Abstract
Objective:
To compare intracerebral hemorrhage (ICH) volume and clinical outcome of non–vitamin K oral anticoagulants (NOAC)–associated ICH to warfarin-associated ICH.
Methods:
In this multicenter cross-sectional observational study of patients with anticoagulant-associated ICH, consecutive patients with NOAC-ICH were compared to those with warfarin-ICH selected from a population of 344 patients with anticoagulant-associated ICH. ICH volume was measured by an observer blinded to clinical details. Outcome measures were ICH volume and clinical outcome adjusted for confounding factors.
Results:
We compared 11 patients with NOAC-ICH to 52 patients with warfarin-ICH. The median ICH volume was 2.4 mL (interquartile range [IQR] 0.3–5.4 mL) for NOAC-ICH vs 8.9 mL (IQR 4.0–21.3 mL) for warfarin-ICH (p = 0.0028). In univariate linear regression, use of warfarin (difference in cube root volume 1.61; 95% confidence interval [CI] 0.69 to 2.53) and lobar ICH location (compared with nonlobar ICH; difference in cube root volume 1.52; 95% CI 2.20 to 0.85) were associated with larger ICH volumes. In multivariable linear regression adjusting for confounding factors (sex, hypertension, previous ischemic stroke, white matter disease burden, and premorbid modified Rankin Scale score [mRS]), warfarin use remained independently associated with larger ICH (cube root) volumes (coefficient 0.64; 95% CI 0.24 to 1.25; p = 0.042). Ordered logistic regression showed an increased odds of a worse clinical outcome (as measured by discharge mRS) in warfarin-ICH compared with NOAC-ICH: odds ratio 4.46 (95% CI 1.10 to 18.14; p = 0.037).
Conclusions:
In this small prospective observational study, patients with NOAC-associated ICH had smaller ICH volumes and better clinical outcomes compared with warfarin-associated ICH.
Intracerebral hemorrhage (ICH) is the most feared complication of oral anticoagulation, with an in-hospital mortality of 42%.1 Despite advances in ICH prevention, the global incidence of ICH has not declined,2 likely secondary to the increase in anticoagulant-related ICH in the elderly.3–5
In large phase 3 randomized trials, patients in atrial fibrillation had half the incidence of ICH when taking non–vitamin K oral anticoagulants (NOACs) compared to warfarin, with similar efficacy in preventing ischemic stroke.6 Data on NOAC-associated ICH (NOAC-ICH) outside randomized trials are limited, and there is widespread concern that, without any currently available specific antidotes, those who have ICH while on NOACs might have larger ICH volumes and worse clinical outcomes than patients with warfarin-associated ICH (warfarin-ICH).7,8
Although experimental models show that dabigatran9 and rivaroxaban10—unlike warfarin—do not increase ICH volume unless given at supratherapeutic doses, few data are available on the clinical and radiologic characteristics of NOAC-ICH. A small study from Japan recently reported that in 5 patients with ICH associated with the NOAC rivaroxaban, the mean hematoma volume was smaller than in a comparison group of ICH associated with warfarin,11 and that functional outcomes were better in the NOAC group.
In this prospective, multicenter cross-sectional observational study of oral anticoagulant-associated ICH, we aim to describe the clinical and radiologic characteristics of NOAC-ICH in comparison to warfarin-ICH. We hypothesized that, in comparison to warfarin-ICH, NOAC-ICH have a smaller volume and a more favorable clinical outcome.
METHODS
Patients were recruited from an ongoing multicenter prospective observational cohort study of 344 patients with oral anticoagulant-related ICH.12 Inclusion criteria for the present study required adult patients (>18 years old) treated at participating centers with ICH confirmed on brain CT or MRI scans with a history of anticoagulant use at the time of the ICH, and informed written consent from the patient or a representative. Exclusion criteria include known underlying structural cause for ICH or major head trauma (causing loss of consciousness and thought to be sufficient to have caused the ICH) in the last 24 hours before presentation. Only patients with CT brain performed within 48 hours of onset of ICH symptoms were included. All consecutively recruited NOAC-ICH cases were considered for inclusion (n = 14) while warfarin-ICH cases were selected randomly from the same study population, recruited over the same time period, in a 4:1 ratio (n = 56), giving a total initial sample size of 70.
Our outcome measures were ICH volume (see below) and clinical outcome at discharge from hospital, measured by the modified Rankin Scale (mRS).11 Other variables included neuroimaging features (hematoma location, severity of white matter hyperintensities of presumed vascular origin), clinical demographics, vascular risk factors, international normalized ratio (INR), C-reactive protein, and immediate management.
Imaging was undertaken at each study center using standard clinical protocols. Anonymized DICOM images were sent to the study center. Two clinical research associates (D.W. and A.C.) blinded to clinical details and trained in neuroimaging undertook quality assurance and all imaging analysis. D.W. rated hematoma size using a validated semiautomated planimetric method13 including only scans less than 48 hours from onset with uniform slice thickness (ranging from 0.625 to 5 mm). Hematoma location was stratified as brainstem, cerebellar, deep, or lobar (cortical or cortical-subcortical and not involving any of the deep gray matter structures), and then further classified into lobar or nonlobar. White matter hyperintensities on plain CT were rated using the simplified Fazekas scale14 by a single trained observer (A.C.).
Statistical analysis.
Hematoma volume was cube root transformed for each patient to satisfy statistical assumptions regarding normality. We compared the characteristics of warfarin-ICH and NOAC-ICH using either t tests or Mann-Whitney tests for continuous variables, and either χ2 or Fisher test for categorical variables. The t tests were undertaken to identify possible predictors of cube root hematoma volume. A multivariable linear regression model was undertaken using the cube root of the hematoma size as the dependent variable and anticoagulant (warfarin or NOAC) as an independent variable. Other variables that were different between the 2 groups, or variables with biological plausibility as confounding factors, were added to the model.
Ordered logistic regression was undertaken using discharge mRS as the dependent variable and anticoagulant (warfarin or NOAC) as an independent variable adjusting for confounding factors (premorbid mRS and baseline NIH Stroke Scale [NIHSS] score). To satisfy the proportional odds assumption we reclassified the mRS as follows: mRS 0–2, mRS 3, mRS 4, and mRS 5–6.
Standard protocol approvals, registrations, and patient consents.
Clinical Relevance Of Microbleeds In Stroke (CROMIS-2) was approved by the Central London Research Ethics Committee, National Research Ethics Service.
Written informed consent for research was obtained from all participants (or guardians of participants) in the study.
RESULTS
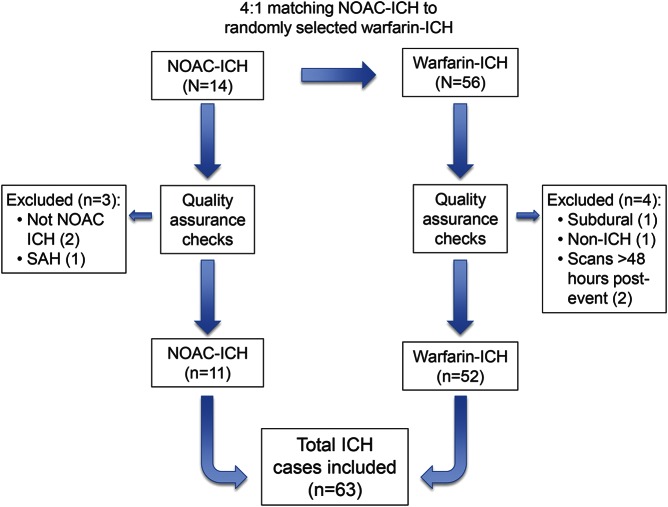
From a total population of 344 patients with ICH associated with oral anticoagulation, we identified 14 cases of NOAC-associated ICH. Blinded to all other clinical and radiologic details, we randomly selected 4 controls with warfarin-associated ICH for each NOAC-ICH case (resulting in a total of 56 warfarin-ICH cases). After quality assurance checks against our inclusion criteria, 3 NOAC-ICH and 4 warfarin-ICH cases were excluded. The final study population thus included 11 NOAC-ICH and 52 warfarin-ICH (figure 1). The NOAC group included 3 patients taking dabigatran, 6 taking rivaroxaban, and 2 taking apixaban from a total of 39 centers, a mixture of small district general hospitals and large central teaching hospitals.
Figure 1. Flow chart of study design and patient selection.
ICH = intracerebral hemorrhage; NOAC = non–vitamin K oral anticoagulant; SAH = subarachnoid hemorrhage.
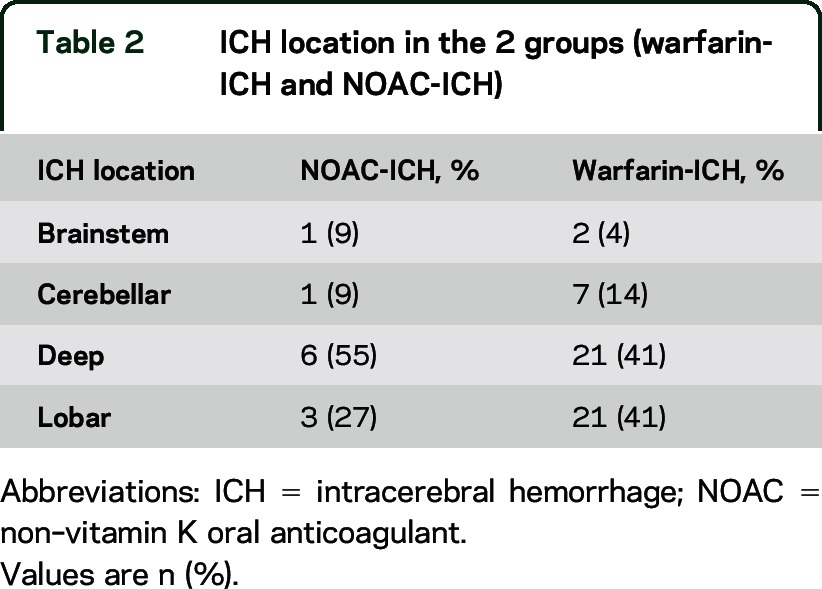
The median age of the participants in the entire cohort was 80.3 years (IQR 72.9 to 84.7); 46% were women. Characteristics were generally similar between the groups, but the NOAC-ICH cohort had a higher prevalence of female sex, hypertension, and previous stroke, but a lower degree of white matter hyperintensities and higher premorbid mRS (table 1). ICH location was similar among the groups (table 2). The mean INR value was 2.5 (95% CI 1.88 to 3.1) for the warfarin group. Of the 52 warfarin-ICH cases, only 13 had an INR >3 upon admission; only 5 had INR >3.5. There was little difference in mean hemorrhage volume (cube root) in warfarin-ICH between those with INR <3 (15 mL; 95% CI 7 to 23) and those >3 (16 mL; 95% CI 9 to 23) (p = 0.85). Time in range was not available.
Table 1.
Baseline characteristics of NOAC-associated and warfarin-associated ICH
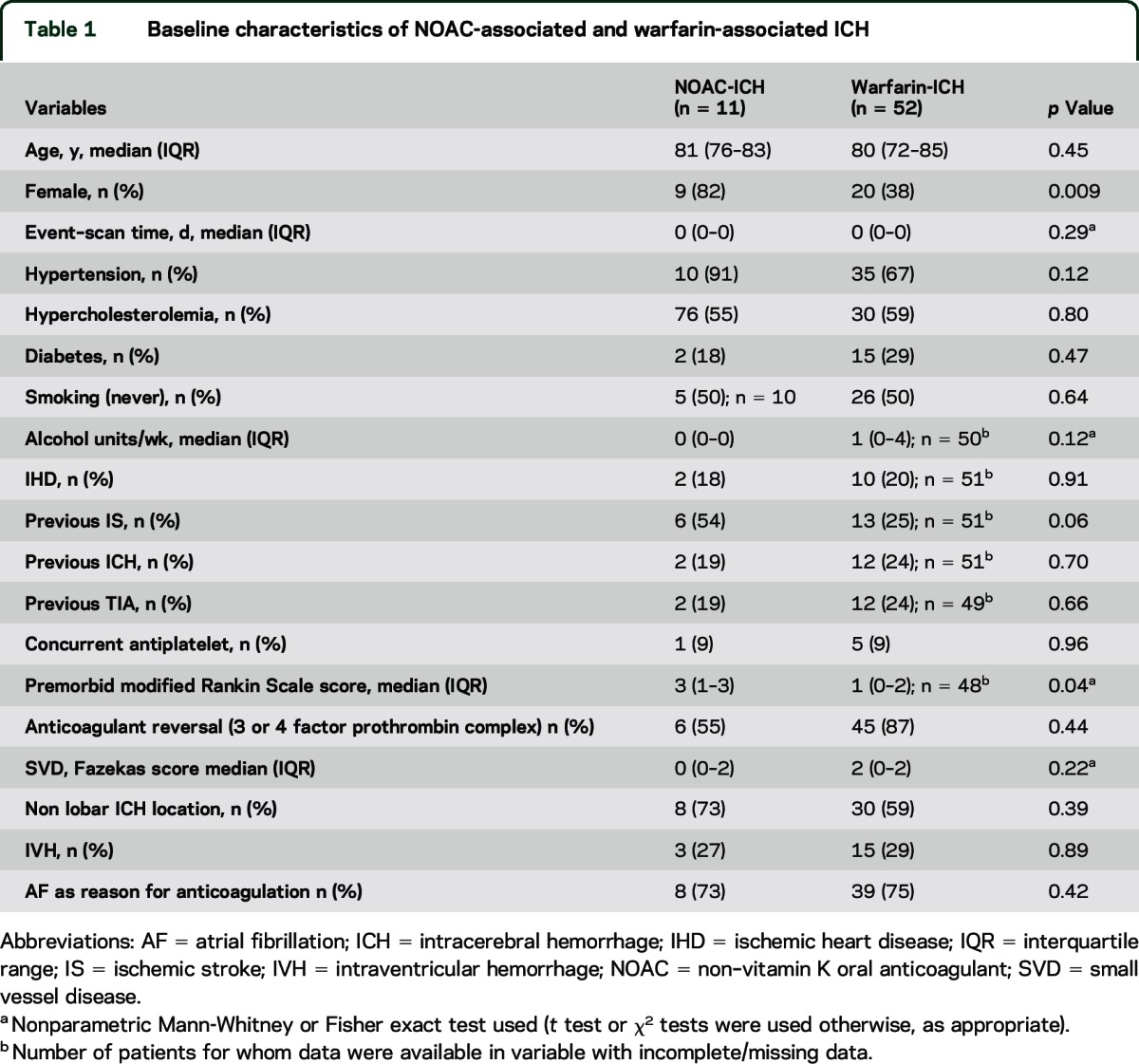
Table 2.
ICH location in the 2 groups (warfarin-ICH and NOAC-ICH)
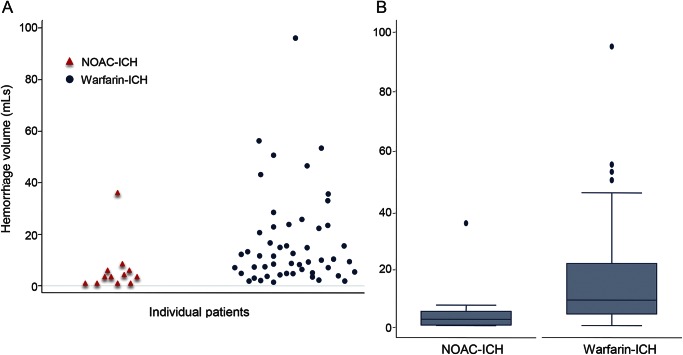
The median ICH volume was 2.4 mL (IQR 0.3–5.4 mL) for NOAC-ICH vs 8.9 mL (IQR 4.0–21.3 mL) for warfarin-ICH (p = 0.0028). Figure 2A shows individual data points for the volume of each ICH; figure 2B (box plot) shows the minimum, median, first quartile, third quartile, and maximum ICH volume in the NOAC-ICH and warfarin-ICH groups. In univariate analysis, only anticoagulant type (warfarin vs NOAC) and lobar ICH location were associated with (cube root) ICH volume (table 3).
Figure 2. Dot plot and box plot.
(A) Dot plot of individual participants and their corresponding hemorrhage sizes. Blue dots show warfarin–intracerebral hemorrhage (ICH); red triangles show non–vitamin K oral anticoagulant (NOAC)–ICH. (B) Box plot shows median, lower and upper quartiles, and minimum and maximum values of hematoma volume for NOAC-ICH cases and warfarin-ICH cases.
Table 3.
Mean cube root of the ICH volume according to different predictor variables of interest
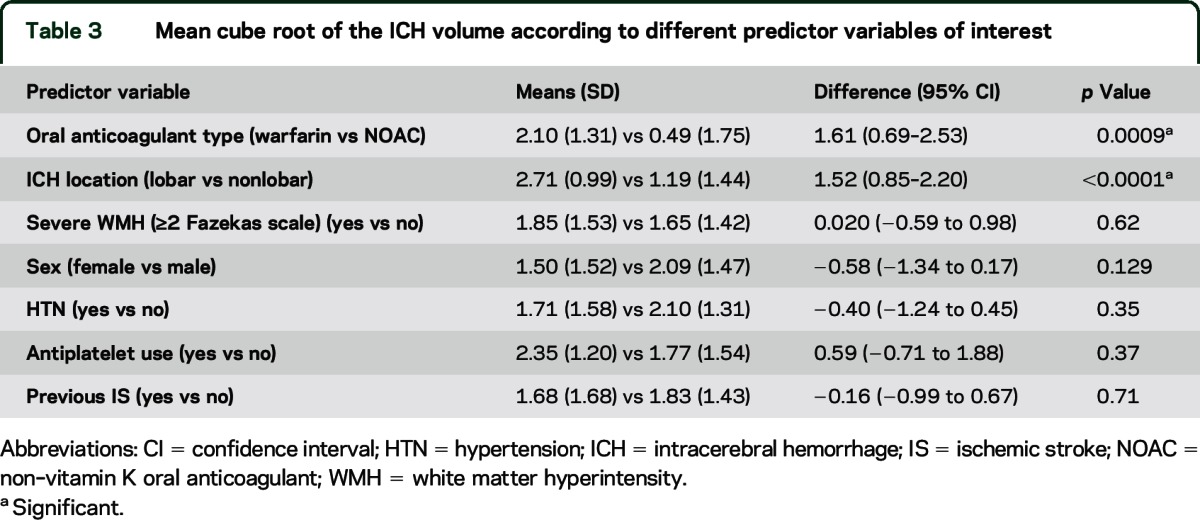
In multivariable linear regression, after adjusting for sex, hypertension, previous ischemic stroke, white matter disease burden, and premorbid mRS, warfarin-ICH (compared to NOAC-ICH) was independently associated with larger (cube root) ICH volume (coefficient 0.64, 95% CI 0.24 to 1.25; p = 0.042).
Ordinal logistic regression, adjusting for premorbid mRS and NIHSS, showed increased odds of a worse outcome in warfarin-ICH compared to NOAC-ICH: odds ratio 4.46 (95% CI 1.10 to 18.14; p = 0.037). The proportional odds assumption was satisfied.
DISCUSSION
Our results from a multicenter prospective observational study show that NOAC-ICH is associated with smaller ICH volume, and better clinical outcome, than warfarin-ICH. Our findings provide preliminary reassurance that NOACs can be used without concern that ICH complicating anticoagulation might be larger and associated with poorer outcomes for NOAC-ICH compared to warfarin-ICH. However, it would be valuable to confirm our findings in larger cohorts and randomized trials.
We are aware of only one previous small study on the clinical and radiologic characteristics of NOAC-ICH from Japan,11 which included 5 patients with rivaroxaban-associated ICH and found smaller ICH volumes and better outcome in comparison to warfarin-ICH. Our study included patients on a range of different NOACs (including both direct thrombin and Xa inhibitors) from multiple hospitals in a prospective cohort study, with regression analyses adjusted for potential confounding factors. Thus, our study strengthens the evidence that NOAC-ICH has a different radiologic and clinical profile vs warfarin-ICH, and may be more widely generalizable to other populations.
The mechanism leading to larger hemorrhage volume in warfarin-ICH compared with NOAC-ICH requires clarification. We postulate that due to the high levels of tissue factor (TF) around blood vessels in the brain,15 the extrinsic or TF-dependent coagulation pathway (the major route by which thrombin generation is initiated in response to vessel damage) plays a vital role in ICH volume. Following vascular injury related to ICH and exposure of TF-presenting cells to the blood, TF comes into contact with factor VII (FVII), a fraction (∼1%) of which circulates in its active form, FVIIa. TF binds both FVII and FVIIa with high affinity, and the trace amount of TF-FVIIa formed is sufficient to activate factor X (FX) both directly (extrinsic pathway) and indirectly via activation of factor IX,16 leading to fibrin formation. Vitamin K antagonists such as warfarin inhibit the vitamin K conversion cycle, inducing hepatic production of partially decarboxylated vitamin K–dependent coagulation proteins II, VII, IX, and X, with reduced coagulant activity.17,18 Thus warfarin, by reducing the amount of factor VII available for interaction with TF (the key system in the initiation of coagulation),19 reduces the extrinsic coagulation pathway and subsequent fibrin formation. NOACs specifically and competitively inhibit FXa (rivaroxaban and apixaban) or thrombin (dabigatran), and thereby reduce the thrombin burst during the downstream propagation phase of coagulation20 or thrombin activity,19 respectively. NOACs do not affect the initial factor VII and VIIa interaction with TF, which may provide an explanation for the smaller NOAC-ICH volumes in our study. Our mean event to scan time of under 24 hours in both groups suggests the shorter half-life of NOAC compared with warfarin is unlikely to play a role in baseline ICH size, although it may contribute toward the trend in improved clinical outcomes.
We hypothesize that the majority of ICH cases in both cohorts is secondary to an interaction of oral anticoagulation (causing impaired homoeostasis) with bleeding-prone cerebral small vessel diseases including a deep perforator (hypertensive) arteriopathy or cerebral amyloid angiopathy.21 While we have data on the baseline INR results for the warfarin cohort (which suggest the majority of warfarin-ICH occurred in the therapeutic range), we did not have access to specific coagulation measures specific for NOAC monitoring (for example, thrombin, activated partial thromboplastin time, and anti-FXa assays). It would have been interesting to examine whether these measures were outside the therapeutic ranges in NOAC-ICH cases. Furthermore, as we did not have access to MRI in these patients, we could not look at the different anticoagulant agents and their interactions with different underlying small vessel disease subtypes.
Strengths of our study include prospective and standardized clinical and imaging data collection. Furthermore, the comparator group (warfarin-related ICH) was drawn at random from the same study population, recruited over the same time period as the NOAC-ICH cases. The major limitations include the small sample size for NOAC-associated ICH, and the inherent selection bias towards ICH survivors. This bias will lead to smaller ICH volumes compared with a population-based cohort of consecutive ICH patients, and might underestimate the true between-group difference in mean ICH volume. As we did not match NOAC-ICH to warfarin-ICH patients, there may be inherent differences between the groups that could conceivably affect the outcomes of interest. However, variables that differed between the groups did not have any significant effect on ICH size in univariate analysis. We considered matching our NOAC-ICH and warfarin-ICH cohorts on a number of variables. However, we are interested in the exposure anticoagulant variable and its relationship with ICH volume and clinical outcome, and concluded that a multivariable regression analysis was the best approach for the following reasons. First, we did not want to overmatch and risk diluting any association of interest. Second, matching on multiple variables would be challenging or impossible due to the small pool of NOAC-ICH patients. Third, matching on only 1 or 2 variables would not fully solve the confounding issues but would necessitate a far more complicated analysis. Fourth, multivariable regression analysis of matched ordinal data was not a standard analysis.
Confounding could arise if hospitals that prescribe NOACs have a higher level of care than those that do not. However, this seems unlikely as the majority of study centers (29 of 39) prescribe NOACs. Furthermore, discharge mRS may not be the most appropriate longer term functional outcome, because ICH has a high 1-month mortality rate (approaching 50%).22 Future studies should include mortality as an outcome measure. Finally, our study is cross-sectional and observational, so cannot demonstrate causality or eliminate all sources of confounding. Randomized control trials are needed to make definitive treatment comparisons. Previous randomized trials show no significant difference in case fatality from ICH among participants assigned dabigatran 150 or 110 mg vs warfarin (41% dabigatran 110 mg vs 35% dabigatran 150 mg vs 36% warfarin),23 or rivaroxaban vs warfarin (48% rivaroxaban vs 50% warfarin).24 The similar fatality rates seen in the above trials suggest that ICH sizes may have been comparable between NOAC-ICH and warfarin-ICH, but radiologic analyses are not available. It is thus possible that our results may not hold true in larger samples. Further larger observational collaborative studies, and randomized control trials of NOACs vs vitamin K antagonists, with detailed clinical and radiologic assessment of ICH events and their outcome, are therefore needed.
Supplementary Material
GLOSSARY
- FVII
factor VII
- FX
factor X
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- NOAC
non–vitamin K oral anticoagulant
- TF
tissue factor
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: On behalf of the CROMIS-2 collaborators, Louise Shaw, Jane Sword, Azlisham Mohd, Pankaj Sharma, Deborah Kelly, Frances Harrington, Marc Randall, Matthew Smith, Karim Mahawish, Abduelbaset Elmarim, Bernard Esisi, Claire Cullen, Arumug Nallasivam, Christopher Price, Adrian Barry, Christine Roffe, John Coyle, Ahamad Hassan, Caroline Lovelock, Jonathan Birns, David Cohen, L Sekaran, Adrian Parry-Jones, Anthea Parry, David Hargroves, Harald Proschel, Prabel Datta, Khaled Darawil, Aravindakshan Manoj, Mathew Burn, Chris Patterson, Elio Giallombardo, Nigel Smyth, Syed Mansoor, Ijaz Anwar, Rachel Marsh, Sissi Ispoglou, Dinesh Chadha, Mathuri Prabhakaran, Sanjeevikumar Meenakishundaram, Janice O'Connell, Jon Scott, Vinodh Krishnamurthy, Prasanna Aghoram, Michael McCormick, Paul O’Mahony, Martin Cooper, Lillian Choy, Peter Wilkinson, Simon Leach, Sarah Caine, Ilse Burger, Gunaratam Gunathilagan, Paul Guyler, Hedley Emsley, Michelle Davis, Dulka Manawadu, Kath Pasco, Maam Mamun, Robert Luder, Mahmud Sajid, Ijaz Anwar, James Okwera, Julie Staals, Elizabeth Warburton, Kari Saastamoinen, Timothy England, Janet Putterill, Enrico Flossman, Michael Power, Krishna Dani, David Mangion, Appu Suman, John Corrigan, Enas Lawrence, and Djamil Vahidassr
AUTHOR CONTRIBUTIONS
Duncan Wilson drafted and revised the manuscript and was involved in study concept, analysis and interpretation of data, and statistical analysis. Andreas Charidimou revised the manuscript and was involved in study concept. Clare Shakeshaft revised the manuscript, was study coordinator, and was involved in acquisition of data. Gareth Ambler revised the manuscript analysis and interpretation of data and statistical analysis. Mark White revised the manuscript and was involved in acquisition of data. Hannah Cohen revised the manuscript and was involved in study design. Tarek Yousry revised the manuscript and was involved in study design. Rustam Al-Shahi Salman revised the manuscript and was involved in study design. Gregory Y.H. Lip revised the manuscript and was involved in study design. Martin M. Brown revised the manuscript and was involved in study design. Hans Rolf Jäger revised the manuscript was involved in study design and analysis of data. David J. Werring revised the manuscript, and was involved in study concept, analysis and interpretation of data, study concept and design, study supervision, and obtaining funding.
STUDY FUNDING
The CROMIS-2 study is funded by the Stroke Association and British Heart Foundation. Rustam Al-Shahi Salman was funded by an MRC senior clinical fellowship.
DISCLOSURE
D. Wilson, A. Charidimou, C. Shakeshaft, G. Ambler, M. White, H. Cohen, T. Yousry, and R. Al-Shahi Salman report no disclosures relevant to the manuscript. G. Lip has served as a consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Biotronic, Portola, and Boehringer Ingelheim and has been on the speakers' bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, and Sanofi-Aventis. M. Brown, R. Jäger, and D. Werring report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dowlatshahi D, Butcher KS, Asdaghi N, et al. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke 2012;43:1812–1817. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology 2007;68:116–121. [DOI] [PubMed] [Google Scholar]
- 4.Lovelock CE, Molyneux AJ, Rothwell PM, Oxford Vascular S. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol 2007;6:487–493. [DOI] [PubMed] [Google Scholar]
- 5.Bejot Y, Cordonnier C, Durier J, Aboa-Eboule C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain 2013;136:658–664. [DOI] [PubMed] [Google Scholar]
- 6.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 7.Schulman S, Majeed A. The oral thrombin inhibitor dabigatran: strengths and weaknesses. Semin Thromb Hemost 2012;38:7–15. [DOI] [PubMed] [Google Scholar]
- 8.Ansell J. New oral anticoagulants should not be used as first-line agents to prevent thromboembolism in patients with atrial fibrillation. Circulation 2012;125:165–170. [DOI] [PubMed] [Google Scholar]
- 9.Lauer A, Cianchetti FA, Van Cott EM, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011;124:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Zorn M, Nawroth P, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke 2013;44:771–778. [DOI] [PubMed] [Google Scholar]
- 11.Hagii J, Tomita H, Metoki N, et al. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke 2014;45:2805–2807. [DOI] [PubMed] [Google Scholar]
- 12.Charidimou A, Shakeshaft C, Werring DJ. Cerebral microbleeds on magnetic resonance imaging and anticoagulant-associated intracerebral hemorrhage risk. Front Neurol 2012;3:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volbers B, Staykov D, Wagner I, et al. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur J Neurol 2011;18:1323–1328. [DOI] [PubMed] [Google Scholar]
- 14.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 15.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res 1990;59:421–437. [DOI] [PubMed] [Google Scholar]
- 16.Morrison SA, Jesty J. Tissue factor-dependent activation of tritium-labeled factor IX and factor X in human plasma. Blood 1984;63:1338–1347. [PubMed] [Google Scholar]
- 17.Friedman PA, Rosenberg RD, Hauschka PV, Fitz-James A. A spectrum of partially carboxylated prothrombins in the plasmas of coumarin-treated patients. Biochim Biophys Acta 1977;494:271–276. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra OP, Nesheim ME, Mann KG. The kinetics of activation of normal and gamma-carboxyglutamic acid-deficient prothrombins. J Biol Chem 1985;260:279–287. [PubMed] [Google Scholar]
- 19.Spyropoulos AC. Investigational treatments of venous thromboembolism. Expert Opin Investig Drugs 2007;16:431–440. [DOI] [PubMed] [Google Scholar]
- 20.Perzborn E, Roehrig S, Straub A, Kubitza D, Mueck W, Laux V. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol 2010;30:376–381. [DOI] [PubMed] [Google Scholar]
- 21.Wilson D, Charidimou A, Werring DJ. Advances in understanding spontaneous intracerebral hemorrhage: insights from neuroimaging. Expert Rev Neurother 2014;14:661–678. [DOI] [PubMed] [Google Scholar]
- 22.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project—1981-86: 2: incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012;43:1511–1517. [DOI] [PubMed] [Google Scholar]
- 24.Hankey GJ. Intracranial hemorrhage and novel anticoagulants for atrial fibrillation: what have we learned? Curr Cardiol Rep 2014;16:480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.