Abstract
Transplantation of neural stem/precursor cells has recently been proposed as a promising, albeit still controversial, approach to brain repair. Human umbilical cord blood could be a source of such therapeutic cells, proven beneficial in several preclinical models of stroke. Intracerebroventricular infusion of neutrally committed cord blood-derived cells allows their broad distribution in the CNS, whereas additional labeling with iron oxide nanoparticles (SPIO) enables to follow the fate of engrafted cells by MRI. A 16-month-old child at 7 months after the onset of cardiac arrest-induced global hypoxic/ischemic brain injury, resulting in a permanent vegetative state, was subjected to intracerebroventricular transplantation of the autologous neutrally committed cord blood cells. These cells obtained by 10-day culture in vitro in neurogenic conditions were tagged with SPIO nanoparticles and grafted monthly by three serial injections (12 × 106 cells/0.5 ml) into lateral ventricle of the brain. Neural conversion of cord blood cells and superparamagnetic labeling efficiency was confirmed by gene expression, immunocytochemistry, and phantom study. MRI examination revealed the discrete hypointense areas appearing immediately after transplantation in the vicinity of lateral ventricles wall with subsequent lowering of the signal during entire period of observation. The child was followed up for 6 months after the last transplantation and his neurological status slightly but significantly improved. No clinically significant adverse events were noted. This report indicates that intracerebroventricular transplantation of autologous, neutrally committed cord blood cells is a feasible, well tolerated, and safe procedure, at least during 6 months of our observation period. Moreover, a cell-related MRI signal persisted at a wall of lateral ventricle for more than 4 months and could be monitored in transplanted brain hemisphere.
Key words: Cord blood, Neural progenitors, Clinical transplantation, Brain ischemia
INTRODUCTION
Hypoxic-ischemic encephalopathy remains one of the most devastating conditions in children, resulting in brain atrophy and persisted functional neurological impairment (48). Current therapeutic strategies include rehabilitation and antiepileptic therapy when needed, although there is no effective strategy to repair the lost brain structures and functions once the injury has occurred. Over the last decade, transplantation of stem/precursor cells has been proposed as an alternative approach to repair damaged neural tissue. Although stem cells have been successfully used for proof of concept in experimental animal models of CNS diseases (8,16,21,22,30), no clinically significant benefits have so far been reported (20).
Several strategies of cell-mediated clinical therapy are currently being investigated, aiming at transplanting cells derived from a variety of different stem cells, including embryonic stem cells, neural stem cells, bone marrow, peripheral blood, as well as umbilical cord blood stem cells (9,11,17,32,34,44,46).
Human umbilical cord blood cell transplantations are being explored as a one of the exceptionally safe and feasible experimental treatments in various central neural system diseases in which no other effective cure could be proposed (26,49). Cord blood cells have been reported to be beneficial for patients with the, genetically determined conditions, like Krabbe’s disease (15) and Wolman disease (40). A study from Duke University is currently investigating the extent to which administration of autologous cord blood influences disease progression in children with infantile cerebral paresis or other cerebral impairments (10).
Based on our previous data showing that indeed human cord blood could be a source of neural stem cells (5,6,13,14,18) and that these cells are functionally and structurally effective in vitro (7,24,38,41) and in vivo after experimental brain injury (29), we feel entitled to hypothesize that cord blood cell transplantation may be helpful also in clinical cases of global brain ischemia.
Several reports announced that local implantation or systemic infusion of cord blood mononuclear cell derivatives results in CNS functional improvement not only by the possible cell replacement mechanism but also (if not mainly) by delivery of various neurotrophic or immunomodulating factors secreted by transplanted cells and beneficial for nerve tissue regeneration (3,4,19,23,28,37). Taking into consideration that severe cerebral ischemia represents a case of disseminate cellular damage confirmed by radiological examination, the ability of transplanted cells to penetrate and survive in relative vicinity of the damaged tissue for prolonged periods of time would be crucial. To date, most of the stem cell tracking studies performed after intraparenchymal cell implantation in animal models revealed mostly local cell engraftment. In contrast, intracerebroventricular transplantation into neonatal or adult recipients caused broad distribution of the engrafted cells, reaching even the most distant brain structures (1,36,45).
Here we report an autologous umbilical cord blood-derived committed (UCB-NC) cell transplantation in a child in a permanent vegetative state resulting from global hypoxic-ischemic brain injury. Based on the previously reported data we decided to transplant UCB-NC cells by a series of repeated injections into the lateral cerebral ventricle. A part of UCB-NC cells were tagged with superparamagnetic iron oxide (SPIO) nanoparticles for visualization of the cell fate in MRI after their transplantation. The child was followed up for 6 months after the last cell injection and his neurological status and MR images have been repeatedly evaluated.
PATIENT AND METHODS
Patient
The first child of healthy, nonrelated parents without relevant perinatal problems was born by spontaneous delivery at term. The birth weight was 2600 g and the Apgar score 9 points. At day 4 he presented classical signs of intestinal obstruction. Double lumen transverse colostomy was performed. Generally satisfactory postoperative period was slightly complicated by inadvertent extravasations of sodium bicarbonate hypertonic solution followed by skin necrosis in the left elbow region. The child was 9 months old when, following the elective dermatoplastic surgery performed under general anesthesia, he developed a cardiac arrest. After prolonged resuscitation the patient remained unresponsive and required mechanical ventilation. Three days after the incidence his breathing became spontaneous but the child stayed unresponsive. The child neither responded to any verbal stimuli nor displayed postures to noxious stimuli. Severe generalized spasticity with contractures and increased tendon reflexes were noted. He also presented high-amplitude and low-frequency nystagmus together with ocular bobbing. Sleep/wake cycles were preserved. Based on these findings, clinical diagnosis of vegetative state was established (2,43). Then he was given a broad rehabilitation program, but without noticeable improvement during the next 6 months of observation. The child also displayed severe epileptic seizures and was given three antiepileptic drugs: clonazepam, valproic acid, and phenobarbital. Despite this treatment seizure control was not satisfactory. EEG recording showed disorganized background activity with global slowing and widely distributed spikes. MRI examination was performed after the insult showed generalized atrophy of the brain. As documented by clinical, neuroradiological, and EEG examinations the brain injury was critical and global without any focal lesions noticed in MRI.
As the child had his umbilical cord blood deposited in a blood bank, autologous cell transplantation was suggested to his parents as an experimental neurorestorative treatment. The procedure was approved and monitored by the Review Board of Ethics Committee designated by the Polish Ministry of Health then performed in the Children’s Memorial Health Institute in Warsaw.
Umbilical Cord Blood Collection and Storage
Umbilical cord blood (UCB) was obtained during the full-time delivery by puncturing the umbilical cord stub. Hydroxyethyl starch sedimentation was performed for erythrocyte depletion. The buffy coat consisting of nucleated cord blood cells was isolated, estimated for cell number, frozen in liquid nitrogen in a concentration of 24.5 × 106/ml of 10% DMSO in PBS, and stored in the Polish Stem Cell Bank (PBKM SA) in Warsaw.
Conditioning Regimens for UCB Cell Culture In Vitro
The sample of UCB was thawed at 37°C, diluted with an equal volume of DMEM/F-12 + 30% FBS + AAS (1:100) medium and spun down. The cell pellet was washed with 10 ml medium and centrifuged again to discard cell debris and DMSO. Viability of UCB cells was estimated by trypan blue staining and the cells were seeded in 25-ml culture flasks at the density of 6−8 × 106/ml in the same medium. After 24 h of culture UCB cells were diluted (1:3) with serum-free medium containing DMEM/F-12 + B27 (1:100) + AAS (1:100) + EGF (40 ng/ml) + βFGF (20 ng/ml) + fibronectin (5 μl/ml) + heparin (5 μ/ml), and then the cells were allowed to grow at 37°C, 5% CO2 in a fully humidified atmosphere with shaking (40 rpm) overnight by an orbital shaker. A half volume of medium was replaced with fresh medium once every 2 days. After 10 days of culture the adherent cells under the name of umbilical cord blood committed (UCB-NC) cells were detached using 0.2% trypsin, washed twice with PBS for phenotypic evaluation, or in PBS with 20% DMSO 1:1 v/v for freezing in liquid nitrogen as samples ready to use for transplantation procedures. The separate sample of cultured UCB-NC cell suspension has been routinely screened for pathogen safety.
PCR Analysis of UCB-NC Cells
Total RNA was isolated from the sample of UCB-NC cells by use of the RNAeasy mini-kit (Qiagen). Reverse transcription was made in 20μl total volume using 1 μg of RNA and 1 μg of oligo-dT, 200 units of SuperScript Rnase H− Reverse Transcriptase (Invitrogen, Paisley, UK) in its associated buffer, 400 μM of dNTPs (Amersham Bioscience), and 40 units of RNAseout (Invitrogen). PCR reactions were carried out in a 25-μl reaction using 1U Taq DNA Polymerase (Fermentas) and identical amount of cDNA per reaction with 1 μM of forward (F) and reverse (R) primers, respectively (primer sequences are listed in Table 1). PCR products were separated by electrophoresis, visualized by ethidium bromide staining, and documented.
Table 1.
Primers Used for PCR Analysis
| Gene (Protein) | Sequence (Forward and Reverse | Product Length (bp) |
|---|---|---|
| Oct4 | 5′-CTCTGAGGAGTGGGGGATTC-3′ 5′-TTGTGCATAGCCACTGCTTG-3′ |
718 |
| Rex1 | 5′-CAGATCCTAAACAGCTCGCAGAAT-3′ 5′-GCGTACGCAAATTAAAGTCCAGA-3′ |
306 |
| SOX2 | 5′-AGTCTCCAAGCGACGAAAAA-3′ 5′-GGA AAG TTG GGA TCG AAC AA-3′ |
410 |
| NANOG | 5′-CCTGTGATTTGTGGGCCTG-3′ 5′-GACAGTCTCCGTGTGAGGCAT-3′ |
153 |
| NeuroD1 | 5′-CGCTGGAGCCCTTCTTTG-3′ 5′-GCGGACGGTTCGTGTTTG-3′ |
118 |
| Neurogenina2 | 5′-CGCATCAAGAAGACCCGTAG-3′ 5′-GTGAGTGCCCAGATGTAGTTGTG-3′ |
173 |
| Nestin | 5′-AGGATGTGGAGGTAGTGAGA-3′ 5′-TGGAGATCTCAGTGGCTCTT-3′ |
266 |
| NF200 | 5′-GAGGAACACCAAGTGGGAGA-3′ 5′-CTTTGCTTCCTCCTTCGTTG-3′ |
850 |
| MAP2 | 5′-TCAGAGGCAATGACCTTACC-3′ 5′-GTGGTAGGCTCTTGGTCTTT-3′ |
325 |
| GAPDH | 5′-TGAAGGTCGGAGTCAACGGATTTGG-3′ 5′-CATGTAGGCCATGAGGTCCACCAC-3′ |
915 |
Immunocytochemical Characteristics of UCB-NC Cells
A part of cultured UCB-NC cells fixed previously with 4% PFA was blocked with serum free blocker (Sigma) at room temperature (RT) for 60 min. The primary antibodies (mouse monoclonal antibodies directed against human nestin, TUJ1, NF200, O4, Ki67, and goat polyclonal antibody against human GFAP) were applied in dilutions listed in Table 2. Then the following secondary antibodies were applied for 60 min at RT in the darkness: goat anti-mouse IgG1, IgG2a, IgM, or goat anti-rabbit IgG (H+L) Alexa Fluor 546 (Molecular Probes) or Alexa Fluor 488 (Molecular Probes). Cell nuclei were counterstained with 5 μM Hoechst 33258 (Sigma) for 20 min. As a control the first antibodies were omitted during immunocytochemical staining and slides were mounted in DAKO medium. To obtain detailed images acquisition and computation the Axiovert 25 and Axioscope 2 microscopes aided by computer-based programmable analyzer Axiovision Rel.4.7 (Carl Zeiss) were used.
Table 2.
Antibodies Used for In Vitro Immunophenotyping
| Antigen | Type | Dilution | Cell Type Marker | Company |
|---|---|---|---|---|
| Nestin | Mono IgG1 | 1:50 | neural progenitors | R&D |
| NF200 | Mono IgG1 | 1:400 | early neural marker | Sigma |
| TUJ1 | Mono IgG2a | 1:500 | neural marker | Covance |
| Class III β-tubulin | Mono IgG2b | 1:1000 | neural marker | Sigma |
| GFAP | Poly rabbit H+L | 1:50 | neural stem cell and astrocyte marker | Cappel |
| S100β | Poly rabbit H+L | 1:1000 | astrocyte marker | Swant |
| Doublecortin | Poly goat H+L | 1:500 | migration marker | SantaCruz |
| Ki67 | Mono IgG1 | 1:100 | proliferation marker | Novocastra |
| CXCR4 | Poly rabbit H+L | 1:200 | SDF-1 ligand | Chemicon |
Labeling of UCB-NC Cells With SPIO Nanoparticles
Serum-free defined medium [DMEM/F-12 + B27 (1:100) + AAS (1:100) + EGF (40 ng/ml) + βFGF (20 ng/ml) + heparin (5 μg/ml)] containing superparamagnetic iron oxide (SPIO Guerbet SA -Endorem) (200 μg/ml) and poly-l-lysine (Sigma P1524 1.5 μg/ml) was prepared and shacked for 60 min. Then SPIO solution was added 1:1 (v/v) to a portion (4 × 106) of freshly thawed UCB-NC cells and incubated for 48 h at 37°C. After incubation the cells were detached, washed two times with PBS, and suspended in 0.5 ml of PBS. For morphological evaluation a sample of SPIO-labeled UCB-NC cells was stained by Perl’s Prussian blue method. Briefly, the cells were fixed in 4% aqueous glutaraldehyde for 15 min, washed three times with PBS, and incubated with 4% potassium ferrocyanide solution containing 10% HCl (1:1) for 30 min. Counterstaining for cell nuclei was performed using neutral red. At the day of first transplantation the SPIO-labeled UCB-NC cells were mixed in 1:2 cell/cell ratios with unlabelled UCB-NC samples and transplanted intracranial in the total number of about 12 × 106 cells in 0.5 ml of PBS. The cells were labeled only once for the first transplantation.
UCB-NC Cell Transplantation Procedure
General anesthesia has been administered to the patient during the transplantation procedures. For surgery the child was laid supine with the head kept in a neutral position. Following a burr hole placed at Kocher point, a proximal shunt catheter was inserted into the lateral ventricle. As cerebrospinal fluid (CSF) appeared 0.5 ml of cell suspension (12 × 106 UCB-NS cells) was injected, followed by 4 ml of saline to push up rest of cells from the catheter to the CSF. Then the drain was removed and water-tight closure of surgical wound was performed. The operation was done under guidance of the magnetic neuronavigation system and was repeated monthly three times, with every one injection being performed to the contralateral cerebral ventricle, alternatively.
MRI Analysis
MRI acquisition has been achieved using 1.5T scanner equipped with 8-channel phased-array head coil (Magnetom, Siemens, Germany). For the detection of iron-oxide nanoparticles SWI T2 gradient-echo sequence (TRI = 58, TE = 44.1 ms, SL = 1.6, voxel size 0.76 × 0.76 × 1.2 mm) was selected.
For in vitro MR imaging of SPIO-labeled UCB-NC cells, the cell pellet was deposited in PCR tubes and placed in the phantom consisting of agar and copper sulfate to stabilize the tubes and avoid artifacts from surrounding air. The cells labeled with SPIO for 12 or 48 h were measured in five different concentrations (103, 104, 105, 106, 4 × 106) each.
In vivo MRI examination of a patient was performed 1 day before, then 1 day, 1 week, and 1, 2, and 4 months after the first transplantation procedure. The child was anesthetized throughout the scanning to reduce motor artifacts. The examination protocol included: SE T1, FSE T2, FSE FLAIR T2, and SWI T2 weighted images. MRI results were assessed independently by two certified radiologists. The level of serial MR images for comparison between successive examinations was selected basing on reference with other brain structures.
RESULTS
Characteristics of Committed Umbilical Cord Blood (UCB-NC) Cells
Before transplantation the patient’s cord blood cells were cultured for 10 days in previously described neurogenic conditions (18) and then evaluated for their stem and neural characteristic by employing standard molecular and immunochemical methods (5,25). The expression of stemness genes involved in self-renewal and maintenance of pluripotent state of stem cells such as Oct3/4, Rex1, Sox2, and hTert revealed (except augmented Nanog) relatively weak signal (Fig. 1). As expected, the 10-day cell culture induced, besides weak expression of pluripotent stem cell markers, the acquisition of several proneural transcripts like nestin, NeuroD1, Neurogenin2, and NF200, never observed in native, noncultured cord blood cells (18).
Figure 1.
RT-PCR analysis of UCB-NC cells after 10 days of culture in vitro.
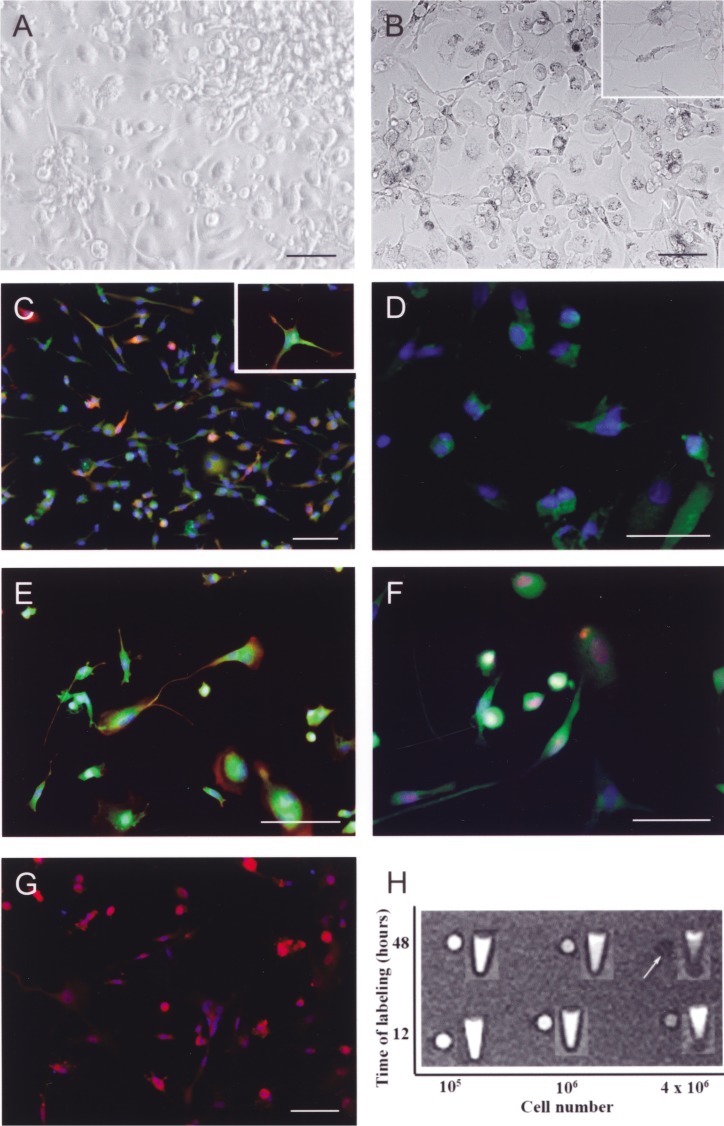
Morphologically, the USC-NC formed a mixed population of partly adherent and partly floating cells (Fig. 2A, B). Spindle-shaped cells with short bipolar processes were frequently found throughout the adherent subpopulation, whereas nondifferentiated round ones remained free or only loosely attached to the surface. The majority of the cells revealed markers of immature neural progenitors such as GFAP and nestin (Fig. 2C, E). Adherent cells with spherical soma and neuronal processes with more ramified morphology coexpressed frequently the early neuronal markers (Fig. 2C–F): NF200 (the high m.w. neurofilament protein found in neuroblasts), doublecortin (characteristic for migrating neuroblasts), and TUJ1 (β-tubulin III expressed in more mature neurons). Few of the cultured UCB cells were positive for S-100β protein distinctive for astroglial cells (Fig. 2E) and manifested proliferating and migrating activity as shown by Ki67 and CXCR4 marker expression (Fig. 2F, G, respectively).
Figure 2.
Phase contrast images of UCB-NC cells after 10 days of culture in vitro (A). SPIO-stained UCB-NC cells for 48 hours (B; inset with higher magnification). Specific immunostaining of UCB-NSC cells (C–G) visualizing the double staining for NF200 (red) and GFAP (green) markers [with insert depicting higher magnification of these cells (C)]; TUJ1-positive cells (D); double staining for nestin (red) and S100β markers (green) (E); double staining for β-tubulin III (green) and Ki67 antigens (red) (F); CXCR4-expressed cells (G). Cells stained with Alexa Fluor 546 (red) and Alexa Fluor 488 (green) were detected simultaneously and together with their nuclei revealed by use of Hoechst 33252 staining (blue). Scale bars: 50 μm for main pictures and 20 μm for insets. In vitro MR imaging of SPIO-tracked UCB-NC cells in phantom experiment (H). Upper column presents the cells incubated with SPIO for 12 h and lower column for 48 h. Increasing cell density from 104 to 4 × 106 in consecutive rows from top to bottom is shown. White arrow indicates the optimal cell detection.
UCB-NC Cell Neuroimaging
For MRI visualization the fate of transplanted UCB-NC cells, part of the cells were tagged with SPIO nanoparticles. In terms of optimizing cell labeling protocol, the efficiency of SPIO internalization by the cells was evaluated by cytochemistry using Perl’s method (Fig. 2B). It was no significant difference in relation with the time of labeling (82% SPIO-positive cells after 24 h, 89% after 48 h, and 93% after 72 h, respectively). Then the threshold of cell visualization by MRI at 1.5 Tesla was determined in phantom experiment. The density up to 4 × 106 cells/ml provided satisfactory signal-to-noise ratio (Fig. 2H).
MRI examination with the use of SWI T2 gradient-echo sequence performed 24 h after the first administration of UCB-NC cells to the right cerebral hemisphere disclosed the SPIO-tagged cell deposits visible as a small area of hypointense signal, extending along the ependyma from the right posterior horn through the corpus to the anterior horn of the lateral ventricle. Seven days later, the signal produced by transplanted UCB-NC cells was weaker but still present. This signal was also visible by MRI examinations performed 30 days and 2 months after the first infusion of UCB-NC cells, but scarcely any signal was left on follow-up investigation performed 4 and 6 months (not shown) after the first surgery (Fig. 3). Similar evolution of MRI signal during the time of observation was reported as typical for the presence of living cells persisted in transplantation area (35,50). No sign of brain edema, hemorrhage, or tumor appearance due to cell transplantation has been found during observation. Also, no MR signal change characteristic for blood extravasations in the consecutive examinations has been observed.
Figure 3.
MRI examination with the use of SWI T2 gradient-echo sequence performed before (A) and 1 day after (B) intracerebroventricular UCB-NC cell transplantation in the brain of a child with global ischemic injury. On the upper left picture the widened sulci between gyri of the cerebral cortex and widened supratentorial ventricular system with 12-mm-wide III ventricle as well as thinning of corpus callosum was seen. The upper right picture reveals SPIO labeled UCB-NC cell cluster visible in the right cerebral hemisphere as a small (3.6 mm in diameter) area of hypointense signal (arrow), extending along the ependyma from the right posterior horn through the body to anterior horn of the lateral ventricle. Longitudinal study of the right posterior horn of lateral ventricle (C) presents two rows of different axial sections through the brain. Transplanted UCB-NC cells were detected as an area of hypointense signal (dotted line and arrows). Gradual disappearance of this signal over 4 months can be noticed.
Neurological Outcome
Full pediatric and neurological examination of the child was performed 1 day before and 1 day after each transplantation procedure. The only complication noticed each time after cell infusion was transient fever up to 38.6°C. The fever lasted for only a few hours and then spontaneously disappeared. Any other clinically relevant side effects were observed during subsequent posttransplantation days. The follow-up examinations were done monthly for 6 months after the last transplantation procedure. Each examination was video-documented and performed by two certified pediatric neurologists and one pediatrician. On each examination slight but gradual improvement in the patient’s neurological state was observed. At the final follow-up examination, although the child was still severely impaired, diagnose of vegetative state was no longer justified. He started to respond to his mother’s voice by smiling. The reduction of seizure frequency by 50% was achieved. Nystagmus, although still present, was milder and the child achieved head and neck control good enough to move in certain positions to dampen the frequency and the amplitude of the nystagmus. When reaching this null point he became calm and apparently satisfied. Spasticity was less pronounced than at baseline examination; however, it should be noted that the patient received a rehabilitation program throughout the project. On the other hand, the same rehabilitation program was implemented soon after the cardiac arrest and had no effect on patient neurological status before UCB cell transplantation.
DISCUSSION
We report here for the first time the case of MRI-monitored intracerebroventricular transplantation of committed cord blood cells in a child with global hypoxic ischemic brain injury.
The UCB-NC cells used for transplantation were extensively evaluated in vitro for their stem and neural characteristic by standard molecular and immunocytochemical methods in parallel cell culture. The maintenance of heterogeneous population of neural lineage-committed progenitor cells being in different stages of their maturation (including even those representing undifferentiated pluripotent developmental stage as revealed by Oct3/4, Rex1, Sox2, Nanog, and hTert gene expression) suggests that transplanted UCB-NC cells probably have a potential to self-renew, survive, and differentiate toward brain-specific cells after their transplantation. Moreover, augmented expression of nestin would mark neuroectodermal conversion of CB cells originally belonging to mesoderm, whereas concomitant appearance of Nanog signal can be attributed to suppression of the cell endodermal differentiation (1,39). This last finding is in line with our previous data on the lack of endodermal marker FoxA1 (Forkhead box protein A1) expression upon CB phenotypic conversion of our CB culture (1,18).
Based on the previously published data we decided to transplant UCB-NC cells by a series of repeated injections into the lateral cerebral ventricle. Intracerebroventricular injection of UCB-NC cells seems to be superior to intravascular because it results in a more local modulating outcome in the lesioned sights. Before the first transplantation procedure a part of UCB-NC cells (30%) was tagged with superparamagnetic iron oxide (SPIO) nanoparticles (Endorem). Then MRI was used for visualizing the cell fate in the host brain. SPIO nanoparticles were confirmed to tag the cells before their transplantation in many experimental studies (27,33,35,42) and also proved to be safe for humans (12,50). However, it is still not clear whether it affects cell proliferation and/or differentiation (47). In our study, SPIO-tagged UCB-NC cells have been transplanted only once during the first surgery. These cells were easily assessed by MRI for several months. Although UCB-NC cells transplanted during the second and third surgical procedure were not labeled, based on our observation with SPIO-tagged cells we could conclude that they also persisted in the brain for four months.
The capacity of cells to home to damaged sites of the CNS is a crucial aspect when attempting to employ cell therapy in neurological diseases. Progenitor cells are known for their migratory properties owing to their chemokine receptors and ligands (31). However, during the time of our observation we did not see any MRI-based evidence of UCB-NC cell migration into brain parenchyma after their transplantation. On the other hand, as the impact of SPIO tagging on cell behavior is still not determined, we cannot definitively exclude that the fate of nonlabeled UCB-NC cells was somewhat different. It should be also noted here that several recent studies report that implantation or infusion of cord blood mononuclear cell derivatives results in CNS functional improvement by their neurotrophic influence. For this reason the persisted presence of transplanted UCB-NC cells at the surface of ventricular wall and also in close vicinity to the subependymal neurogenic brain region may be beneficial for endogenous neurogenesis and thus for repairing processes. Thus, the paracrine effect of these cells could mediate the slight clinical improvement observed in a child after UCB-NC cell transplantation.
In conclusion, although the follow-up of this first case of intraventricular UCB-NC cells transplantation was only 6 months, we were able to demonstrate here the short-term safety and feasibility of this novel clinical approach. In addition, intracerebroventricular cell transplantation into injured central nervous system was generally a well-tolerated procedure. The only clinically demonstrated side effect of the transplantation was transient and moderate fever.
According to our knowledge, this is the first report of intraventricular autologous UCB cells transplantation in child with global ischemic brain injury. Our neuroimaging data demonstrated that committed cord blood-derived cells transplanted intracerebroventricularly remained in the host brain by at least 4 months. Moreover, this autologous UCB-NC cell transplantation into lateral ventricle of the brain seems to be safe when done under neuronavigation and may represent a novel promising tool for management of brain ischemic injury. However, despite the signs of neurological improvement being noticed by the parents and neurologists after cell transplantation, the only one case does not allow us to predict true efficiency of such treatment and further studies are needed to establish the most beneficial timing and protocols for UCB-NC transplantation into the injured brain.
ACKNOWLEDGMENTS
The authors wish to thank to Prof. Irena Hausmanowa-Petrusewicz for critical review and encouraging comments to our work and the manuscript. We also thank Dr. Piotr Walczak from Johns Hopkins University School of Medicine for his advice on using superparamagnetic iron oxide nanoparticles for tagging UCB-NC cells. This work was supported by grants from the Polish Ministry of Scientific Research and Higher Education (0141/B/P01/2008/35; 0142/B/P01/2008/35).
REFERENCES
- 1. Ashwal S.; Obenaus A.; Snyder E. Y. Neuroimaging as a basis for rational stem cell therapy. Pediatr. Neurol. 40:227–236; 2009. [DOI] [PubMed] [Google Scholar]
- 2. Bates D. The vegetative state and the Royal College of Physicians guidance. Neuropsychol. Rehabil. 15:175–183; 2005. [DOI] [PubMed] [Google Scholar]
- 3. Bliss T. M.; Andres R. H.; Steinberg G. K. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 37:275–283; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borlongan C. V.; Hadman M.; Sanberg C. D.; Sanberg P. R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35:2385–2389; 2004. [DOI] [PubMed] [Google Scholar]
- 5. Buzanska L.; Jurga M.; Stachowiak E. K.; Stachowiak M. K.; Domanska-Janik K. Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 15:391–406; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Buzanska L.; Machaj E. K.; Zablocka B.; Pojda Z.; Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J. Cell Sci. 115:2131–2138; 2002. [DOI] [PubMed] [Google Scholar]
- 7. Buzanska L.; Ruiz A.; Zychowicz M.; Rauscher H.; Ceriotti L.; Rossi F.; Colpo P.; Domanska-Janik K.; Coecke S. Patterned growth and differentiation of human cord blood-derived neural stem cells on bio-functionalized surfaces. Acta Neurobiol. Exp. 69:24–36; 2009. [DOI] [PubMed] [Google Scholar]
- 8. Cho S. R.; Kim Y. R.; Kang H. S.; Yim S. H.; Park C. I.; Min Y. H.; Lee B. H.; Shin J. C.; Lim J. B. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone barrow in a rat model of spinal cord injury. Cell Transplant. 18:1359–1368; 2009. [DOI] [PubMed] [Google Scholar]
- 9. Christie B. Study on stem cells for stroke patients to start in Scotland later in 2009. BMJ 338:b245; 2009. [DOI] [PubMed] [Google Scholar]
- 10. Copeland N.; Harris D.; Gaballa M. A. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin. Med. 9:342–345; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deda H.; Inci M. C.; Kurekci A. E.; Sav A.; Kayihan K.; Ozgun E.; Ustunsoy G. E.; Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: A 1-year follow-up. Cytotherapy 11:18–25; 2009. [DOI] [PubMed] [Google Scholar]
- 12. de Vries I. J.; Lesterhuis W. J.; Barentsz J. O.; Verdijk P.; van Krieken J. H.; Boerman O. C.; Oyen W. J.; Bonenkamp J. J.; Boezeman J. B.; Adema G. J.; Bulte J. W.; Scheenen T. W.; Punt C. J.; Heerschap A.; Figdor C. G. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 23:1407–1413; 2005. [DOI] [PubMed] [Google Scholar]
- 13. Domanska-Janik K.; Buzanska L.; Lukomska B. A novel, neural potential of non-hematopoietic human umbilical cord blood stem cells. Int. J. Dev. Biol. 52:237–248; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Domanska-Janik K.; Habich A.; Sarnowska A.; Janowski M. Neural commitment of cord blood stem cells (HUCB-NSC/NP): Therapeutic perspectives. Acta Neurobiol. Exp. 66:279–291; 2006. [DOI] [PubMed] [Google Scholar]
- 15. Escolar M. L.; Poe M. D.; Provenzale J. M.; Richards K. C.; Allison J.; Wood S.; Wenger D. A.; Pietryga D.; Wall D.; Champagne M.; Morse R.; Krivit W.; Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N. Engl. J. Med. 352:2069–2081; 2005. [DOI] [PubMed] [Google Scholar]
- 16. Farin A.; Liu C. Y.; Elder J. B.; Langmoen I. A.; Apuzzo M. L. The biological restoration of central nervous system architecture and function: Part 1—foundations and historical landmarks in contemporary stem cell biology. Neurosurgery 64:15–39; 2009. [DOI] [PubMed] [Google Scholar]
- 17. Geffner L. F.; Santacruz P.; Izurieta M.; Flor L.; Maldonado B.; Auad A. H.; Montenegro X.; Gonzalez R.; Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 17:1277–1293; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Habich A.; Jurga M.; Markiewicz I.; Lukomska B.; Bany-Laszewicz U.; Domanska-Janik K. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp. Hematol. 34:914–925; 2006. [DOI] [PubMed] [Google Scholar]
- 19. Hau S.; Reich D. M.; Scholz M.; Naumann W.; Emmrich F.; Kamprad M.; Boltze J. Evidence for neuroprotective properties of human umbilical cord blood cells after neuronal hypoxia in vitro. BMC Neurosci. 9:30; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hess D. C.; Borlongan C. V. Cell-based therapy in ischemic stroke. Expert Rev. Neurother. 8:1193–1201; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hess D. C.; Borlongan C. V. Stem cells and neurological diseases. Cell Prolif. 41(Suppl. 1):94–114; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janowski M.; Date I. Systemic neurotransplantation–a problem-oriented systematic review. Rev. Neurosci. 20:39–60; 2009. [DOI] [PubMed] [Google Scholar]
- 23. Janowski M.; Walczak P.; Date I. Intravenous route of cell delivery for treatment of neurological disorders: A meta-analysis of preclinical results. Stem Cells Dev. 19:5–16; 2010. [DOI] [PubMed] [Google Scholar]
- 24. Jurga M.; Lipkowski A. W.; Lukomska B.; Buzanska L.; Kurzepa K.; Sobanski T.; Habich A.; Coecke S.; Gajkowska B.; Domanska-Janik K. Generation of functional neural artificial tissue from human umbilical cord blood stem cells. Tissue Eng. Part C Methods 15:365–372; 2009. [DOI] [PubMed] [Google Scholar]
- 25. Jurga M.; Markiewicz I.; Sarnowska A.; Habich A.; Kozlowska H.; Lukomska B.; Buzanska L.; Domanska-Janik K. Neurogenic potential of human umbilical cord blood: Neural-like stem cells depend on previous long-term culture conditions. J. Neurosci. Res. 83:627–637; 2006. [DOI] [PubMed] [Google Scholar]
- 26. Kang K. S.; Kim S. W.; Oh Y. H.; Yu J. W.; Kim K. Y.; Park H. K.; Song C. H.; Han H. A 37-year-old spinal cord-injured female patient, transplanted of multi-potent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: A case study. Cytotherapy 7:368–373; 2005. [DOI] [PubMed] [Google Scholar]
- 27. Kim D.; Chun B. G.; Kim Y. K.; Lee Y. H.; Park C. S.; Jeon I.; Cheong C.; Hwang T. S.; Chung H.; Gwag B. J.; Hong K. S.; Song J. In vivo tracking of human mesenchymal stem cells in experimental stroke. Cell Transplant. 16:1007–1012; 2008. [PubMed] [Google Scholar]
- 28. Koh S. H.; Kim K. S.; Choi M. R.; Jung K. H.; Park K. S.; Chai Y. G.; Roh W.; Hwang S. J.; Ko H. J.; Huh Y. M.; Kim H. T.; Kim S. H. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 1229:233–248; 2008. [DOI] [PubMed] [Google Scholar]
- 29. Kozlowska H.; Jablonka J.; Janowski M.; Jurga M.; Kossut M.; Domanska-Janik K. Transplantation of a novel human cord blood-derived neural-like stem cell line in a rat model of cortical infarct. Stem Cells Dev. 16:481–488; 2007. [DOI] [PubMed] [Google Scholar]
- 30. Lee J. P.; McKercher S.; Muller F. J.; Snyder E. Y. Neural stem cell transplantation in mouse brain. Curr. Protoc. Neurosci. 42:3.10.1–3.10.23; 2008. [DOI] [PubMed] [Google Scholar]
- 31. Li M.; Ransohoff R. M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 84:116–131; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mackay-Sim A.; Feron F.; Cochrane J.; Bassingth-waighte L.; Bayliss C.; Davies W.; Fronek P.; Gray C.; Kerr G.; Licina P.; Nowitzke A.; Perry C.; Silburn P. A.; Urquhart S.; Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 131:2376–2386; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mai X. L.; Ma Z. L.; Sun J. H.; Ju S. H.; Ma M.; Teng G. J. Assessments of proliferation capacity and viability of New Zealand rabbit peripheral blood endothelial progenitor cells labeled with superparamagnetic particles. Cell Transplant. 18:171–181; 2009. [DOI] [PubMed] [Google Scholar]
- 34. Mazzini L.; Fagioli F.; Boccaletti R.; Mareschi K.; Oliveri G.; Olivieri C.; Pastore I.; Marasso R.; Madon E. Stem cell therapy in amyotrophic lateral sclerosis: A methodological approach in humans. Amyotroph. Lateral. Scler. Other Motor Neuron Disord. 4:158–161; 2003. [DOI] [PubMed] [Google Scholar]
- 35. McAteer M. A.; Sibson N. R.; von Zur M. C.; Schneider J. E.; Lowe A. S.; Warrick N.; Channon K. M.; Anthony D. C.; Choudhury R. P. In vivo magnetic resonance imaging of acute brain inflammation using micro-particles of iron oxide. Nat. Med. 13:1253–1258; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morita E.; Watanabe Y.; Ishimoto M.; Nakano T.; Kitayama M.; Yasui K.; Fukada Y.; Doi K.; Karunaratne A.; Murrell W. G.; Sutharsan R.; kay-Sim A.; Hata Y.; Nakashima K. A novel cell transplantation protocol and its application to an ALS mouse model. Exp. Neurol. 213:431–438; 2008. [DOI] [PubMed] [Google Scholar]
- 37. Park D. H.; Borlongan C. V.; Willing A. E.; Eve D. J.; Cruz L. E.; Sanberg C. D.; Chung Y. G.; Sanberg P. R. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 18:985–998; 2009. [DOI] [PubMed] [Google Scholar]
- 38. Sarnowska A.; Jurga M.; Buzanska L.; Filipkowski R. K.; Duniec K.; Domanska-Janik K. Bilateral interaction between cord blood-derived human neural stem cells and organotypic rat hippocampal culture. Stem Cells Dev. 18:1191–1200; 2009. [DOI] [PubMed] [Google Scholar]
- 39. Singh A. M.; Hamazaki T.; Hankowski K. E.; Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells 25:2534–2542; 2007. [DOI] [PubMed] [Google Scholar]
- 40. Stein J.; Garty B. Z.; Dror Y.; Fenig E.; Zeigler M.; Yaniv I. Successful treatment of Wolman disease by unrelated umbilical cord blood transplantation. Eur. J. Pediatr. 166:663–666; 2007. [DOI] [PubMed] [Google Scholar]
- 41. Sun W.; Buzanska L.; Domanska-Janik K.; Salvi R. J.; Stachowiak M. K. Voltage-sensitive and ligand-gated channels in differentiating neural stem-like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells 23:931–945; 2005. [DOI] [PubMed] [Google Scholar]
- 42. Sykova E.; Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog. Brain Res. 161:367–383; 2007. [DOI] [PubMed] [Google Scholar]
- 43. The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. N. Engl. J. Med. 330:1499–1508; 1994.7818633 [Google Scholar]
- 44. Wagner J. E.; Gluckman E. Umbilical cord blood transplantation: The first 20 years. Semin. Hematol. 47:3–12; 2010. [DOI] [PubMed] [Google Scholar]
- 45. Walczak P.; Kedziorek D. A.; Gilad A. A.; Barnett B. P.; Bulte J. W. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: The case of the shiverer dysmyelinated mouse brain. Magn. Reson. Med. 58:261–269; 2007. [DOI] [PubMed] [Google Scholar]
- 46. Watt F. M.; Driskell R. R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365:155–163; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weissleder R.; Stark D. D.; Engelstad B. L.; Bacon B. R.; Compton C. C.; White D. L.; Jacobs P.; Lewis J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 152:167–173; 1989. [DOI] [PubMed] [Google Scholar]
- 48. Young K. D.; Gausche-Hill M.; McClung C. D.; Lewis R. J. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardio-pulmonary arrest. Pediatrics 114:157–164; 2004. [DOI] [PubMed] [Google Scholar]
- 49. Yu G.; Borlongan C. V.; Stahl C. E.; Hess D. C.; Ou Y.; Kaneko Y.; Yu S. J.; Yang T.; Fang L.; Xie X. Systemic delivery of umbilical cord blood cells for stroke therapy: A review. Restor. Neurol. Neurosci. 27:41–54; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu J.; Zhou L.; XingWu F. Tracking neural stem cells in patients with brain trauma. N. Engl. J. Med. 355:2376–2378; 2006. [DOI] [PubMed] [Google Scholar]