Abstract
Objective:
To determine the value of neurologist ambulatory care in chronic neurologic diseases in a large administrative claims dataset detailing costs, adverse events, and health care utilization.
Methods:
The Optum proprietary claims dataset (2010–2012) was examined to describe direct health care costs, as well as specific outcome metrics for a large population of persons with chronic neurologic illnesses. In phase I of the study, we detail neurologist involvement and differences in annualized allowed third--party payments within episode treatment groups (ETGs) for 10 neurologic illnesses. For phase II, we examined health care utilization for ETGs of epilepsy, Parkinson disease (PD), stroke, and multiple sclerosis (MS) with and without neurologist involvement. Reported outcomes were unadjusted differences and odds ratios between treatment groups.
Results:
For phase I, a total of 1,913,605 ETGs for 10 neurologic conditions were identified, 30.1% meeting criteria for neurologist involvement. All conditions had higher direct costs when neurologists were involved with care, ranging from a 25% increase for Alzheimer dementia to 100% more for MS care. In phase II, fractures, infections, emergent care, and inpatient admission were less with neurologist ambulatory care, while neurologist care was associated with greater utilization of disease-specific treatments (immunotherapies in MS anticoagulation in atrial fibrillation–associated stroke, deep brain stimulation and dopaminergic therapies in PD).
Conclusion:
Neurologist involvement with care is associated with greater unadjusted allowed payments, but fewer adverse events and less acute care utilization.
The effect of neurologist care on health care costs and utilization outcomes in persons with chronic neurologic illness is not well-characterized. Aggregation of health care information in large administrative claims datasets presents an opportunity to assess the effect of specialist physicians on downstream usage of health resources related to the diseases they treat. Payers, from the US Government to third-party private insurers and employers, are looking to this kind of big data analysis to inform coverage, decisions, regulatory actions, and policymaking decisions.1–5
Exploration of large datasets requires access to specialized expertise, programmers, and computing unavailable to clinicians in daily practice. Physician professional organizations, on behalf of their members, have the resources to engage in analysis of these data and advocate for value and quality.6 Therefore, the American Academy of Neurology (AAN) engaged Optum, Inc. (Philadelphia, PA), a health care consulting firm, for a guided exploratory analysis of their proprietary administrative claims dataset to assess the value of neurologist care.
The study was staged in 2 separate phases. Phase I was an economic burden of illness (BOI) study of a number of neurologic conditions assessing neurologist care and related health care costs. Phase II examined disease-specific metrics of health care utilization, therapeutics, and screening for selected neurologic illnesses.
METHODS
Data source.
We utilized the Optum proprietary administrative claims database for 2010–2012, detailing health care for 21.4 million unique persons. Data are from persons enrolled in commercial insurance as well as a Medicare supplemental insurance package. Most enrollees are 18–64 years old, but children and retirement-age persons are included. Components of this dataset include emergency department (ED), inpatient, outpatient, and office-based medical care, durable medical equipment, pharmacy records, and hospital and office-based medical procedures.
Study design.
This was a cross-sectional study design.
Funding and approvals.
The study was approved and funded by the AAN. Project data analysis was approved by internal review of Optum.
Identification of cohort.
Optum uses Symmetry episode treatment group (ETG) software7 for determining episodes of care for persons with specific medical conditions. ETGs aggregate inpatient, outpatient, and pharmacy services attributable to specific diagnoses over time, and are structurally similar to diagnosis-related groups used in the inpatient setting. Approximately 85% of US insurance claims are assigned to ETGs based on groups of relevant ICD-9-CM codes for billing. To be identified within an ETG, a person must have a billable claim for a relevant disease-specific ETG. Each ETG spans exactly 1 calendar year; therefore, the same patients may be counted for separate ETGs in multiple calendar years. Only persons with complete data for the calendar year were included. The unit of analysis was therefore the ETG, not the individual with the medical condition. We looked for ETGs indicating neurologic disorders.
For phase I (economic burden of illness study), the AAN relied on expert opinion to select 10 neurologic disorders of interest: Alzheimer disease, amyotrophic lateral sclerosis (ALS), autism, developmental delay, stroke (defined by presence of a stroke hospitalization), dementia (all), epilepsy, migraine headache, multiple sclerosis (MS), and Parkinson disease (PD). Four disorders were selected from among the phase I disorders by AAN expert opinion (largely on the basis of a high proportion of neurologist involvement and prospect for continued chronic care after diagnosis) for in-depth accounting of health care utilization and outcomes focused on 4 neurologic diseases: MS, epilepsy, PD, and stroke. Epilepsy ETGs with epilepsy surgeries were excluded for phase II.
Determination of exposure.
Exposure was defined as an office-based or outpatient face-to-face encounter with a neurologist for all conditions except stroke in phase I (but not phase II), where a billed inpatient consultation or visit identified neurologist involvement.
Outcomes of interest.
Phase I of the study examined the numbers of specific neurologic ETGs within the dataset, the incidence of neurologist involvement, and the mean total direct health care costs stratified over ETGs with and without neurologist involvement in care. Costs were defined as allowed payments from third-party payers, indexed using the Consumer Price Index for Medical Expenditure to 2012 dollars.8 Cost outliers (<5th and >95th percentile) were excluded.
Phase II focused on condition-specific metrics of incident adverse event and related health care utilization, or use of disease-specific treatments and screening in consultation with subject matter experts from the AAN. Occurrence and counts of ICD-9-CM diagnostic codes, Current Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes within ETGs were dichotomized over neurologist involvement. Pharmaceutical-related outcomes were determined for ETGs where persons had pharmacy insurance benefits.
Severity determination.
Optum utilizes regression analysis of diagnostic codes encompassed within a particular ETG, adjusted for patient demographics, condition status, and comorbidities to differentiate severity levels (higher severity correlating with higher costs). For ETGs with only 1 associated ICD-9-CM diagnostic code (i.e., MS, PD), only 1 severity level can be determined. An ETG could have up to 4 levels of increasing severity.
Statistical analysis.
The results of the project were presented to the AAN as summary count data by Optum. Observation-level data were not available for subsequent review or analysis.
The analysis for phase I depicts ETG counts, proportions, and unadjusted mean costs for neurologic diseases and neurologist involvement. Costs were not adjusted based on differences in third-party payers. This descriptive analysis is stratified for year, where appropriate. No hypothesis testing was performed.
Descriptive analysis for phase II included ETG count data, and outcome counts by neurologist involvement stratified within-ETG severity level where available and appropriate. Secondary analysis using 2 × 2 table odds ratio estimation tested for significant differences between nonoverlapping ETG count categories, when possible. Significance was set at α = 0.05 and p values reported for χ2 test for homogeneity. Further adjustment using Mantel-Haenszel techniques for severity levels was not performed due to (1) only 1 level of severity for the disorder (MS, PD), (2) no important differences were ascertained among severity levels (stroke), or (3) the data reporting did not lend itself to inferential testing (epilepsy). All dataset queries and descriptive analysis was performed using SAS 9.3 (SAS Institute, Cary, NC). Secondary analysis of phase II results were performed using Stata 13.0 (StataCorp, College Park, TX).
RESULTS
Phase I.
A total of 1,913,605 ETGs met inclusion criteria. Of these, 593,101 had neurologist involvement among 10 index neurologic conditions. Relative to the overall demographic of the Optum Database, persons seeing neurologists were older (mean age 46.9 years vs 36.5 years), more often female (59.6% vs 52.3%), and had Medicare as primary insurance (22.2% vs 8.7%) (table 1). ETG counts were largest for migraine (n = 998,036) and epilepsy (n = 279,173), and smallest for ALS (n = 3,271) (figure 1). Two or more severity levels were differentiated in epilepsy (high and low severity), stroke (4 graduated levels of severity), and migraine (3 levels of severity) (table e-1 on the Neurology® Web site at Neurology.org). Neurologist involvement was highest for stroke and MS (>70%), and least for dementias, developmental disorders, and autism (<12%). ALS, epilepsy, and PD had neurologists involved with ≥50% of episodes (figure 1). In migraine and epilepsy, neurologist involvement was greater at higher severity levels (table e-2).
Table 1.
Demographic and insurance information of persons seen by neurologists for 10 identified conditions compared to the entire Optum dataset
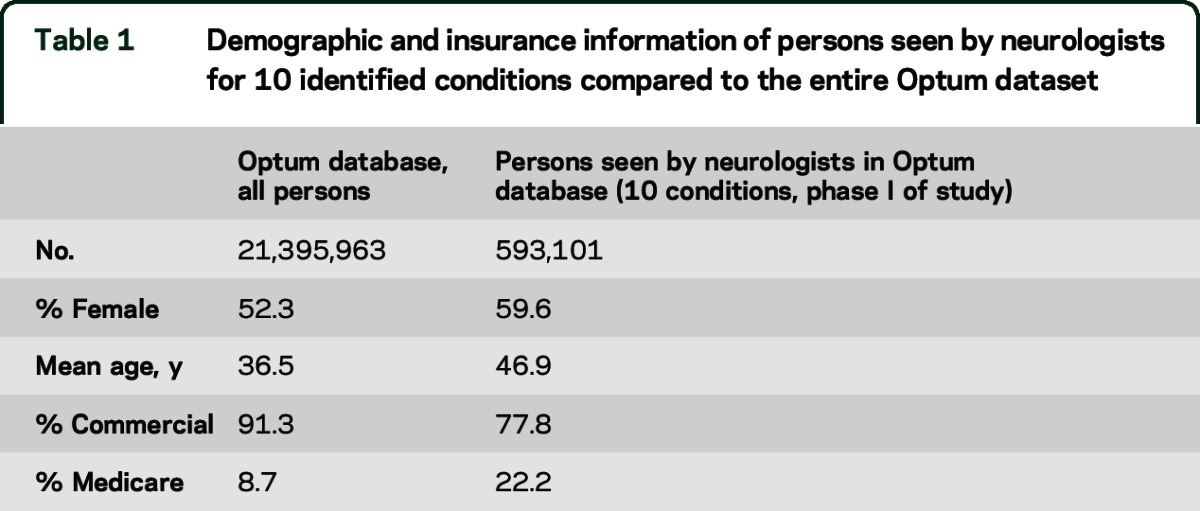
Figure 1. Episode treatment groups (ETGs) (bar height) and percentage neurologist involvement (top of bars) for 10 neurologic conditions.
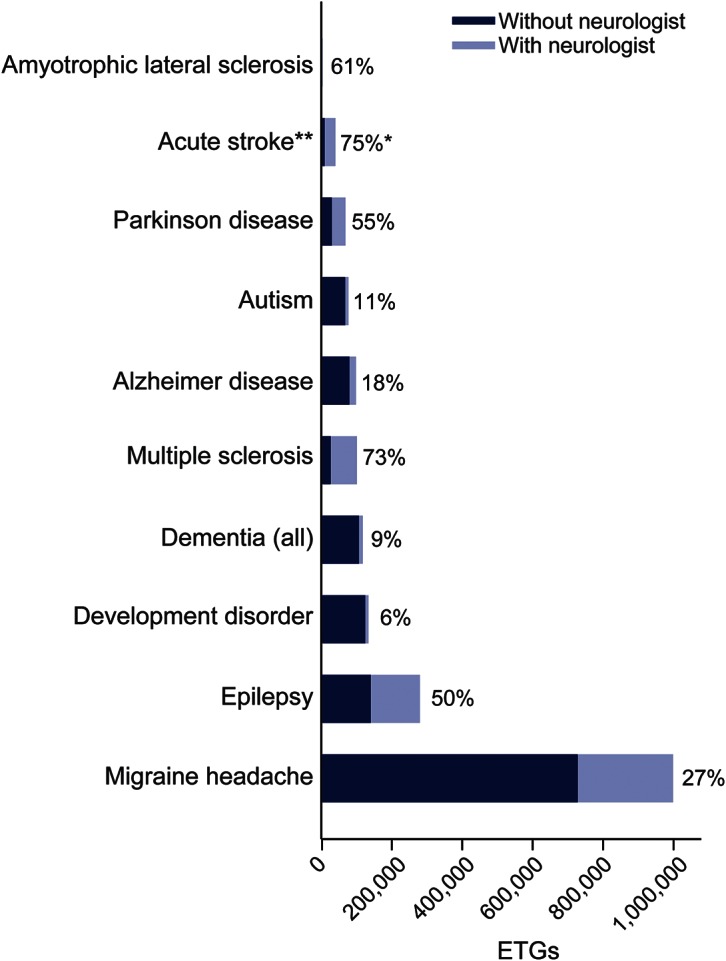
*Involvement defined as ambulatory care for all conditions except acute stroke, where inpatient neurologist care was defined as involvement. **Acute stroke defined as having an inpatient stay with a discharge diagnosis-related group for stroke in a calendar year.
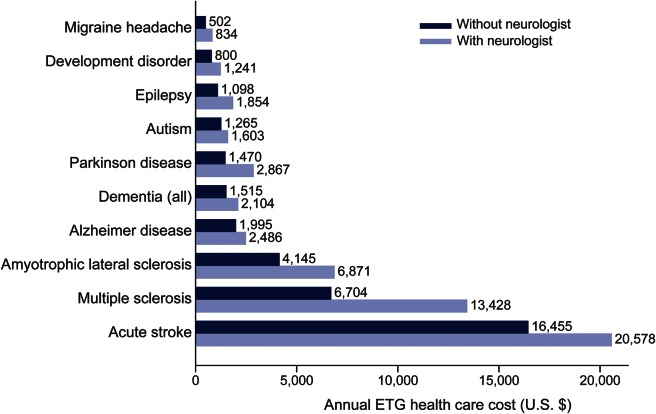
Total allowed payments for condition-related health care during the ETG calendar year were greater with neurologist involvement for all conditions. Neurologists had the largest percentage effect on annual costs for multiple sclerosis (+100% costs), and the least among Alzheimer disease and acute stroke (+25% costs) (figure 2). Among the lowest severity level ETGs for epilepsy and migraine, costs were ≥40% greater with neurologist involvement (table e-2).
Figure 2. Average annual episode treatment group (ETG) costs (allowed third-party payments, in 2012 US dollars) by neurologist involvement.
Phase II: Focused metrics for specific conditions.
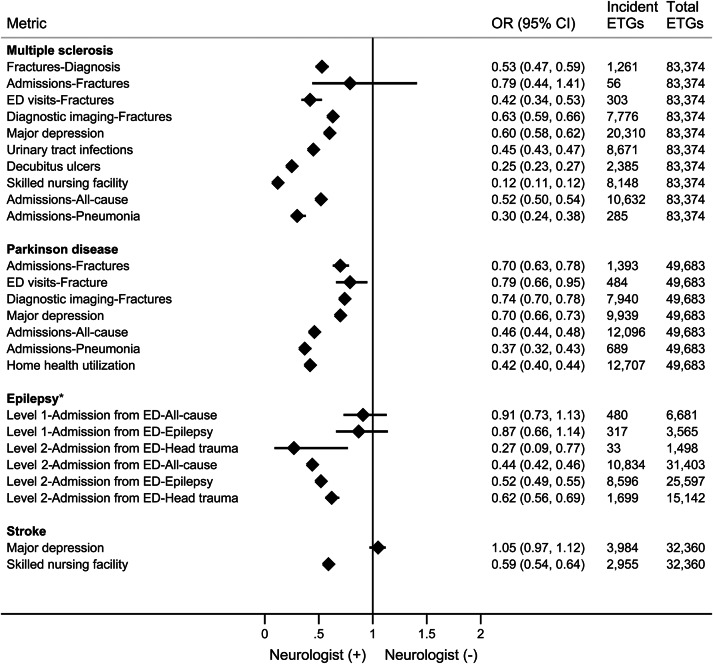
Adverse event reporting and related health care utilization was less for ETGs with ambulatory neurologist care, while usage of disease-specific treatments and screening was substantially greater for selected conditions (figures 3 and 4). Notably, only 32% of ETGs for stroke included an outpatient neurologist, meeting the case definition for phase II neurologist involvement.
Figure 3. Adverse events and health care utilization metrics, and association with neurologist ambulatory care, by condition.
*Epilepsy has 2 severity levels; total episode treatment groups (ETGs) depicted here for epilepsy are emergency visits. CI = confidence interval; ED = emergency department; OR = odds ratio.
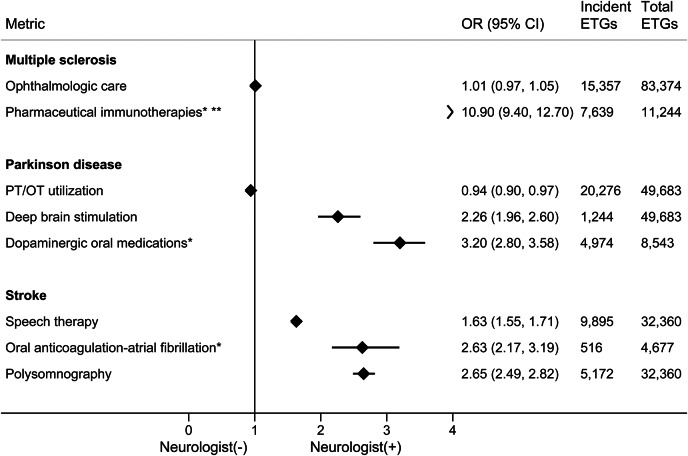
Figure 4. Disease-specific therapeutics and related screening metrics, by condition.
*For episode treatment groups (ETGs) with included pharmacy insurance benefit. **Immunotherapies not including steroid treatments. CI = confidence interval; OR = odds ratio; OT = occupational therapy; PT = physical therapy.
ETGs with neurologist ambulatory care had significantly fewer overall hospitalizations, fewer diagnosed fractures and related health care, fewer pneumonia admissions, and less coincident major depression in MS and PD. Postacute care (skilled nursing facility [SNF] or home health agency [HHA]) utilization was less when an outpatient neurologist was involved in ETGs for MS (SNF odds ratio [OR] 0.12, 95% confidence interval [CI] 0.11–0.12), stroke (SNF OR 0.59, 95% CI 0.54–0.64), and PD (HHA OR 0.42, 95% CI 0.34–0.53). Epilepsy ETGs with neurologist care had lower mean annual rates of ED usage (0.22 vs 0.27) and, at the higher severity level, significantly less inpatient admission from the ED (OR 0.44, 95% CI 0.42–0.46).
Neurologist care was associated with greater utilization of disease-specific therapies, including immunotherapies for MS (OR 10.9, 95% CI 9.4–12.7), deep brain stimulation in PD (OR 2.26, 95% CI 1.96–2.60), dopaminergic pharmacotherapy in PD (OR 3.16, 95% CI 2.79–3.58), and anticoagulation in atrial fibrillation–associated stroke (OR 2.63, 95% CI 2.17–3.19). EEGs were more commonly utilized in epileptic patients with outpatient neurologists. Neurologist ambulatory care in stroke was associated with more use of speech therapy and sleep studies (OR 1.63, 95% CI 1.55–1.71, p < 0.001; OR 2.65, 95% CI 2.49–2.82, p < 0.001, respectively). However, yearly ophthalmologic screening and liver function and blood count testing in MS (if receiving immunotherapies), physical and occupational therapy for PD, and medication compliance rates in epilepsy and MS were not substantially different or were slightly worse in the group with identified neurologist care (table e-3).
DISCUSSION
This study is part of the AAN's ongoing effort to determine if and how a neurologist provides value to health care. Phase I results informed selection of index neurologic conditions for phase II, where epilepsy, MS, PD, and stroke ETGs were relatively common, with a high proportion of ambulatory neurologist care. Ambulatory care by neurologists was selected as the exposure of interest, because the majority of neurologist care occurs in an outpatient or office-based medical setting.9 The findings give a bird's eye view of commercial claims data pertinent to neurologists by examining ETGs, the most common classification of disease-related services for commercial payers, and a likely candidate for use in Medicare and Medicaid in the near future.10 The major study findings are costs of care when a neurologist is involved, the effect of neurologist care on acute and postacute health care utilization, and the effects on disease-specific therapies and screening.
The first major finding is that ETGs with neurologist involvement have greater allowed third-party payment. This result should be tempered by several factors. (1) Analysis compares neurologist involvement with noninvolvement, including ETGs where no physician care is identified. These ETGs will invariably have less expense than those with physician involvement. (2) Neurologists deliver disease-specific care, where diagnostic testing (i.e., EEGs and MRIs) and disease-specific drugs are more likely to be captured as expenses of the ETG than other care. (3) Many cost-savings effects of neurologist care mediated by reduced acute health care utilization are not reflected in the disease-specific ETG payments. Fewer inpatient admissions and emergency visits for pneumonia and fractures would not be included in the ETG payments. (4) By looking only at cross-sectional data confined within the calendar year, we may not be capturing the longitudinal effects of neurologist care on bending the curve of disease costs over the lifetime of the disorder. (5) These results are unadjusted for patient disease severity, age, or comorbid conditions. (6) We look only at allowed third-party payments, not indirect health costs such as caretaker costs, work absenteeism, reduced productivity, or opportunity losses from missed school. Nor does this study quantify diminished quality of life.
Certainly, ETGs with neurologist involvement may have greater costs even if all of these issues were addressed, but the beneficial effects of neurologist care may not be reducible to costs alone. The goal of health care, then, is not to provide care at zero or net negative expenditure, but rather to improve health and quality of life at acceptable costs. Through administrative claims data, we cannot look at clinical improvement directly, but we can deduce better health in avoided ED visits and unplanned hospitalizations, where these data show a meaningful and significant effect for neurologist ambulatory care, especially in MS and PD. For health economists, this becomes part of a familiar formulation, where the cost per beneficial health effect is not zero or negative, but improved health is achieved at some increased cost.11
The second major finding is that neurologist ambulatory care is associated with decreased adverse events and usage of acute and postacute health care resources. Patients who visit neurologists for MS have fewer urinary tract infections and decubitus ulcers, less pneumonias and major depression in MS and PD, and fewer disease-specific hospitalizations for MS, PD, and epilepsy (including epilepsy monitoring unit admissions). That all-cause hospitalizations are less for these patients with ambulatory neurologist care suggests that neurologist visits may have beneficial effects even outside of the treated neurologic disorder. ED visits are reduced with neurologist care for severe epilepsy, a disorder defined by its paroxysmal events, in all-cause, epilepsy-specific, and head-trauma ED visits. Even when an ED visit occurs, subsequent inpatient hospitalization is less likely for patients with epilepsy under a neurologist's care. Postacute care is also meaningfully affected for patients with neurologist outpatient care, where SNFs were less likely to be used for MS and stroke, and the likelihood of HHA use was less for patients with PD. Only the odds of major depression in stroke patients were (nonsignificantly) higher with neurologist outpatient care, which may be due to greater identification by neurologists of underlying major depression.
The third major finding is that neurologist involvement is associated with improvement in utilization of disease-specific therapies and screening. Neurologist care is significantly associated with greater usage of symptom-ameliorating and disease-modifying medication usage for MS, PD, and stroke. Poststroke patients are more likely to have polysomnography for sleep-disordered breathing if they are taken care of by neurologists. Deep brain stimulation is more common among patients with PD with outpatient neurologists. In contrast, patients under neurologist care are no more likely to have routine ophthalmologic care, cognitive testing, or complete blood counts or liver function testing for MS, are less likely to have physical or occupational therapy for PD, and had no better medication compliance rates in epilepsy. While a number of these metrics are coincident with AAN Physician Performance Quality Measures (https://www.aan.com/practice/quality-measures/), the metrics were not based on these published guidelines.
The results depicted here provide a substantial contribution to health services research on neurologist involvement with care and provide a model to study the value of specialist care in general. Access to a neurologist has been an outcome of interest in a number of studies,9,12–14 but the differential effect of neurologist involvement on clinical, cost, and downstream health care utilization outcomes have been evaluated in few studies for specific diseases. The VA Stroke Study15 showed neurologist involvement with inpatient stroke care was associated with more diagnostic testing and greater costs, but improved patient outcomes (less mortality and disability). In a survey of 121 Dutch patients with MS,16 69% of those seeing a neurologist reported health care use (inpatient and outpatient) in the subsequent 6 months, compared to 62% of those seeing a general practitioner. For a cohort of 103 patients diagnosed with pseudoseizures by an epileptologist,17 emergency and inpatient visits were reduced by 40% in the year after diagnosis compared to the year prior. In studies of Medicare claims for patients with PD,18,19 health care use for hip fracture, SNF usage, and inpatient stay for PD-related events were significantly reduced when a neurologist was involved with care.
Our study coincides with the recent emphasis on acute and postacute care with implications for health care policy. The Center for Medicare and Medicaid Services (CMS) assesses 30-day readhmission rates for modifications in Medicare Part A payments to hospitals, while basing the value-based payment modifier to Part B physician payments in part on inpatient care usage.20 Payments to SNFs and home health agencies have grown at twice the annual rate of hospital and physician payments, and account for 40% of the geographic variation in Medicare spending.21,22 Ambulatory neurologist care could be incentivized by CMS through inclusion of neurologists in accountable care organizations, the Bundled Payment for Care Improvement program, and patient-centered medical home new care models,23 through inclusion in the cognitive care bonus that primary care and medicine subspecialist physicians enjoyed and through Medicare–Medicaid physician payment–parity initiatives.24
There are several limitations to this study. Administrative claims data pose unique challenges to researchers. Sample sizes are enormous, and can overemphasize minor differences.25 The absence of clinical information creates a need for proxies based on coding of billed services. To be counted, services must first be billed, then the coding must be assumed to be accurate. Outcomes are limited to health care resource utilization, and patient-reported outcomes, such as disability (e.g., Expanded Disability Status Scale in MS), cannot be determined. The data for researchers are de-identified; there can be no independent chart review process to validate data. Furthermore, we cannot presume random selection of an intervention or exposure, and often the limited demographic and patient information is not enough to estimate the latent biases in selection. Here, the reported results are unadjusted and selection bias is likely.
Despite attempts at stratification, severity of neurologic disease in these data is mostly unknown. Where severity levels could be differentiated in ETGs for migraine, stroke, and epilepsy, neurologist care was more prevalent at higher severity levels. This suggests that neurologists see patients with greater disease severity. While this may explain the increased unadjusted costs for ETGs with neurologist visits in phase I, greater patient disease severity would bias the effects on adverse events and acute/postacute health care utilization in phase II toward the null. For equal severity patients, the true beneficial effects of neurologist care are likely greater in magnitude than those depicted here.
Other limitations are related to the persons represented in the data and the analysis. As a commercial dataset, the proportion of Medicare-eligible patients is smaller than in the total population, leading to underrepresentation of diseases associated with the elderly, including stroke and PD. Optum provided results in phase II as summary count data only; post hoc inferential analysis was performed on ETG counts where possible. Finally, where inferential analysis shows statistical significance, we cannot infer causation from these unadjusted observational claims data.
Although many of our findings were significant in post hoc analysis, the effects of neurologist ambulatory care on health care utilization should be demonstrated in private and public payer datasets with appropriate adjustment for demographic, clinical comorbidity, and location factors. Attempts to eliminate the selection bias of the exposure can be addressed through pseudorandomization approaches of propensity scoring and instrumental variable methodology26,27 in addition to multiple regression modeling. A registry with combined clinical and administrative data, prospectively collected from disease onset and linked forward to health care events over years, would help to further validate our findings and address expenditures on a longer timeline, albeit with considerable cost for the scale required for statistical validity. These data and methods may help to confirm that neurologists provide high-quality care with fewer adverse events.
Supplementary Material
ACKNOWLEDGMENT
Dr. Knabel and B. Johnson take full responsibility for the integrity of the summary data and the accuracy of the descriptive data analysis presented to the AAN. Dr. Ney takes responsibility for the accuracy of the secondary inferential analyses presented in the article. The authors thank the American Academy of Neurology Value of Neurology Work Group members: Jeffrey R. Buchhalter, MD, FAAN; Eric Cheng, MD, MS; Dan Hoch, MD, PhD; Mark Homonoff, MD; Joel M. Kaufman, MD, FAAN; John Ney, MD, MPH; Joel Laura B. Powers, MD; Marc Raphaelson, MD; and Heidi Schwarz, MD.
GLOSSARY
- AAN
American Academy of Neurology
- ALS
amyotrophic lateral sclerosis
- BOI
burden of illness
- CI
confidence interval
- CMS
Center for Medicare and Medicaid Services
- CPT
Current Procedural Terminology
- ED
emergency department
- ETG
episode treatment group
- HCPCS
Healthcare Common Procedure Coding System
- HHA
home health agency
- ICD-9-CM
International Classification of Diseases–9–clinical modification
- MS
multiple sclerosis
- OR
odds ratio
- PD
Parkinson disease
- SNF
skilled nursing facility
Footnotes
Supplemental data at Neurology.org
Editorial, page 320
AUTHOR CONTRIBUTIONS
Dr. Ney: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. B. Johnson: acquisition of data, study concept and design, analysis and interpretation. Dr. Knabel: acquisition of data, study concept and design, analysis and interpretation. K. Craft: study concept and design, critical revision of the manuscript for important intellectual content, study supervision. Dr. Kaufman: study concept and design, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
The study was approved and funded by the American Academy of Neurology.
DISCLOSURE
J. Ney has been a consultant for AxelaCare, a home intravenous infusion company, SpecialtyCare, a surgical services company, and Alliance Life Sciences Consulting. Dr. Ney has received honoraria for speaking at the AAN and ACNS national meetings. B. Johnson, T. Knabel, and K. Kraft report no disclosures relevant to the manuscript. J. Kaufman reports receiving honoraria for speaking at the AAN national meeting. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Langel SJ. Crunching the really big numbers at CMS. Health Aff 2013;32:100–101. [DOI] [PubMed] [Google Scholar]
- 2.Riley GF. Administrative and claims records as sources of health care cost data. Med Care 2009;47:S51–S55. [DOI] [PubMed] [Google Scholar]
- 3.Serxner S, Alberti A, Weinberger S. Medical cost savings for participants and nonparticipants in health risk assessments, lifestyle management, disease management, depression management, and nurseline in a large financial services corporation. Am J Health Promot 2012;26:245–252. [DOI] [PubMed] [Google Scholar]
- 4.Fallik D. For big data, big questions remain. Health Aff 2014;33:1111–1114. [DOI] [PubMed] [Google Scholar]
- 5.Nash DB. Harnessing the power of big data in healthcare. Am Health Drug Benefits 2014;7:69–70. [PMC free article] [PubMed] [Google Scholar]
- 6.Marcotte L, Moriates C, Milstein A. Professional organizations' role in supporting physicians to improve value in health care. JAMA 2014;312:231–232. [DOI] [PubMed] [Google Scholar]
- 7.MaCurdy T, Kerwin J, Gibbs J, et al. Evaluating the Functionality of the Symmetry ETG and Medstat MEG Software in Forming Episodes of Care Using Medicare Data. Burlingame, CA: Accumen, LLC; 2008. [Google Scholar]
- 8.Bureau of Labor Statistics. Consumer Price Index. Available at: http://www.bls.gov/cpi/. Accessed August 17, 2014. [Google Scholar]
- 9.Dall TM, Storm MV, Chakrabarti R, et al. Supply and demand analysis of the current and future US neurology workforce. Neurology 2013;81:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobbs MR. Episode-based payment for ischemic stroke care with implications for neurologists. Neurol Clin Pract 2014;4:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 12.Hirsch LJ, Laroche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 13.Minden SL, Hoaglin DC, Hadden L, Frankel D, Robbins T, Perloff J. Access to and utilization of neurologists by people with multiple sclerosis. Neurology 2008;70:1141–1149. [DOI] [PubMed] [Google Scholar]
- 14.Dorsey ER, George BP, Leff B, Willis AW. The coming crisis: obtaining care for the growing burden of neurodegenerative conditions. Neurology 2013;80:1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein LB, Matchar DB, Hoff-Lindquist J, Samsa GP, Horner RD. VA Stroke Study: neurologist care is associated with increased testing but improved outcomes. Neurology 2003;61:792–796. [DOI] [PubMed] [Google Scholar]
- 16.Beckerman H, van Zee IE, de Groot V, van den Bos GAM, Lankhorst GJ, Dekker J. Utilization of health care by patients with multiple sclerosis is based on professional and patient-defined health needs. Mult Scler 2008;14:1269–1279. [DOI] [PubMed] [Google Scholar]
- 17.Ahmedani BK, Osborne J, Nerenz DR, et al. Diagnosis, costs, and utilization for psychogenic non-epileptic seizures in a US health care setting. Psychosomatics 2013;54:28–34. [DOI] [PubMed] [Google Scholar]
- 18.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis AW, Schootman M, Tran R, et al. Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology 2012;79:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Physician Value-Based Payment Modifier Under the Medicare Physician Fee Schedule, 2013 Final Rule. Available at: www.cms.gov. Accessed January 1, 2014. [Google Scholar]
- 21.Feder J. Bundle with care: rethinking Medicare incentives for post-acute care services. N Engl J Med 2013;369:400–401. [DOI] [PubMed] [Google Scholar]
- 22.Landers S. The future of the Medicare home health program. JAMA 2013;310:1443–1444. [DOI] [PubMed] [Google Scholar]
- 23.Hoch D, Homonoff M, Moawad M, et al. The neurologist as medical home neighbor. Neurol Clin Pract 2013;3:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakhan SE, Schwindt M, Alshareef BN, Tepper D, Mays M. Opinion & Special Articles: neurologist: specialized primary care provider vs consultant. Neurology 2013;81:e1–e2. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis JP. Are mortality differences detected by administrative data reliable and actionable? JAMA 2013;309:1410–1411. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 2005;24:1563–1578. [DOI] [PubMed] [Google Scholar]
- 27.Hogan JW, Lancaster T. Instrumental variables and inverse probability weighting for causal inference from longitudinal observational studies. Stat Methods Med Res 2004;13:17–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.