIRIS study team A randomized, double-blind, placebo-controlled clinical trial of a chemokine receptor 5 antagonist to decrease the occurrence of immune reconstitution inflammatory syndrome in HIV-infection: the CADIRIS study. Lancet HIV. 2014;1(2):e60–e67. doi: 10.1016/S2352-3018(14)70027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.T., Koh S., Goh W. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J. Hepatol. 2010;52(3):330–339. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Van Den Bout-Van Den Beukel C.J., Fievez L., Michels M. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res. Hum. Retrovir. 2008;24(11):1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- Vignesh R., Kumarasamy N., Lim A. TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J. Acquir. Immune Defic. Syndr. 2013;64(3):241–248. doi: 10.1097/QAI.0b013e31829f6df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bahr L., Blennow O., Alm J. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50(9):1217–1223. doi: 10.1038/bmt.2015.123. [DOI] [PubMed] [Google Scholar]
- Yang C.Y., Leung P.S., Adamopoulos I.E., Gershwin M.E. The implication of vitamin D and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 2013;45(2):217–226. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wu N., Yang L. Study on the T-helper cell 1/2 cytokine profile in blister fluid of patients with herpes zoster and its clinical significance. J. Dermatol. 2011;38(12):1158–1162. doi: 10.1111/j.1346-8138.2011.01289.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman correlations used to build the interactome graphs.