Abstract
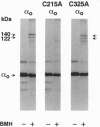
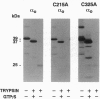
The reversible association of alpha and beta gamma subunits of GTP-binding proteins is important for signal transmission from a variety of cell-surface receptors to intracellular effectors. Previous work showed that 1,6-bis(maleimido)hexane, which crosslinks cysteine residues, crosslinks alpha o and alpha i-1 to beta gamma. These crosslinks are likely to form through a conserved cysteine because 1,6-bis(maleimido)hexane can also crosslink alpha i-2, alpha 1, alpha s and Drosophila alpha 1 to give products of the same apparent molecular weight as crosslinked alpha o beta gamma and alpha i-1 beta gamma. These proteins have only two cysteines in common. Therefore, we mutated each of the two conserved cysteines of alpha o to alanines. Mutation of Cys215 prevents crosslinking to beta gamma, but does not affect binding of guanosine 5'-[gamma-thio]triphosphate or the ability of the mutated alpha subunit to bind beta gamma. In models of the alpha subunit based on the crystal structure of p21ras, Cys215 is located on the face opposite to the GTP-binding site and near an area that changes conformation depending on the nucleotide bound. This surface on the alpha subunit overlaps a putative effector binding region, raising important questions about the spatial organization of the proteins as they form ternary complexes. Mutation of Cys325 has no effect on crosslinking but, surprisingly, decreases by a factor of 10 the affinity of the mutated protein for GDP, relative to wild type, without changing the affinity for guanosine 5'-[gamma-thio]triphosphate. This mutation falls within a region thought to contact receptors and may represent a site through which receptors enhance the release of GDP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlot C. H., Bourne H. R. Identification of effector-activating residues of Gs alpha. Cell. 1992 Mar 6;68(5):911–922. doi: 10.1016/0092-8674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Kroll S., Rajaram R., Unson C., Goldsmith P., Spiegel A. M. An antibody directed against the carboxyl-terminal decapeptide of the alpha subunit of the retinal GTP-binding protein, transducin. Effects on transducin function. J Biol Chem. 1988 Jul 5;263(19):9345–9352. [PubMed] [Google Scholar]
- Denker B. M., Neer E. J., Schmidt C. J. Mutagenesis of the amino terminus of the alpha subunit of the G protein Go. In vitro characterization of alpha o beta gamma interactions. J Biol Chem. 1992 Mar 25;267(9):6272–6277. [PubMed] [Google Scholar]
- Denker B. M., Schmidt C. J., Neer E. J. Promotion of the GTP-liganded state of the Go alpha protein by deletion of the C terminus. J Biol Chem. 1992 May 15;267(14):9998–10002. [PubMed] [Google Scholar]
- Deretic D., Hamm H. E. Topographic analysis of antigenic determinants recognized by monoclonal antibodies to the photoreceptor guanyl nucleotide-binding protein, transducin. J Biol Chem. 1987 Aug 5;262(22):10839–10847. [PubMed] [Google Scholar]
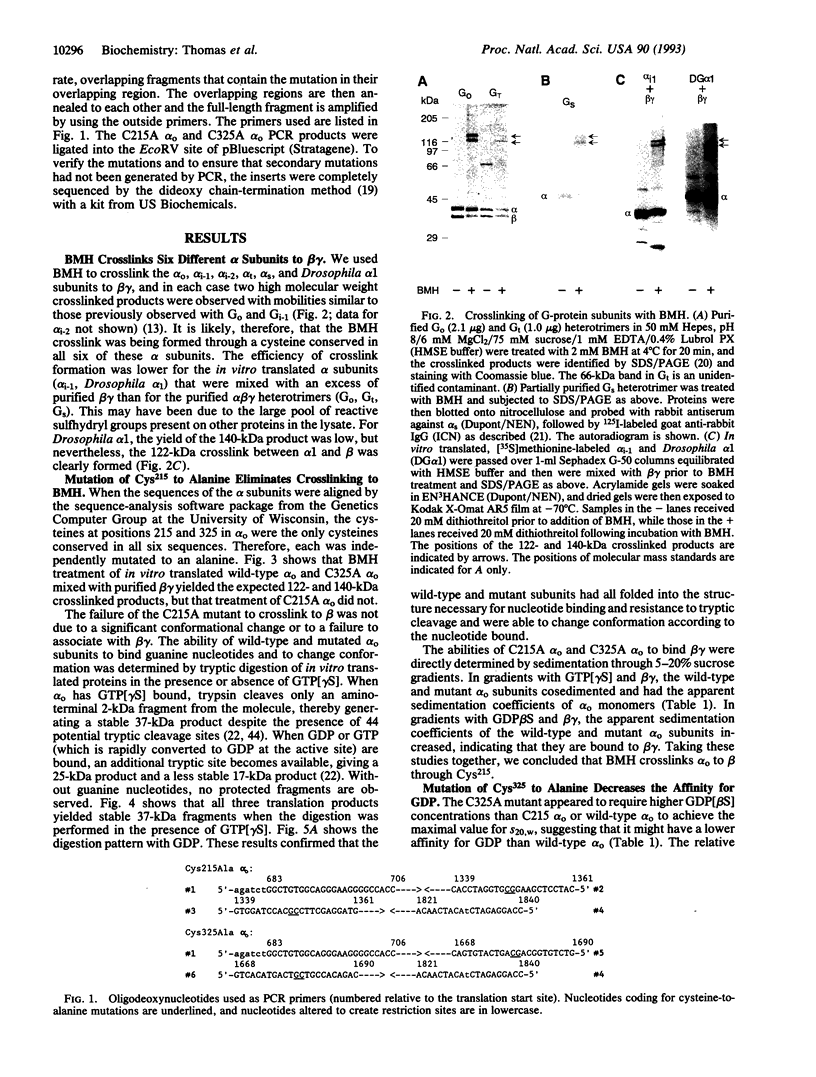
- Dhanasekaran N., Wessling-Resnick M., Kelleher D. J., Johnson G. L., Ruoho A. E. Mapping of the carboxyl terminus within the tertiary structure of transducin's alpha subunit using the heterobifunctional cross-linking reagent, 125I-N-(3-iodo-4-azidophenylpropionamido-S-(2-thiopyridyl) cysteine. J Biol Chem. 1988 Dec 5;263(34):17942–17950. [PubMed] [Google Scholar]
- Dratz E. A., Furstenau J. E., Lambert C. G., Thireault D. L., Rarick H., Schepers T., Pakhlevaniants S., Hamm H. E. NMR structure of a receptor-bound G-protein peptide. Nature. 1993 May 20;363(6426):276–281. doi: 10.1038/363276a0. [DOI] [PubMed] [Google Scholar]
- Graf R., Mattera R., Codina J., Estes M. K., Birnbaumer L. A truncated recombinant alpha subunit of Gi3 with a reduced affinity for beta gamma dimers and altered guanosine 5'-3-O-(thio)triphosphate binding. J Biol Chem. 1992 Dec 5;267(34):24307–24314. [PubMed] [Google Scholar]
- Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988 Aug 12;241(4867):832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani V. N., Tobias D. T., Henderson J. T., Ho Y. K. Chemical cross-linking of bovine retinal transducin and cGMP phosphodiesterase. J Biol Chem. 1988 May 15;263(14):6916–6926. [PubMed] [Google Scholar]
- Holbrook S. R., Kim S. H. Molecular model of the G protein alpha subunit based on the crystal structure of the HRAS protein. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1751–1755. doi: 10.1073/pnas.86.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff R. M., Axton J. M., Neer E. J. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J Biol Chem. 1985 Sep 5;260(19):10864–10871. [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Itoh H., Gilman A. G. Expression and analysis of Gs alpha mutants with decreased ability to activate adenylylcyclase. J Biol Chem. 1991 Aug 25;266(24):16226–16231. [PubMed] [Google Scholar]
- Journot L., Pantaloni C., Bockaert J., Audigier Y. Deletion within the amino-terminal region of Gs alpha impairs its ability to interact with beta gamma subunits and to activate adenylate cyclase. J Biol Chem. 1991 May 15;266(14):9009–9015. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E., Taussig R., Gilman A. G. The G226A mutant of Gs alpha highlights the requirement for dissociation of G protein subunits. J Biol Chem. 1992 Jan 15;267(2):1212–1218. [PubMed] [Google Scholar]
- Mazzoni M. R., Hamm H. E. Tryptophan207 is involved in the GTP-dependent conformational switch in the alpha subunit of the G protein transducin: chymotryptic digestion patterns of the GTP gamma S and GDP-bound forms. J Protein Chem. 1993 Apr;12(2):215–221. doi: 10.1007/BF01026043. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Tong L., deVos A. M., Brünger A., Yamaizumi Z., Nishimura S., Kim S. H. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990 Feb 23;247(4945):939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Heukeroth R. O., Gordon J. I., Gilman A. G. G-protein alpha-subunit expression, myristoylation, and membrane association in COS cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon S. E., Fung B. K. Characterization of transducin from bovine retinal rod outer segments. Participation of the amino-terminal region of T alpha in subunit interaction. J Biol Chem. 1987 Nov 15;262(32):15746–15751. [PubMed] [Google Scholar]
- Navon S. E., Fung B. K. Characterization of transducin from bovine retinal rod outer segments. Use of monoclonal antibodies to probe the structure and function of the subunit. J Biol Chem. 1988 Jan 5;263(1):489–496. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Neer E. J., Pulsifer L., Wolf L. G. The amino terminus of G protein alpha subunits is required for interaction with beta gamma. J Biol Chem. 1988 Jun 25;263(18):8996–8970. [PubMed] [Google Scholar]
- Osawa S., Dhanasekaran N., Woon C. W., Johnson G. L. G alpha i-G alpha s chimeras define the function of alpha chain domains in control of G protein activation and beta gamma subunit complex interactions. Cell. 1990 Nov 16;63(4):697–706. doi: 10.1016/0092-8674(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Krengel U., Petsko G. A., Goody R. S., Kabsch W., Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990 Aug;9(8):2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost N. M., Somers D. E., Hurley J. B. A Drosophila melanogaster G protein alpha subunit gene is expressed primarily in embryos and pupae. J Biol Chem. 1988 Aug 25;263(24):12070–12076. [PubMed] [Google Scholar]
- Rarick H. M., Artemyev N. O., Hamm H. E. A site on rod G protein alpha subunit that mediates effector activation. Science. 1992 May 15;256(5059):1031–1033. doi: 10.1126/science.1317058. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. J., Neer E. J. In vitro synthesis of G protein beta gamma dimers. J Biol Chem. 1991 Mar 5;266(7):4538–4544. [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Slepak V. Z., Wilkie T. M., Simon M. I. Mutational analysis of G protein alpha subunit G(o) alpha expressed in Escherichia coli. J Biol Chem. 1993 Jan 15;268(2):1414–1423. [PubMed] [Google Scholar]
- Spiegel A. M., Shenker A., Weinstein L. S. Receptor-effector coupling by G proteins: implications for normal and abnormal signal transduction. Endocr Rev. 1992 Aug;13(3):536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Thomas T. C., Sladek T., Yi F., Smith T., Neer E. J. G protein beta gamma subunit: physical and chemical characterization. Biochemistry. 1993 Aug 24;32(33):8628–8635. doi: 10.1021/bi00084a034. [DOI] [PubMed] [Google Scholar]
- Vaillancourt R. R., Dhanasekaran N., Johnson G. L., Ruoho A. E. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of alpha interacts with both alpha and gamma subunits. Proc Natl Acad Sci U S A. 1990 May;87(10):3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meurs K. P., Angus C. W., Lavu S., Kung H. F., Czarnecki S. K., Moss J., Vaughan M. Deduced amino acid sequence of bovine retinal Go alpha: similarities to other guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Allosteric behavior in transducin activation mediated by rhodopsin. Initial rate analysis of guanine nucleotide exchange. J Biol Chem. 1987 Mar 15;262(8):3697–3705. [PubMed] [Google Scholar]
- Winslow J. W., Van Amsterdam J. R., Neer E. J. Conformations of the alpha 39, alpha 41, and beta.gamma components of brain guanine nucleotide-binding proteins. Analysis by limited proteolysis. J Biol Chem. 1986 Jun 5;261(16):7571–7579. [PubMed] [Google Scholar]
- Yi F., Denker B. M., Neer E. J. Structural and functional studies of cross-linked Go protein subunits. J Biol Chem. 1991 Feb 25;266(6):3900–3906. [PubMed] [Google Scholar]