Abstract
Objective:
To study the safety profile and characterize the immunologic effects of high- vs low-dose cholecalciferol supplementation in patients with multiple sclerosis (MS).
Methods:
In this double-blind, single-center randomized pilot study, 40 patients with relapsing-remitting MS were randomized to receive 10,400 IU or 800 IU cholecalciferol daily for 6 months. Assessments were performed at baseline and 3 and 6 months.
Results:
Mean increase of 25-hydroxyvitamin D levels from baseline to final visit was larger in the high-dose group (34.9 ng/mL; 95% confidence interval [CI] 25.0–44.7 ng/mL) than in the low-dose group (6.9 ng/mL; 95% CI 1.0–13.7 ng/mL). Adverse events were minor and did not differ between the 2 groups. Two relapses occurred, one in each treatment arm. In the high-dose group, we found a reduction in the proportion of interleukin-17+CD4+ T cells (p = 0.016), CD161+CD4+ T cells (p = 0.03), and effector memory CD4+ T cells (p = 0.021) with a concomitant increase in the proportion of central memory CD4+ T cells (p = 0.018) and naive CD4+ T cells (p = 0.04). These effects were not observed in the low-dose group.
Conclusions:
Cholecalciferol supplementation with 10,400 IU daily is safe and tolerable in patients with MS and exhibits in vivo pleiotropic immunomodulatory effects in MS, which include reduction of interleukin-17 production by CD4+ T cells and decreased proportion of effector memory CD4+ T cells with concomitant increase in central memory CD4+ T cells and naive CD4+ T cells.
Classification of evidence:
This study provides Class I evidence that cholecalciferol supplementation with 10,400 IU daily is safe and well-tolerated in patients with MS and exhibits in vivo pleiotropic immunomodulatory effects.
Low serum 25-hydroxyvitamin D levels [25(OH)D] are associated with an increased risk of multiple sclerosis (MS), and in established disease are associated with increased disability and clinico-radiologic disease activity.1–5 Administration of vitamin D prevents or ameliorates experimental autoimmune encephalitis (EAE; murine model of MS).6,7 These observations may reflect the pleiotropic immunomodulatory effects of vitamin D, involving the innate and adaptive immune system.7–9
The majority of studies examining the immunologic effects of vitamin D have been conducted either in vitro or in animal models, and human studies have employed differing methods and produced discrepant results.10–12 The immunologic effects of high-dose compared to low-dose vitamin D in patients with MS remain unclear. We also sought to confirm that cholecalciferol at 10,000 IU daily is well-tolerated in patients with MS.13
METHODS
Standard protocol approvals, registrations, and patient consents.
Johns Hopkins University Institutional Review Board approval was obtained for the study protocol and written informed consent was obtained from all participants. The trial was registered with ClinicalTrials.gov (NCT01024777). An investigational new drug application (IND) was obtained from the Food and Drug Administration (IND 105,978) (P.A.C.).
Participants.
Inclusion criteria were a diagnosis of relapsing-remitting MS,14 age 18–55 years, and screening (within 1 month of baseline) serum 25(OH)D level of 20–50 ng/mL. Exclusion criteria were high-dose vitamin D supplementation (daily intake >1,000 IU) or change of immunomodulatory therapy within the past 3 months, systemic glucocorticoid therapy or relapse within 30 days, pregnancy, serum creatinine >1.5 mg/dL, hypersensitivity to vitamin D preparations, and history of hyperparathyroidism, tuberculosis, sarcoidosis, or nephrolithiasis.
Design.
This was a single-center, randomized, double-blind pilot study conducted between April 2010 and January 2013 at the Johns Hopkins Hospital. Forty patients were randomized to receive 10,000 IU or 400 IU of cholecalciferol (Continental Vitamin Company, Vernon, CA) daily for 6 months. In addition, all study participants received a daily multivitamin (LuckyVitamin, Conshohocken, PA) including 400 IU cholecalciferol and 1,000 mg calcium.
Sample size was calculated based on in vitro data of our group that treatment of immune cells with vitamin D results in a >50% absolute reduction in interferon (IFN)-γ-producing cells. Based on this, a sample size of 20 per group would have a 90% power to detect a 50% or greater reduction in the high-dose group, provided that no change would occur in the low-dose group.
The primary outcomes of this study were the change in the proportion of IFN-γ+ and interleukin (IL)-17+CD4+ T cells in the high- vs low-dose group and the relative frequency of adverse events between the 2 groups. Secondary outcomes were changes in other immune cell subtypes (outlined below). The classification of evidence assigned to these outcomes is Class I.
Participants were stratified by sex and randomized by blocks of 4, to ensure a similar sex ratio in the 2 intervention groups, since the immunologic effects of cholecalciferol may differ by sex.15,16 Randomization was performed by the Johns Hopkins Investigational Drug Pharmacy using a validated, automated system. Study personnel and participants were blinded to the study intervention dose.
Study visits were performed at baseline, 3 months, and 6 months. Patients were interviewed by telephone monthly between visits to assess compliance, additional vitamin D intake, and adverse events. Figure e-1 on the Neurology® Web site at Neurology.org outlines the study design.
Treatment was discontinued for adverse events possibly related to the study drug, including but not limited to gastrointestinal disturbances, hypercalcemia, and nephrolithiasis. Spot urine calcium:creatinine ratios were checked at the mid- and end-study visit, and if elevated (>0.21 mg/mL), a 24-hour urine calcium measurement was performed. If the 24-hour urine calcium was also elevated (>300 mg/24 h), the dosing frequency was decreased to every other day.
Laboratory assays and immunologic outcomes.
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood as previously described.17 PBMC were frozen with a cryoprotectant in liquid nitrogen until assays were performed. Serum was isolated from whole blood and stored at −80°C. Discussion of the effects of cryopreservation on PBMC may be found elsewhere.18,19
Serum 25(OH)D levels were measured by chemiluminescent immunoassay at the Johns Hopkins Hospital Clinical Pathology Laboratory.
Assays of CD4+ memory T-cell subsets and cytokine production.
Cryopreserved PBMC were thawed and cultured with Dynabeads (anti-CD3/anti-CD28; Life Technologies, Carlsbad, CA) for 5 days as previously described.9 At the end of the culture duration, cells were either collected and stained with antibodies to CD4, CCR7, and CD45RO (Biolegend, San Diego, CA) to characterize memory T-cell subsets (CD45RO−CCR7+ Tnaive cells, CD45RO+CCR7+ TCM cells, and CD45RO+CCR7− TEM cells) or stimulated for 4 hours with phorbol myristate acetate and ionomycin (BD Biosciences, Franklin Lakes, NJ) to induce cytokine expression, which was captured within cells using brefeldin A and monensin (BD Biosciences) and then surface stained with anti-CD4 (Biolegend) and fixed and permeabilized using the Foxp3 staining buffer kit (eBioscience, San Diego, CA) to enable intracellular cytokine staining using anti-human IFN-γ and IL-17 (eBioscience). Appropriate unstained and fluorescence minus one controls were also prepared. Cells were acquired on a BD Biosciences FACS Calibur and data were analyzed using FlowJo (Treestar, Ashland, OR).
Immunophenotyping of cryopreserved PBMC without immune stimulation and serum cytokine measurement.
Immunophenotyping without immune stimulation (antibodies shown in table e-1) and serum cytokine measurement were performed by the Human Immune Monitoring Center at Stanford University. Details regarding the utilized protocols are available at http://iti.stanford.edu/himc/protocols.html.
Statistical methods.
25(OH)D levels were deseasonalized and calculated as the predicted level on January 1 as previously described.3 The Shapiro-Wilk test was used to evaluate the normality of distributions. Comparisons were performed utilizing appropriate statistical methods, including paired or unpaired t tests, Wilcoxon signed-rank test, Mann-Whitney U test, or Fisher exact test. Comparisons of the changes from baseline to final visit of immunologic parameters between the study arms were performed utilizing generalized estimating equations. Immunologic data were censored at the time of change/discontinuation of immunomodulatory therapy or discontinuation of vitamin D supplementation. Statistical significance was defined as p < 0.05. A locally weighted scatterplot smoothing (LOWESS) plot was used to visualize the relationship between changes in serum 25(OH)D and IL-17+CD4+ T cells and a linear regression model with a spline term was used to study the relationship between these 2 variables.
Serum cytokine changes were analyzed utilizing significance analysis of microarrays (statweb.stanford.edu/∼tibs/SAM/). Other analyses were performed with Stata version 11 (StataCorp, College Station, TX) or GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS
Study population.
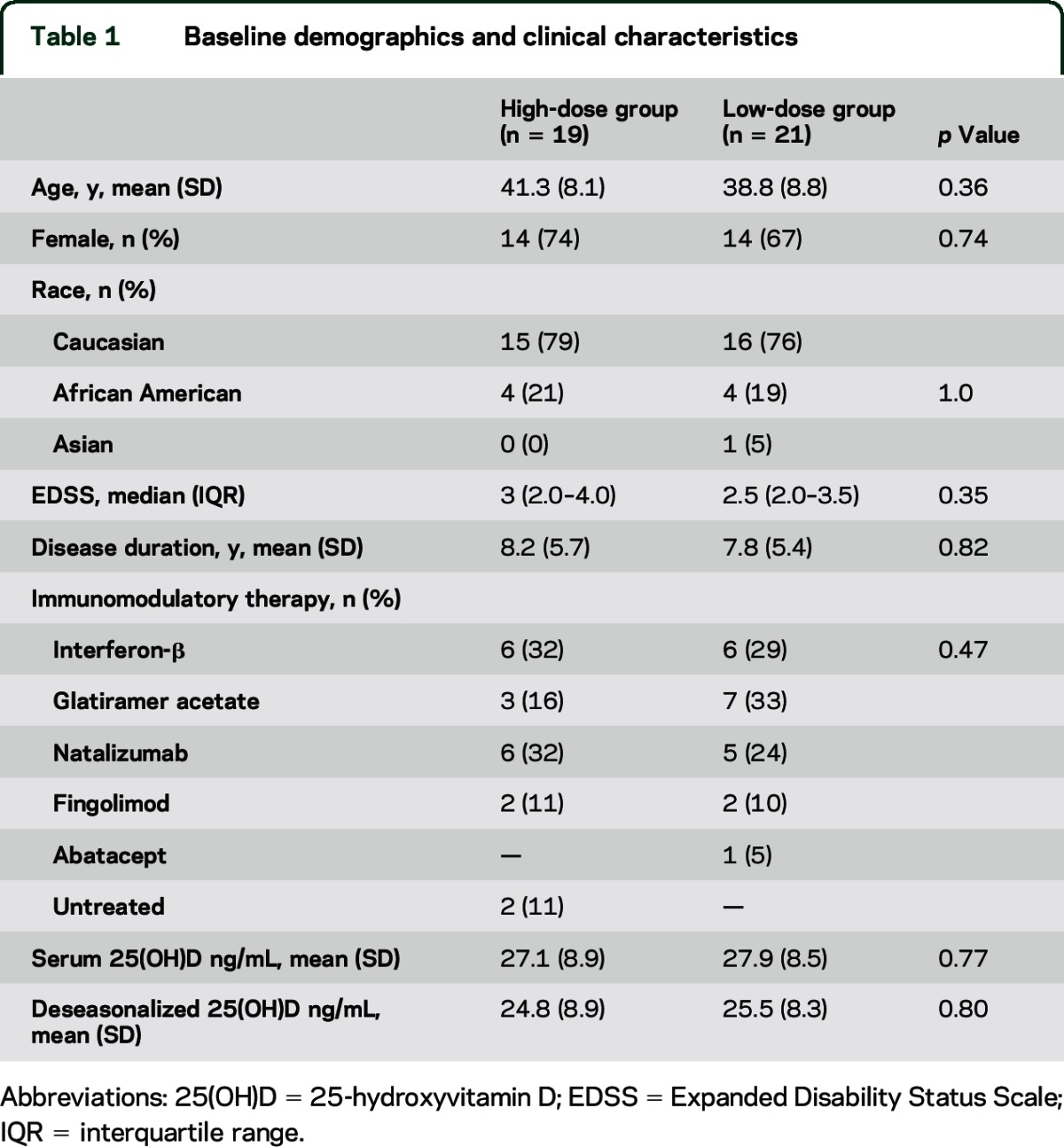
Nineteen patients were randomized to the high-dose group and 21 patients to the low-dose group. Baseline characteristics did not differ between the 2 groups (table 1).
Table 1.
Baseline demographics and clinical characteristics
Participant flow, adverse events, and safety outcomes.
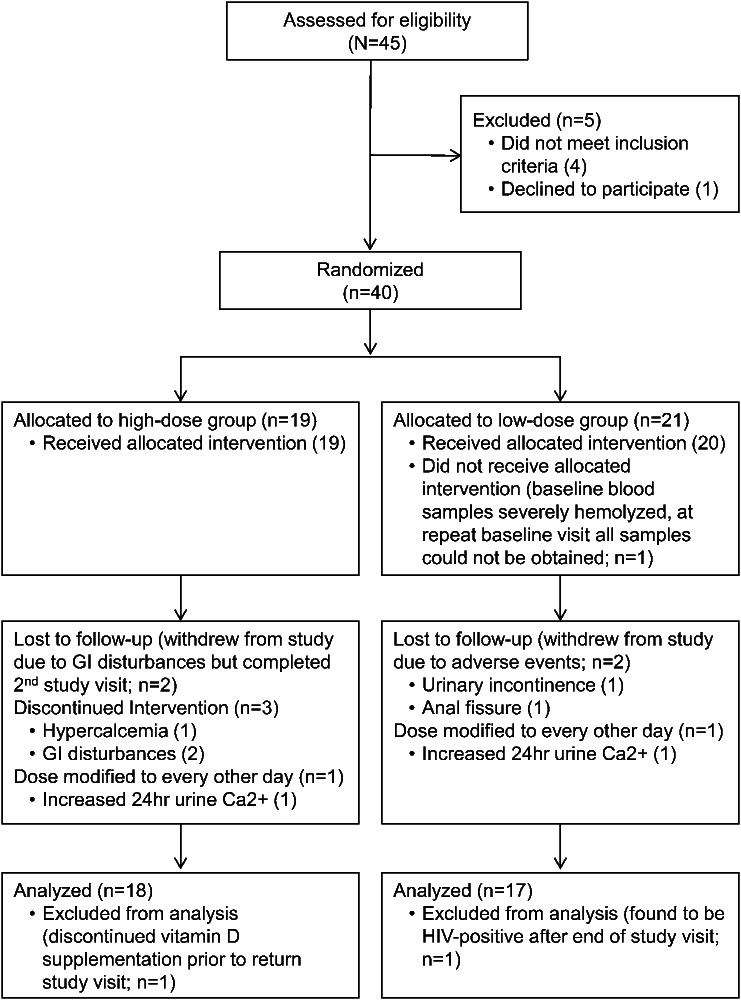
The trial profile is shown in the flow diagram (figure 1). Adverse events are summarized in table e-2. Thirty-five patients completed all study visits, 2 patients completed the baseline and mid-study visits only, and 3 patients completed only the baseline visit. Two patients completed end-study visits at 4 months (1 high-dose, 1 low-dose) due to impending change of immunomodulatory therapy by their treating neurologist due to radiologic progression of disease.
Figure 1. Study flow diagram.
Flow diagram of the progress of the 2 study groups through the phases of this study including enrollment, randomization, follow-up, and data analysis. GI = gastrointestinal.
Three patients developed nausea that resolved after discontinuation of supplementation. Of these patients, 1 in the low-dose group discontinued the intervention after the mid-study visit but completed the 6-month follow-up, 1 in the high-dose group discontinued the intervention after the mid-study visit and withdrew from the study, and 1 in the high-dose group discontinued the intervention 1 week after the baseline visit and withdrew after the mid-study visit.
Two patients in the low-dose group withdrew prior to the mid-study visit due to adverse events (urinary incontinence and anal fissure), which were not believed by the treating physician to be related to vitamin D supplementation.
One patient in the low-dose group was found to be HIV-seropositive shortly after completing the study and was excluded from immunologic analyses.
One patient in the high-dose group was found at the mid-study visit to have a serum calcium level of 10.6 mg/dL (reference range 8.4–10.5 mg/dL; baseline visit calcium 10 mg/dL) with a normal urine calcium:creatinine ratio and a serum 25(OH)D level of 83 ng/mL. Supplementation was discontinued per study protocol and at 6 month follow-up serum calcium normalized to 10.0 mg/dL and serum 25(OH)D level was 28 ng/mL.
Fourteen patients had increased spot urine calcium:creatinine ratios at either the mid- or end-study visit, (9 high-dose, 5 low-dose). Eight of these completed 24-hour urine calcium measurements with 4 being elevated (3 of 6 high-dose, 1 of 2 low-dose). The frequency of elevated urine calcium:creatinine ratio and increased 24-hour urine calcium excretion did not differ between treatment groups (p = 0.31 and p = 0.32, respectively). The dosing frequency for 1 patient in each treatment group with elevated 24-hour urine calcium excretion detected prior to study completion was decreased to every other day per study protocol.
End-study urine calcium:creatinine ratio was greater in the high-dose relative to the low-dose group (p = 0.03) and serum parathyroid hormone, calcium, phosphate, and creatinine levels did not differ between visits or groups (table e-3).
Serum 25(OH)D level changes in response to treatment.
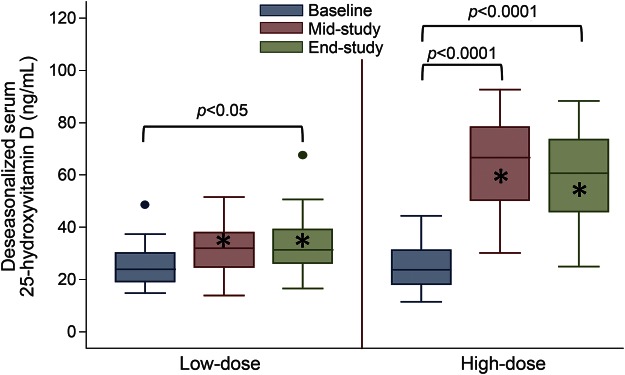
Serum 25(OH)D levels did not differ at baseline between the 2 treatment groups (table 1), but at subsequent visits 25(OH)D levels increased more in the high-dose group compared to the low-dose group (figure 2, table e-3). Mean change from baseline to the end-study visit in the high-dose group was 34.9 ng/mL (95% confidence interval [CI] 25.0–44.7 ng/mL) and in the low-dose group 6.9 ng/mL (95% CI 1.0–13.7 ng/mL).
Figure 2. Serum 25-hydroxyvitamin D level in the treatment groups.
This figure provides boxplots of the serum 25-hydroxyvitamin D [25(OH)D] levels at each visit in the 2 study groups and shows significant p values for comparisons of the serum 25(OH)D at the mid- and end-study visits compared to baseline for each group, as well as for comparisons between groups at each visit. *Significant difference between low-dose and high-dose group at given visit (p < 0.0001).
Relapses.
One relapse in each treatment arm occurred during the study. One patient in the high-dose group experienced a relapse 7 weeks prior to the mid-study visit and received glucocorticoids and completed the study as scheduled and 1 patient in the low-dose group experienced a relapse that coincided with the end-study visit and blood samples were collected prior to glucocorticoid administration.
High-dose cholecalciferol reduces the proportion of IL-17-producing CD4+ T cells.
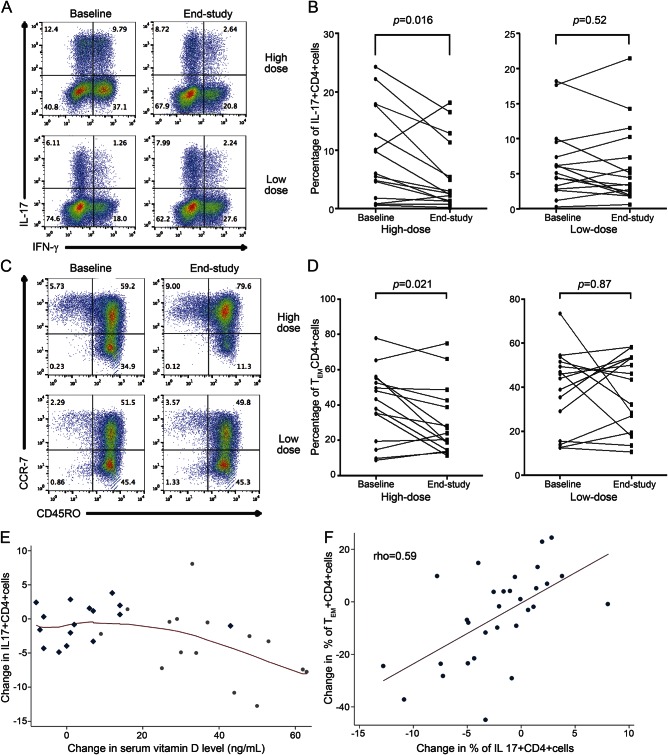
In the high-dose group, the proportion of IL-17+CD4+ T cells decreased between baseline and final visits while in the low-dose group a similar effect was not noted (figure 3, A and B; table 2). The change was greater in the high-dose group compared with the low-dose group (table 2). Greater reductions in the proportion of IFN-γ+CD4+ T cells and double-positive IFN-γ+IL-17+CD4+ T cells occurred in the high-dose group as compared to the low-dose group, but these changes were not significant (table 2).
Figure 3. Flow cytometry plots and line graphs of changes in immune cell subtypes and scatterplots showing relationships among change in IL-17+CD4+ T cells, change in serum 25-hydroxyvitamin D (25(OH)D), and TEM cells.
(A) Representative flow cytometry plots of interleukin (IL)–17 and interferon (IFN)–γ staining in CD4+ cells in the high- and low-dose groups at baseline and end-study visits. Note the large decrease in the IL-17+ proportion of CD4+ cells in the high-dose patient. (B) Line graphs with the percentage of IL-17+CD4+ cells at baseline and end-study visit, with each line representing an individual patient. (C) Representative flow cytometry plots of CCR7 and CD45RO staining in CD4+ cells in the high- and low-dose groups at baseline and end-study visits. Note the large decrease in the CD45RO+CCR7− TEM cells with a concomitant increase in the CD45RO+CCR7+ TCM cells in the high-dose patient. (D) Line graphs with the percentage of TEM CD4+ cells at baseline and end-study visit, with each line representing an individual patient. (E) A locally weighted scatterplot smoothing plot was used to visualize the relationship between changes in serum 25(OH)D and IL-17+CD4+ T cells. Low-dose patients are demonstrated with rhombi and high-dose patients with circles. Given the nonlinear relationship depicted, this relationship was modeled with a linear spline model with a breakpoint at 18 ng/mL that showed that when the increase in serum 25(OH)D was greater than 18 ng/mL, every 5 ng/mL increase led to 1.0% absolute decrease in the percentage of IL-17+CD4+ T cells (95% confidence interval [CI] −0.4% to −1.4%, p = 0.003), while an increase in serum 25(OH)D less than 18 ng/mL did not result in a significant change in IL-17+CD4+ T cells (−0.5%, 95% CI −1.5% to 0.5%, p = 0.31). (F) A scatterplot shows the positive correlation of the change in IL-17+CD4+ cells and the change in TEM CD4+ cells (Spearman rho = 0.59).
Table 2.
Immune cell subtype changes during the study
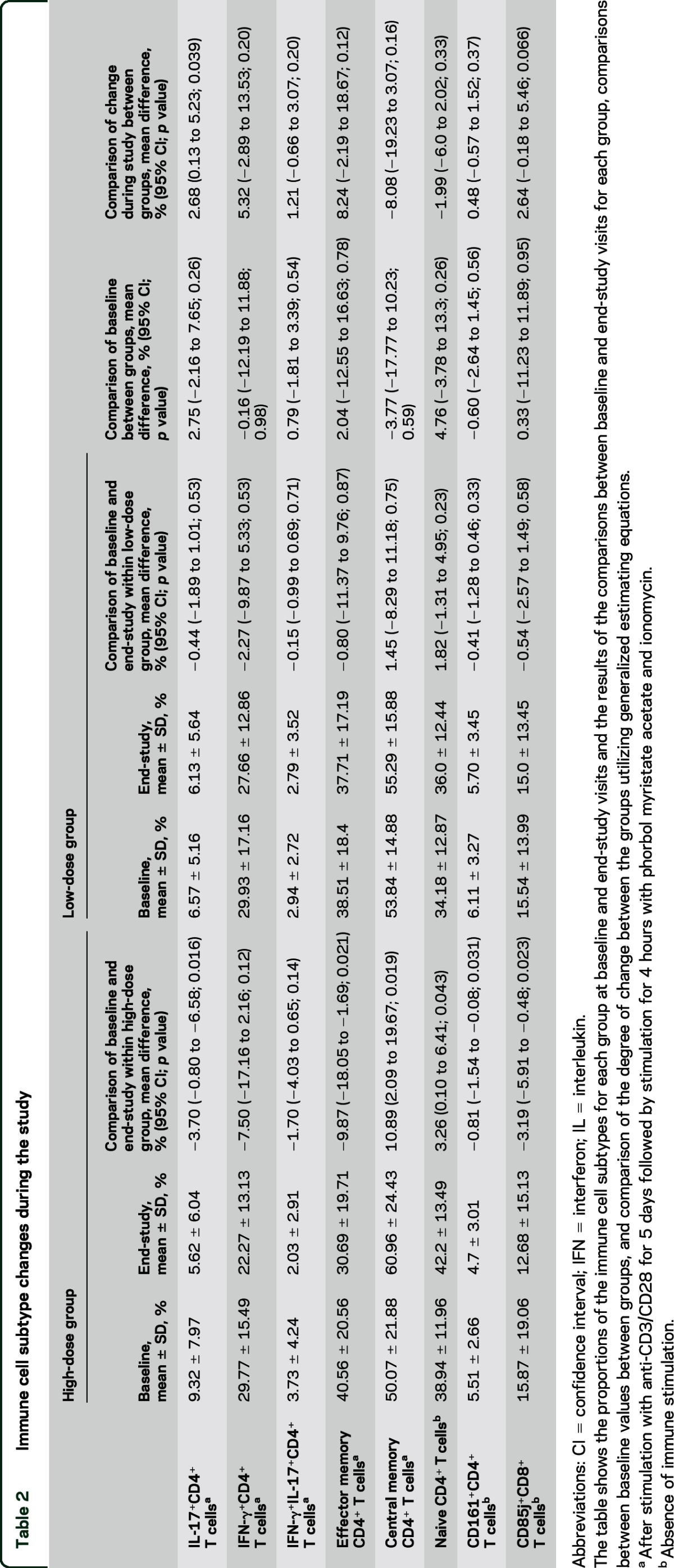
Immunophenotyping of PBMC in the absence of immune stimulation demonstrated a decrease in the CD161+CD4+ cells in the high-dose group but not the low-dose group (table 2). The CD161 gene is highly upregulated in Th17 clones and is considered to be a membrane marker of IL-17-producing cells, further corroborating the finding of decreased IL-17 production in CD4+ cells from high-dose treated patients.20 However, when comparing the degree of change of CD4+CD161+ cells between groups, this was not significant (p = 0.37).
Reductions in IL-17 production correlate with increase in serum 25(OH)D.
As depicted in the LOWESS plot (figure 3E), the relationship between the change in IL-17+CD4+ T cells and the change in serum 25(OH)D is best approximated by a linear spline and hence this relationship was modeled using linear regression with a breakpoint at 18 ng/mL of 25(OH)D. This demonstrated that when the increase in serum 25(OH)D was greater than 18 ng/mL, every 5 ng/mL increase led to 1.0% absolute decrease in the percentage of IL-17+CD4+ T cells (95% CI −0.4% to −1.4%, p = 0.003), while an increase in serum 25(OH)D less than 18 ng/mL did not result in a significant change in IL-17+CD4+ T cells (−0.5%, 95% CI −1.5% to 0.5%, p = 0.31).
High-dose cholecalciferol treatment results in a reduction in the proportion of effector memory CD4+ T cells, an increase in central memory CD4+ T cells, and naive CD4+ T cells.
In the high-dose group, there was a reduction in the proportion of CD4+ TEM cells between baseline and end-study visits, while in the low-dose group no change was noted (figure 3, C and D; table 2). Concomitantly, an increase in the proportion of CD4+ TCM cells was noted in the high-dose group but not in the low-dose group (table 2). A strong correlation was noted between the reduction in CD4+ TEM cells and the reduction in IL-17+CD4+ T cells (Spearman rho = 0.59, p = 0.0005; figure 3F).
Immunophenotyping of PBMC without immune stimulation demonstrated increased CD45RA+CD4+ cells (i.e., naive CD4+ T cells) in the high-dose group but not the low-dose group (table 2). Analyses comparing the degree of change in these immunologic populations between the 2 treatment arms were not significant (table 2).
High-dose vitamin D treatment results in a decrease in the CD85j+ proportion of CD8+ T cells.
We observed a decrease in the CD85j+ proportion of CD8+ cells in the high-dose group but not in the low-dose group (table 2). CD85j, also known as LILRB1 or LIR-1, is an inhibitory receptor for major histocompatibility complex (MHC) Class I molecules and modulates both cytotoxic and regulatory responses.21,22 Comparison of the change in the CD85j+ proportion of CD8+ cells between the 2 groups was not significant (p = 0.07).
We did not observe any significant changes in the other immune cell subtypes that were studied (table e-1).
Vitamin D treatment does not affect serum concentration of cytokines.
Fifty-one cytokines were measured in the serum of the treated patients at baseline and at end-study. No differences were observed in either of the 2 treatment groups. Results are presented in a post-cluster heat map in figure e-2.
DISCUSSION
In this randomized, double-blind, active comparator-controlled trial of high- vs low-dose cholecalciferol supplementation in MS, we have demonstrated that 10,400 IU daily cholecalciferol is safe and tolerable in patients with MS and increases serum 25(OH)D levels to levels proposed to be sufficient in MS.23 Furthermore, the high-dose group exhibited reductions of IL-17 production by CD4+ cells and decreased proportion of effector memory CD4+ T cells with concomitant increase in central memory CD4+ T cells and naive CD4+ T cells.
The optimal levels of serum 25(OH)D in MS are currently unclear. Observational studies have demonstrated an inverse linear relationship between 25(OH)D levels and clinico-radiologic disease activity, with no threshold effect observed. However, an important caveat is that the majority of patients in these studies had levels below 60 ng/mL.3–5 Thus it has been proposed that 25(OH)D levels between 40 and 60 ng/mL may be the optimal target for patients with MS.23 In our study, supplementation with 800 IU daily was inadequate to achieve this goal, whereas supplementation with 10,400 IU daily achieved the desired levels, with no difference in safety outcomes. Randomized controlled trials are currently underway to examine the effects of vitamin D supplementation on clinical and radiologic outcomes, with dosages of 5,000 to 10,000 IU daily.23–25
CD4+IL-17+ T cells (Th17) have been implicated as a major contributor to the immunopathogenesis of MS. IL-17 appears to play a crucial role in the development of EAE and IL-17+ T cells and IL-17 gene expression are increased in MS lesions.26–28 Additionally, IL-17 expression is augmented in the peripheral blood and the CSF of patients with MS during clinical exacerbations.29 Furthermore, single nucleotide polymorphisms in the gene encoding CD161 (KLRB1), which is considered to be a surface marker of IL-17-producing cells and is present on a steroid refractory subset of Th17s expressing P-glycoprotein, have been associated with an increased risk of MS.20,30,31 Thus, our finding that high-dose cholecalciferol leads to a decrease in the proportion of CD4+IL-17+ T cells in the peripheral blood of patients with MS suggests that this may be a major mechanism underlying the possible therapeutic role of vitamin D in MS. Moreover, our finding that high-dose cholecalciferol decreases the CD161+ proportion of CD4+ cells further corroborates this finding.
These in vivo findings serve as validation of the observation that in vitro vitamin D treatment of PBMC cultures leads to an increase in naive CD4+ T cells and TCM and a decrease in TEM.9 TEM cells are thought to play a pivotal role in the pathogenesis of MS and are predominant in MS lesions.32,33
Finally, we have shown that high-dose cholecalciferol results in a decrease in the CD85j+ proportion of CD8+ T cells. CD85j is an inhibitory MHC Class I receptor of the immunoglobulin superfamily expressed by multiple immune cell subsets.21,22 In MS, lower CD85j expression by CD8+ T cells has been associated with IFN-β treatment.34 Also, longer MS disease duration has been associated with increased CD85j expression.34 Further studies are necessary to corroborate this finding and further characterize the role of CD85j in the immunology of MS.
Of note, we did not observe any changes in serum cytokine levels; however, these measures are known to vary significantly throughout the day due to numerous exogenous factors, limiting our ability to reliably detect changes.35
A limitation of this study is the exclusion of patients with severe vitamin D deficiency (<20 ng/mL), who would be expected to demonstrate the most dramatic immunomodulatory effect in response to cholecalciferol supplementation; however, it was believed that given the existing evidence it would not be appropriate to identify these patients and withhold sufficient vitamin D supplementation for the 6-month study period. Another limitation is the variety of immunomodulatory therapies utilized by patients in the trial. However, this did not differ between the 2 treatment arms, and our study only included patients who had been receiving the same disease-modifying therapy for at least 3 months prior to enrollment to minimize any contribution to the observed immunologic effects. It has been suggested that IFN-β and vitamin D may interact and exert a synergistic immunomodulatory effect.36,37 Our sample size was not powered to investigate this possible interaction; however, the proportion of patients treated with IFN-β was similar in the 2 treatment arms, thus eliminating possible confounding of this study's findings. In the high-dose group, a more pronounced immunologic effect of cholecalciferol was observed in a subset of patients; however, this study was not powered to perform subgroup analyses and this should be further investigated in future studies. A further limitation is the fact that calcium:creatinine ratios were not obtained at baseline visits. We observed that the urine calcium:creatinine ratio was significantly higher at the end-study visit in patients in the high-dose group, a finding that is difficult to interpret without baseline measurements. Of note, the frequency of abnormally elevated calcium:creatinine ratios did not differ between the 2 groups and thus the difference in the calcium:creatinine ratios between the 2 groups is mainly accounted for by elevations within the reference range.
We have found that high-dose cholecalciferol supplementation is safe and exerts in vivo pleiotropic immunomodulatory effects in patients with MS. Future studies are warranted to further elucidate the molecular mechanisms of these effects and ongoing randomized controlled clinical trials will be instrumental to establish the clinical utility of cholecalciferol as a novel immunomodulatory therapy for MS.
Supplementary Material
GLOSSARY
- 25(OH)D
25-hydroxyvitamin D
- CI
confidence interval
- EAE
experimental autoimmune encephalitis
- IFN
interferon
- IL
interleukin
- IND
investigational new drug application
- LOWESS
locally weighted scatterplot smoothing
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cells
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Conceptualization of the study/study concept and design: Peter Calabresi. Acquisition of data: Elias Sotirchos, Pavan Bhargava, Christopher Eckstein, Keith Van Haren, Moira Baynes, Achilles Ntranos. Analysis and interpretation of the data: Elias Sotirchos, Pavan Bhargava, Keith Van Haren, Peter Calabresi. Drafting/revising the manuscript: Elias Sotirchos, Pavan Bhargava, Christopher Eckstein, Keith Van Haren, Moira Baynes, Achilles Ntranos, Anne Gocke, Lawrence Steinman, Ellen Mowry, Peter Calabresi. Critical revision of the manuscript for important intellectual content: Elias Sotirchos, Pavan Bhargava, Christopher Eckstein, Keith Van Haren, Moira Baynes, Achilles Ntranos, Anne Gocke, Lawrence Steinman, Ellen Mowry, Peter Calabresi. Study supervision: Peter Calabresi.
STUDY FUNDING
The Kenneth and Claudia Silverman Family Foundation and Montel Williams Foundation (P.A.C.); National Multiple Sclerosis Society Sylvia Lawry Physician Fellowship (FP-1787-A-1) (P.B.).
DISCLOSURE
E. Sotirchos, P. Bhargava, and C. Eckstein report no disclosures relevant to the manuscript. K. Van Haren is funded by the Child Neurology Society, the Lucile Packard Foundation Sprague-McHugh Multiple Sclerosis Fund, and the National Institutes of Health (NINDS K23 NS087151). He is an ad hoc consultant for Viking Pharmaceuticals. M. Baynes, A. Ntranos, and A. Gocke report no disclosures relevant to the manuscript. L. Steinman has received travel funding and/or speaker honoraria from Biogen, Bayhill, Bayer, and Cellgene; has a patent pending for cytokines and type 1 interferons; is on the speakers' bureau for EMD Serono; and received research support from JT Pharma, Pfizer, Biogen, and NIH. E. Mowry is funded by the National MS Society and the NIH (NINDS K23 NS067055). She receives free medication from Teva Neuroscience Inc. for a clinical trial and has research funding from Biogen Idec. P. Calabresi has received personal compensation for consulting and serving on scientific advisory boards from Vertex, Vaccinex, Merck, Prothena, and Abbvie, and has received research funding from Biogen-Idec, Medimmune, and Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 2.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008;14:1220–1224. [DOI] [PubMed] [Google Scholar]
- 3.Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol 2010;67:618–624. [DOI] [PubMed] [Google Scholar]
- 4.Simpson S, Jr, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010;68:193–203. [DOI] [PubMed] [Google Scholar]
- 5.Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol 2012;72:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantorna MT, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA 1996;93:7861–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci USA 2013;110:21101–21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev 2011;10:733–743. [DOI] [PubMed] [Google Scholar]
- 9.Bhargava P, Gocke A, Calabresi PA. 1,25-dihydroxyvitamin D3 impairs the differentiation of effector memory T cells in vitro in multiple sclerosis patients and healthy controls. J Neuroimmunol 2015;279:20–24. [DOI] [PubMed] [Google Scholar]
- 10.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 2003;134:128–132. [DOI] [PubMed] [Google Scholar]
- 11.Smolders J, Peelen E, Thewissen M, et al. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One 2010;5:e15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimball S, Vieth R, Dosch HM, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab 2011;96:2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr 2007;85:6–18. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 2005;175:4119–4126. [DOI] [PubMed] [Google Scholar]
- 16.Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 2010;185:4948–4958. [DOI] [PubMed] [Google Scholar]
- 17.Calabresi PA, Yun SH, Allie R, Whartenby KA. Chemokine receptor expression on MBP-reactive T cells: CXCR6 is a marker of IFN-gamma-producing effector cells. J Neuroimmunol 2002;127:96–105. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg A, Song LY, Wilkening C, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol 2009;16:1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol 2012;12:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 2008;205:1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colonna M, Navarro F, Bellon T, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997;186:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anfossi N, Doisne JM, Peyrat MA, et al. Coordinated expression of Ig-like inhibitory MHC Class I receptors and acquisition of cytotoxic function in human CD8+ T cells. J Immunol 2004;173:7223–7229. [DOI] [PubMed] [Google Scholar]
- 23.Bhargava P, Cassard S, Steele SU, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials 2014;39:288–293. [DOI] [PubMed] [Google Scholar]
- 24.Smolders J, Hupperts R, Barkhof F, et al. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: a phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci 2011;311:44–49. [DOI] [PubMed] [Google Scholar]
- 25.Dorr J, Ohlraun S, Skarabis H, Paul F. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS trial): study protocol for a randomized controlled trial. Trials 2012;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002;8:500–508. [DOI] [PubMed] [Google Scholar]
- 27.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008;172:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006;177:566–573. [DOI] [PubMed] [Google Scholar]
- 29.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999;5:101–104. [DOI] [PubMed] [Google Scholar]
- 30.Ramesh R, Kozhaya L, McKevitt K, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 2014;211:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007;357:851–862. [DOI] [PubMed] [Google Scholar]
- 32.Kivisakk P, Mahad DJ, Callahan MK, et al. Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann Neurol 2004;55:627–638. [DOI] [PubMed] [Google Scholar]
- 33.Rus H, Pardo CA, Hu L, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci USA 2005;102:11094–11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Rodriguez JE, Saez-Borderias A, Munteis E, Romo N, Roquer J, Lopez-Botet M. Natural killer receptors distribution in multiple sclerosis: Relation to clinical course and interferon-beta therapy. Clin Immunol 2010;137:41–50. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 2010;13:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart N, Simpson S, Jr, van der Mei I, et al. Interferon-beta and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012;79:254–260. [DOI] [PubMed] [Google Scholar]
- 37.Waschbisch A, Sanderson N, Krumbholz M, et al. Interferon beta and vitamin D synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PLoS One 2014;9:e115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.