Abstract
Human T-cell Lymphotropic Virus type 1 (HTLV-1) is a human retrovirus that infects at least 5–10 million people worldwide, and is the etiological agent of a lymphoproliferative malignancy; Adult T-cell Leukemia/Lymphoma (ATLL); and a chronic neuromyelopathy, HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP), as well as other inflammatory diseases such as infective dermatitis and uveitis. Besides sexual intercourse and intravenous transmission, HTLV-1 can also be transmitted from infected mother to child during prolonged breastfeeding. Some characteristics that are linked to mother-to-child transmission (MTCT) of HTLV-1, such as the role of proviral load, antibody titer of the infected mother, and duration of breastfeeding, have been elucidated; however, most of the mechanisms underlying HTLV-1 transmission during breast feeding remain largely unknown, such as the sites of infection and cellular targets as well as the role of milk factors. The present review focuses on the latest findings and current opinions and perspectives on MTCT of HTLV-1.
Keywords: human, HTLV-1, intestinal barrier, retrovirus, breastfeeding
1. Introduction
Human T-cell Leukemia Virus Type 1 (HTLV-1) infects at least 5–10 million people worldwide, mainly in highly endemic areas such as southern Japan, West/Central Africa, the Caribbean region, and parts of South America and Melanesia [1]. HTLV-1 infection is mostly associated with two distinct diseases: a lymphoproliferation, Adult T cell Leukemia/Lymphoma (ATLL), and an inflammatory neurological disease, tropical spastic paraparesis or HTLV-1 associated myelopathy (HAM/TSP). Additionally, HTLV-1 is associated with other inflammatory diseases such as infective dermatitis, some uveitis and some myositis. Although HTLV-1 preferentially infects CD4+ T cells [2], CD8+ T-cells may play an important role as reservoir in the host [3], and to a lesser extent, infected monocytes and B lymphocytes, dendritic cells, and endothelial cells may be found [4,5]. Different modes of transmission have been identified for HTLV-1: (1) sexual contact; (2) transfusion of contaminated blood; and (3) from mother to child (MTCT) [6]. In each case, such a transmission involves the transfer of infected body fluid (semen, blood, and milk, respectively). In the case of MTCT, cohort studies on HTLV-1 infected carriers indicate that infection during childhood is a potent risk factor for the development of ATLL [7]. It is now clear that HTLV-1 MTCT mainly involves prolonged breastfeeding, as demonstrated by epidemiological, virological and experimental data. However, the mechanisms of such a transmission remain largely unknown. For instance, the nature of the infected cells present in the milk, the anatomical sites of viral entry through the mucosa, the first cellular targets of infection, the role of anti-HTLV-1 antibodies present in breast milk, and the role of other milk factors that may influence MTCT have not been completely addressed. The present review focuses on such mechanisms, current studies and perspectives.
2. Evidence of HTLV-1 MTCT during Breastfeeding
First evidence of HTLV-1 transmission from infected mother to children during lactation has been brought by epidemiological studies. HTLV-1 infection was more prevalent among breastfed children than bottle-fed children in Japan [8,9], with a rate of seroconversion of 15.7% among children that had been breastfed for 12 months, compared to 3.6% for bottle-fed children for similar period [10]. Moreover, there is a correlation between MTCT rate and breastfeeding duration. Thus, in a prospective study in Jamaica, Wiktor et al. [11] reported that breastfeeding beyond 12 months was associated with a transmission rate of 32%, compared to a transmission rate of 9% for shorter breastfeeding durations. Similarly, Takahashi et al. [12] showed that a six-month duration of breastfeeding was a critical point in the rate of seroconversion, since rates of 4.4% and 14.4% were found for children that had been breastfed for periods under six months or over seven months, respectively. A major piece of evidence supporting HTLV-1 transmission through breastfeeding has been brought in the 1980s in Japan, where Hino and coworkers started a pilot study to screen pregnant women in Nagasaki Prefecture for anti-HTLV-1 antibodies (for a review, see [13]). HTLV-1 prevalence was around 4%. Interestingly, HTLV-1 prevalence among the elder children of the HTLV-1 carrier mothers was approximately 20%, and mothers of the HTLV-1 positive children were usually HTLV-1 positive (92%), thus showing evidence of MTCT. More importantly, in 1987, the ATLL Prevention Program Nagasaki, which aimed to refrain seropositive mothers from breastfeeding in the Nagasaki Prefecture, resulted in a huge reduction of HTLV-1 MTCT from 20.3% to 2.5% [14]. The major importance of breastfeeding in HTLV-1 MTCT was later confirmed in other areas [15]. Of note, this residual rate (2.5%) of MTCT in the absence of breastfeeding raised the possibility of minor secondary routes, such as contamination during delivery, or intrauterine transmission. This latter route remains controversial, since contradictory studies on the presence of HTLV-1 in cord-blood samples from seropositive babies have been reported [16,17,18].
From a virological point of view, it is known that many retroviruses may be transmitted via breast milk, such as Moloney murine leukemia virus [19,20], Mouse Mammary Tumor Virus [21], or Caprine Arthritis Encephalitis Virus [22]. Concerning HTLV-1, viral antigens [23], and antibodies to HTLV-1 were found in the milk of seropositive mothers [24]. The proviral load in breast milk is strongly predictive of the risk of MTCT, increasing from 4.7/1000 person-months for a provirus load in milk lower than 0.18% to 28.7/1000 person-months for a provirus load higher than 1.5% [25].
From an experimental point of view, oral inoculation of peripheral blood lymphocytes isolated from ATLL patients to adult common marmosets (Callithrix jacus) was able to induce seroconversion within 2.5 months, and the virus was detected in the animal peripheral blood lymphocytes [26]. This study also demonstrated that 5.6 × 107 cells from ATLL patients were sufficient to infect these animals. Similarly, oral inoculation of concentrated fresh milk from HTLV-1 seropositive mothers in the same animal model could transmit the infection [27]. Oral transmission of HTLV-1 could be likewise observed in other animal models. Oral inoculation of four rabbits for eight weeks with an HTLV-1-infected rabbit lymphoid cell line resulted in the seroconversion of one animal, and it was possible to generate from this animal a lymphoid cell line productively infected with HTLV-1 [28]. Similarly, oral inoculation of HTLV-1-infected lymphoid cell line (i.e., MT-2) to rats induced a persistent HTLV-1 infection in the absence of both humoral and cellular immune responses [29].
3. The Mechanisms of HTLV-1 MTCT
Altogether, these data indicate that breastfeeding is a major route for HTLV-1 MTCT. However, the mechanisms of HTLV-1 passage through the digestive tract remain largely unknown.
A first point to address concerns the source of HTLV-1 infection in breast milk. It is known that cell-to-cell contact is required for efficient viral transmission in vivo [30] as well as in vitro [31,32], except for dendritic cells that can be infected directly with cell-free HTLV-1 virions [33]. Cell free virions have not been detected so far in breast milk, thus the potential source of infection in breast milk may come from infected cells, such as lymphocytes, macrophages, or breast epithelial mammary cells. Since it has been estimated that breastfed children ingest an average of 108 leucocytes a day, considering prolonged breastfeeding [34,35], infected lymphocytes could provide a strong source of infection in milk [36]. HTLV-1 infected mononuclear cells can be found in the milk from seropositive mothers during early lactation [23,37]. Of note, cellular components in breast milk can be found even after long-term lactation (over 5 years) [38], even if the ratio between the different cell types varies along the time: for example, the major part of cells in mother’s early milk and colostrum is constituted of macrophages [39]. It has been found that leukocytes and epithelial cells from the mammary gland are susceptible to HTLV-1 infection [38]. This observation was confirmed by the evidence that mammary basal epithelial cells can be productively infected with HTLV-1 and are able to transfer infection to peripheral blood lymphocytes [40,41]. In addition, in a case report of an ATLL male patient with pseudogynecomasty, breast biopsy revealed the presence of mammary epithelial cells productively infected with HTLV-1 [42]. Such data support the hypothesis that basal and/or luminal epithelial cells may constitute a reservoir of HTLV-1 infectivity. The importance of mammary epithelial cells in viral transmission during lactation has also been evoked for Bovine Leukemia Virus (BLV), another deltaretrovirus that is transmitted from BLV-infected cows to calves during lactation [43]. Whatever the cell types involved, this can provide a more or less continuous source of infection.
A second question concerning the mechanisms of HTLV-1 transmission through the digestive tract is the anatomical site of viral entry. Up until now, no studies have addressed this question; in fact, animal models studies (marmoset, rabbit, and rat) on oral inoculation of HTLV-1 were mainly focused on seroconversion, progression towards an ATLL-like disease, and immune status of the host. Among the different possible sites of entry, the palatine tonsils and gut seem to be of particular importance due to their enrichment in possible targets (lymphoid cells and M cells), their function in antigen sampling [44] and their structure. Moreover, although it does not constitute a proof of viral entry, HTLV-1 may be retrieved in these structures, as shown in the tonsils for HTLV-1 infected humans [45], in the intestine and mesenteric lymph nodes of squirrel monkeys inoculated intravenously with HTLV-1 [46] and in Gut-Associated-Lymphoid Tissues from orally inoculated rabbits [47]. Whatever the primary sites of HTLV-1 passage/infection, tonsils and/or gut, the virus encounters an epithelium, pluristratified or monostratified, respectively. This allows in vitro studies using classical models of human epithelial cell monolayers.
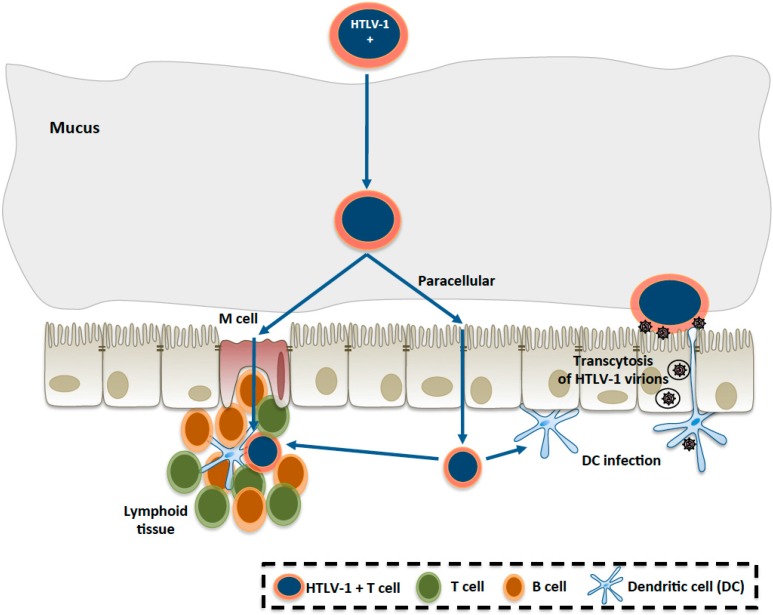
Very few in vitro studies have been published concerning the mechanisms of passage of HTLV-1 across the intestinal barrier. In 1992, Zacharopoulos et al. [48] indicated that a human enterocytic cell lines (i.e., I407) was susceptible to HTLV-1 infection in vitro, as shown by electron microscopy, in situ hybridization, and PCR amplification. However, it was unclear if the cells were fully differentiated as no data on the epithelium integrity and tight junctions were shown. In contrast, our laboratory performed studies on the susceptibility of three different human enterocytic cell lines on compartmentalized culture devices, with assessment of the enterocyte differentiation: existence of a tight epithelial barrier was checked by electron and confocal microscopy, and the trans-epithelial resistance was measured [49]. In this study, as summarized in Figure 1, it was demonstrated that HTLV-1 infected lymphocytes were unable to disrupt the epithelial barrier integrity or infect human enterocytes, in contrast to previous studies on a blood–brain barrier model in human brain endothelial cells [5,50]. However, it was shown that HTLV-1 virions were able to cross the epithelial barrier by transcytosis mechanism, and productively infect underlying human dendritic cells [49].
Figure 1.
Hypothetical mechanisms of HTLV-1 (Human T-cell Lymphotropic Virus type 1 ) passage through the intestinal epithelium.
HTLV-1-infected lymphocytes, once they have crossed the mucus layer, could either pass through the epithelium via M cells, or in-between enterocytes (paracellular passage), to reach the lamina propria where potential targets of HTLV-1 infection, such as T lymphocytes (and/or dendritic cells and B lymphocytes) are located. From in vitro studies [49], the infection of enterocytes or the epithelial layer disruption seems to be excluded. Another possibility, suggested in the same study, could be viral transcytosis through the enterocyte, with infection of underlying dendritic cells.
Interestingly, viral transcytosis through enterocytes was observed only in the presence of HTLV-1 infected lymphocytes, and not in the case of purified virions. Such a mechanism of viral transcytosis is reminiscent of previous work showing transcytosis of HIV across an epithelial barrier, without infection of enterocytes, and subsequent infection of macrophages or CD4 lymphocytes located to the basal side of the epithelium [51]. These in vitro results highlight one of the potential mechanisms proposed for HTLV-1 passage, which are summarized in Figure 1.
4. Determinants of HTLV-1 MTCT
Studies on HTLV-1 MTCT determinants have focused mainly on genetic host factors, immunological host factors, lactation duration and milk components.
The genetic host factors that control HTLV-1 infection by breastfeeding have been investigated by Plancoulaine et al. [52], who began in the 1990s a large epidemiological study in endemic villages of French Guiana. The authors found a dominant major gene predisposing to HTLV-1 infection, in addition to the expected familial correlations due to the transmission routes (mother to child and spouse to spouse) [53]. Previous studies had shown that HLA (Human Leukocyte Antigen) genes distribution was different for ATLL or HAM/TSP patients compared to asymptomatic carriers (for example, see [54]), but the study by Plancoulaine et al. [53] indicated a genetic predisposition for HTLV-1 infection itself for 1.5% of the population, which concerned almost all infected children under 10 years of age, i.e., infected through breastfeeding. Further studies allowed mapping a major susceptibility locus for HTLV-1 infection during childhood to chromosome 6q27 [55].
Concerning the immunological factors involved in HTLV-1 MTCT, the role of maternal anti-HTLV-1 antibodies may appear controversial. Such studies have to take into account the duration of breastfeeding, since the protective role of anti-HTLV-1 antibodies has been demonstrated in a rabbit model of infection, where passive immunization was shown to prevent milk-borne transmission of HTLV-1 to offspring [56]. Moreover, it has been shown in vitro that the addition of HTLV-1 serum cord blood plasma is able to prevent the infection of human neonatal lymphocytes when co-cultured with breast-milk cells of HTLV-1 carrier mothers [12]. However, it has been suggested that higher anti-HTLV-1 antibodies titer in the serum of the mother, as well as the presence of anti-Tax antibodies, is associated with a higher risk of children infection [11,52,57,58,59]. However, a high anti-HTLV-1 antibody titer in the serum may be correlated with a high provirus load in PBMCs, which is a risk factor for HTLV-1 MTCT [57]. In an analysis including the provirus load in maternal PBMCs, the presence of anti-Tax antibodies and the anti-HTLV-1 titers, it was found that a higher maternal proviral load and a higher anti-HTLV-1 antibody titer were independently associated with a higher risk of HTLV-1 MTCT, whereas the presence of anti-Tax antibodies was not [60].
Another point to take into account concerns the other milk components that may influence HTLV-1 MTCT transmission. As an example, it has been shown that lactoferrin, an iron-binding milk glycoprotein, was able to enhance HTLV-1 replication, by transcriptional activation of HTLV-1 Long Terminal Repeat (LTR), the viral promoter [61]. This effect on HTLV-1 infection seems to be specific since lactoferrin did not show any effect on HIV-1 LTR, and was even able to inhibit HIV-1 infection, probably by interfering with viral fusion and entry steps [61]. As an interesting example of “positive feedback”, the same authors further demonstrated that lactoferrin expression was up-regulated during HTLV-1 infection, probably in a paracrine manner involving Tax-induced NF-κB activation [62].
5. Ongoing Research on HTLV-1 MTCT and Perspectives
One of the major remaining questions on MTCT concerns the sites of primary passage/infection of HTLV-1 in the digestive tract. The mechanisms of HTLV-1 infection after oral inoculation should be addressed in vivo using a humanized mouse as a model of HTLV-1 infection [63,64,65], in complement to the rabbit model. In particular, combination of histopathological studies and bioluminescence imaging will allow determining the preferential sites of HTLV-1 entry (palatine tonsils, and/or gut). In parallel, the use of transgenic, knock-out, and knock-in mice depleted for different cell types (M cells, dendritic cells, and macrophages) will allow assessing the role of the different cell types in the first steps of infection. These studies will also benefit from an in vitro approach, such as differentiation of M cells from enterocytic cell lines, on compartmentalized culture devices, as already done to show the role of these cells in virus transport across the epithelium [66]. Combination of in vivo/in vitro studies will also allow delineating the role of factors such as milk components (e.g., lactoperoxidase), the proviral load, and the antibody titer in HTLV-1 transport through the intestinal epithelium.
Another perspective concerns the comprehension of the role of breastfeeding duration on HTLV-1 MTCT. It seems rather clear that such a role corresponds to a combination of the cumulative viral input, the changes over time in milk composition in infected cell types, maternal antibodies, and the immune status and maturation of the neonate’s gut. It is known that neonatal life (as well as prenatal) organizes and controls mucosal homeostasis through endogenous and exogenous factors that drive the development and maturation of the intestinal immune system (for a review, see [67]). Recent studies have shown the effects of intestinal microbiota in the development of the immune system and intestinal architecture [68,69]. Gut microbiota could have an impact on HTLV-1 MTCT efficiency, as reported for HIV-1 [70] or Mouse Mammary Tumor Virus [71], as well as the human milk microbiota, which participate in the neonate’s gut microbiota constitution [72].
Altogether, these further studies could delineate new preventive strategies to counteract HTLV-1 MTCT, as well as provide new data on the general mechanisms of pathogenic agents in MTCT.
Acknowledgments
The authors thank Florence Buseyne, Olivier Cassar and Catherine Cecilio for helpful advices and improvement of the manuscript. Florent Percher is a recipient of a DIM Malinf (Région Ile de France) Ph.D. fellowship. Part of this work is financially supported by the Ligue contre le Cancer.
Author Contributions
All the authors conceived and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 Infection. Front. Microbiol. 2012;3:1–23. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson J.H., Edwards A.J., Cruickshank J.K., Rudge P., Dalgleish A.G. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai M., Brennan M.B., Sakai J.A., Mora C.A., Jacobson S. CD8+ T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood. 2001;98:1858–1861. doi: 10.1182/blood.V98.6.1858. [DOI] [PubMed] [Google Scholar]
- 4.Koyanagi Y., Yoshida T., Suzuki M., Uma A., Ananthasubramaniam L., Ramajayam S., Yamamoto N. Dual infection of HIV-1 and HTLV-I in south India: A study on a patient with AIDS-related complex. Microbiol. Immunol. 1993;37:983–986. doi: 10.1111/j.1348-0421.1993.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 5.Afonso P.V., Ozden S., Cumont M.C., Seilhean D., Cartier L., Rezaie P., Mason S., Lambert S., Huerre M., Gessain A., et al. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008;4:40. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carneiro-Proietti A.B., Amaranto-Damasio M.S., Leal-Horiguchi C.F., Bastos R.H., Seabra-Freitas G., Borowiak D.R., Ribeiro M.A., Proietti F.A., Ferreira A.S., Martins M.L. Mother-to-Child transmission of human T-Cell lymphotropic viruses-1/2: What we know, and what are the gaps in understanding and preventing this route of infection. J. Pediatr. Infect. Dis. Soc. 2014;3(Suppl. 1):S24–S29. doi: 10.1093/jpids/piu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E.L., Hanchard B., Figueroa J.P., Gibbs W.N., Lofters W.S., Campbell M., Goedert J.J., Blattner W.A. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 8.Ando Y., Nakano S., Saito K., Shimamoto I., Ichijo M., Toyama T., Hinuma Y. Transmission of adult T-cell leukemia retrovirus (HTLV-I) from mother to child: Comparison of bottle- with breast-fed babies. Jpn. J. Cancer Res. 1987;78:322–324. [PubMed] [Google Scholar]
- 9.Hino S., Sugiyama H., Doi H., Ishimaru T., Yamabe T., Tsuji Y., Miyamoto T. Breaking the cycle of HTLV-I transmission via carrier mothers’ milk. Lancet. 1987;2:158–159. doi: 10.1016/S0140-6736(87)92358-0. [DOI] [PubMed] [Google Scholar]
- 10.Hino S., Katamine S., Miyata H., Tsuji Y., Yamabe T., Miyamoto T. Primary prevention of HTLV-I in Japan. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;13(Suppl. 1):S199–S203. doi: 10.1097/00042560-199600001-00030. [DOI] [PubMed] [Google Scholar]
- 11.Wiktor S.Z., Pate E.J., Rosenberg P.S., Barnett M., Palmer P., Medeiros D., Maloney E.M., Blattner W.A. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J. Hum. Virol. 1997;1:37–44. [PubMed] [Google Scholar]
- 12.Takahashi K., Takezaki T., Oki T., Kawakami K., Yashiki S., Fujiyoshi T., Usuku K., Mueller N., Osame M., Miyata K., et al. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. The Mother-to-Child Transmission Study Group. Int. J. Cancer. 1991;49:673–677. doi: 10.1002/ijc.2910490508. [DOI] [PubMed] [Google Scholar]
- 13.Hino S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): The ATL Prevention Program Nagasaki. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011;87:152–166. doi: 10.2183/pjab.87.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hino S., Katamine S., Miyata H., Tsuji Y., Yamabe T., Miyamoto T. Primary prevention of HTLV-1 in Japan. Leukemia. 1997;11(Suppl. 3):57–59. doi: 10.1097/00042560-199600001-00030. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro M.A., Martins M.L., Teixeira C., Ladeira R., Oliveira Mde F., Januario J.N., Proietti F.A., Carneiro-Proietti A.B. Blocking vertical transmission of human T cell lymphotropic virus type 1 and 2 through breastfeeding interruption. Pediatr. Infect. Dis. J. 2012;31:1139–1143. doi: 10.1097/INF.0b013e318263215e. [DOI] [PubMed] [Google Scholar]
- 16.Hino S., Yamaguchi K., Katamine S., Sugiyama H., Amagasaki T., Kinoshita K., Yoshida Y., Doi H., Tsuji Y., Miyamoto T. Mother-to-child transmission of human T-cell leukemia virus type-I. Jpn. J. Cancer Res. 1985;76:474–480. [PubMed] [Google Scholar]
- 17.Komuro A., Hayami M., Fujii H., Miyahara S., Hirayama M. Vertical transmission of adult T-cell leukaemia virus. Lancet. 1983;1:240. doi: 10.1016/S0140-6736(83)92613-2. [DOI] [PubMed] [Google Scholar]
- 18.Satow Y., Hashido M., Ishikawa K., Honda H., Mizuno M., Kawana T., Hayami M. Detection of HTLV-I antigen in peripheral and cord blood lymphocytes from carrier mothers. Lancet. 1991;338:915–916. doi: 10.1016/0140-6736(91)91775-p. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty J., Clark S., Okonta H., Duggan J. A small animal model for mother-to-fetus transmission of ts1, a murine retrovirus. Viral Immunol. 2003;16:191–201. doi: 10.1089/088282403322017929. [DOI] [PubMed] [Google Scholar]
- 20.Duggan J., Okonta H., Chakraborty J. Transmission of Moloney murine leukemia virus (ts-1) by breast milk. J. Gen. Virol. 2006;87 Pt 9:2679–2684. doi: 10.1099/vir.0.82015-0. [DOI] [PubMed] [Google Scholar]
- 21.Bittner J.J. Relation of nursing to the extra-chromosomal theory of breast cancer in mice. Am. J. Cancer. 1939;35:90–97. [PubMed] [Google Scholar]
- 22.Le Jan C., Bellaton C., Greenland T., Mornex J.F. Mammary transmission of caprine arthritis encephalitis virus: A 3D model for in vitro study. Reprod. Nutr. Dev. 2005;45:513–523. doi: 10.1051/rnd:2005035. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita K., Hino S., Amagaski T., Ikeda S., Yamada Y., Suzuyama J., Momita S., Toriya K., Kamihira S., Ichimaru M. Demonstration of adult T-cell leukemia virus antigen in milk from three sero-positive mothers. Gann. 1984;75:103–105. [PubMed] [Google Scholar]
- 24.Matsubara F., Haraguchi K., Harada K., Koizumi A. Screening for antibodies to human T-cell leukemia virus type I in Japanese breast milk. Biol. Pharm. Bull. 2012;35:773–776. doi: 10.1248/bpb.35.773. [DOI] [PubMed] [Google Scholar]
- 25.Li H.C., Biggar R.J., Miley W.J., Maloney E.M., Cranston B., Hanchard B., Hisada M. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J. Infect. Dis. 2004;190:1275–1278. doi: 10.1086/423941. [DOI] [PubMed] [Google Scholar]
- 26.Yamanouchi K., Kinoshita K., Moriuchi R., Katamine S., Amagasaki T., Ikeda S., Ichimaru M., Miyamoto T., Hino S. Oral transmission of human T-cell leukemia virus type-I into a common marmoset (Callithrix jacchus) as an experimental model for milk-borne transmission. Jpn. J. Cancer Res. 1985;76:481–487. [PubMed] [Google Scholar]
- 27.Kinoshita K., Yamanouchi K., Ikeda S., Momita S., Amagasaki T., Soda H., Ichimaru M., Moriuchi R., Katamine S., Miyamoto T., et al. Oral infection of a common marmoset with human T-cell leukemia virus type-I (HTLV-I) by inoculating fresh human milk of HTLV-I carrier mothers. Jpn. J. Cancer Res. 1985;76:1147–1153. [PubMed] [Google Scholar]
- 28.Uemura Y., Kotani S., Yoshimoto S., Fujishita M., Yano S., Ohtsuki Y., Miyoshi I. Oral transmission of human T-cell leukemia virus type I in the rabbit. Jpn. J. Cancer Res. 1986;77:970–973. [PubMed] [Google Scholar]
- 29.Kato H., Koya Y., Ohashi T., Hanabuchi S., Takemura F., Fujii M., Tsujimoto H., Hasegawa A., Kannagi M. Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats. J. Virol. 1998;72:7289–7293. doi: 10.1128/jvi.72.9.7289-7293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okochi K., Sato H., Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: Seroconversion in recipients. Vox Sang. 1984;46:245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 32.Popovic M., Sarin P.S., Robert-Gurroff M., Kalyanaraman V.S., Mann D., Minowada J., Gallo R.C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 33.Jones K.S., Petrow-Sadowski C., Huang Y.K., Bertolette D.C., Ruscetti F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4+ T cells. Nat. Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 34.Jarvinen K.M., Geller L., Bencharitiwong R., Sampson H.A. Presence of functional, autoreactive human milk-specific IgE in infants with cow’s milk allergy. Clin. Exp. Allergy. 2012;42:238–247. doi: 10.1111/j.1365-2222.2011.03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogra S.S., Weintraub D.I., Ogra P.L. Immunologic aspects of human colostrum and milk: Interaction with the intestinal immunity of the neonate. Adv. Exp. Med. Biol. 1978;107:95–107. doi: 10.1007/978-1-4684-3369-2_12. [DOI] [PubMed] [Google Scholar]
- 36.Proietti F.A., Carneiro-Proietti A.B., Catalan-Soares B.C., Murphy E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita K., Amagasaki T., Hino S., Doi H., Yamanouchi K., Ban N., Momita S., Ikeda S., Kamihira S., Ichimaru M., et al. Milk-borne transmission of HTLV-I from carrier mothers to their children. Jpn. J. Cancer Res. 1987;78:674–680. [PubMed] [Google Scholar]
- 38.Southern S.O., Southern P.J. Persistent HTLV-I infection of breast luminal epithelial cells: A role in HTLV transmission? Virology. 1998;241:200–214. doi: 10.1006/viro.1997.8978. [DOI] [PubMed] [Google Scholar]
- 39.Satomi M., Shimizu M., Shinya E., Watari E., Owaki A., Hidaka C., Ichikawa M., Takeshita T., Takahashi H. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J. Infect. Dis. 2005;191:174–181. doi: 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 40.LeVasseur R.J., Southern S.O., Southern P.J. Mammary epithelial cells support and transfer productive human T-cell lymphotropic virus infections. J. Hum. Virol. 1998;1:214–223. [PubMed] [Google Scholar]
- 41.Takeuchi H., Takahashi M., Norose Y., Takeshita T., Fukunaga Y., Takahashi H. Transformation of breast milk macrophages by HTLV-I: Implications for HTLV-I transmission via breastfeeding. Biomed. Res. 2010;31:53–61. doi: 10.2220/biomedres.31.53. [DOI] [PubMed] [Google Scholar]
- 42.Loureiro P., Southern S.O., Southern P.J., Pombo-de-Oliveira M.S. Clinicopathological studies of a patient with adult T-cell leukemia and pseudogynecomasty. Am. J. Hematol. 2000;65:256–259. doi: 10.1002/1096-8652(200011)65:3<256::AID-AJH14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 43.Buehring G.C., Kramme P.M., Schultz R.D. Evidence for bovine leukemia virus in mammary epithelial cells of infected cows. Lab. Invest. 1994;71:359–365. [PubMed] [Google Scholar]
- 44.Schulz O., Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Takenouchi N., Matsuoka E., Moritoyo T., Nagai M., Katsuta K., Hasui K., Ueno K., Eizuru Y., Usuku K., Osame M., et al. Molecular pathologic analysis of the tonsil in HTLV-I-infected individuals. J. Acquir. Immune Defic. Syndr. 1999;22:200–207. doi: 10.1097/00126334-199910010-00014. [DOI] [PubMed] [Google Scholar]
- 46.Kazanji M. HTLV type 1 infection in squirrel monkeys (Saimiri sciureus): A promising animal model for HTLV type 1 human infection. AIDS Res. Hum. Retrovir. 2000;16:1741–1746. doi: 10.1089/08892220050193245. [DOI] [PubMed] [Google Scholar]
- 47.Haynes R.A., 2nd, Ware E., Premanandan C., Zimmerman B., Yu L., Phipps A.J., Lairmore M.D. Cyclosporine-induced immune suppression alters establishment of HTLV-1 infection in a rabbit model. Blood. 2010;115:815–823. doi: 10.1182/blood-2009-07-230912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zacharopoulos V.R., Perotti M.E., Phillips D.M. Lymphocyte-facilitated infection of epithelia by human T-cell lymphotropic virus type I. J. Virol. 1992;66:4601–4605. doi: 10.1128/jvi.66.7.4601-4605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Latil S., Gnadig N.F., Mallet A., Desdouits M., Guivel-Benhassine F., Jeannin P., Prevost M.C., Schwartz O., Gessain A., Ozden S., et al. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood. 2012;120:572–580. doi: 10.1182/blood-2011-08-374637. [DOI] [PubMed] [Google Scholar]
- 50.Afonso P.V., Ozden S., Prevost M.C., Schmitt C., Seilhean D., Weksler B., Couraud P.O., Gessain A., Romero I.A., Ceccaldi P.E. Human blood-brain barrier disruption by retroviral-infected lymphocytes: Role of myosin light chain kinase in endothelial tight-junction disorganization. J. Immunol. 2007;179:2576–2583. doi: 10.4049/jimmunol.179.4.2576. [DOI] [PubMed] [Google Scholar]
- 51.Hocini H., Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J. Infect. Dis. 1999;179(Suppl. 3):S448–S453. doi: 10.1086/314802. [DOI] [PubMed] [Google Scholar]
- 52.Plancoulaine S., Buigues R.P., Murphy E.L., van Beveren M., Pouliquen J.F., Joubert M., Remy F., Tuppin P., Tortevoye P., de The G., et al. Demographic and familial characteristics of HTLV-1 infection among an isolated, highly endemic population of African origin in French Guiana. Int. J. Cancer. 1998;76:331–336. doi: 10.1002/(SICI)1097-0215(19980504)76:3<331::AID-IJC8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 53.Plancoulaine S., Gessain A., Joubert M., Tortevoye P., Jeanne I., Talarmin A., de The G., Abel L. Detection of a major gene predisposing to human T lymphotropic virus type I infection in children among an endemic population of African origin. J. Infect. Dis. 2000;182:405–412. doi: 10.1086/315741. [DOI] [PubMed] [Google Scholar]
- 54.Jeffery K.J., Usuku K., Hall S.E., Matsumoto W., Taylor G.P., Procter J., Bunce M., Ogg G.S., Welsh K.I., Weber J.N., et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plancoulaine S., Gessain A., Tortevoye P., Boland-Auge A., Vasilescu A., Matsuda F., Abel L. A major susceptibility locus for HTLV-1 infection in childhood maps to chromosome 6q27. Hum. Mol. Genet. 2006;15:3306–3312. doi: 10.1093/hmg/ddl406. [DOI] [PubMed] [Google Scholar]
- 56.Sawada T., Iwahara Y., Ishii K., Taguchi H., Hoshino H., Miyoshi I. Immunoglobulin prophylaxis against milkborne transmission of human T cell leukemia virus type I in rabbits. J. Infect. Dis. 1991;164:1193–1196. doi: 10.1093/infdis/164.6.1193. [DOI] [PubMed] [Google Scholar]
- 57.Ureta-Vidal A., Angelin-Duclos C., Tortevoye P., Murphy E., Lepere J.F., Buigues R.P., Jolly N., Joubert M., Carles G., Pouliquen J.F., et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. Int. J. Cancer. 1999;82:832–836. doi: 10.1002/(SICI)1097-0215(19990909)82:6<832::AID-IJC11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Sawada T., Tohmatsu J., Obara T., Koide A., Kamihira S., Ichimaru M., Kashiwagi S., Kajiyama W., Matsumura N., Kinoshita K., et al. High risk of mother-to-child transmission of HTLV-I in p40tax antibody-positive mothers. Jpn. J. Cancer Res. 1989;80:506–508. doi: 10.1111/j.1349-7006.1989.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirata M., Hayashi J., Noguchi A., Nakashima K., Kajiyama W., Kashiwagi S., Sawada T. The effects of breastfeeding and presence of antibody to p40tax protein of human T cell lymphotropic virus type-I on mother to child transmission. Int. J. Epidemiol. 1992;21:989–994. doi: 10.1093/ije/21.5.989. [DOI] [PubMed] [Google Scholar]
- 60.Hisada M., Maloney E.M., Sawada T., Miley W.J., Palmer P., Hanchard B., Goedert J.J., Manns A. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin. Infect. Dis. 2002;34:1551–1557. doi: 10.1086/340537. [DOI] [PubMed] [Google Scholar]
- 61.Moriuchi M., Moriuchi H. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 2001;166:4231–4236. doi: 10.4049/jimmunol.166.6.4231. [DOI] [PubMed] [Google Scholar]
- 62.Moriuchi M., Moriuchi H. Induction of lactoferrin gene expression in myeloid or mammary gland cells by human T-cell leukemia virus type 1 (HTLV-1) tax: Implications for milk-borne transmission of HTLV-1. J. Virol. 2006;80:7118–7126. doi: 10.1128/JVI.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee P., Tripp A., Lairmore M.D., Crawford L., Sieburg M., Ramos J.C., Harrington W., Jr., Beilke M.A., Feuer G. Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood. 2010;115:2640–2648. doi: 10.1182/blood-2009-10-246959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Villaudy J., Wencker M., Gadot N., Gillet N.A., Scoazec J.Y., Gazzolo L., Manz M.G., Bangham C.R., Dodon M.D. HTLV-1 propels thymic human T cell development in “human immune system” Rag2−/− λc−/− mice. PLoS Pathog. 2011;7:40. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodon M.D., Villaudy J., Gazzolo L., Haines R., Lairmore M. What we are learning on HTLV-1 pathogenesis from animal models. Front. Microbiol. 2012;3:320. doi: 10.3389/fmicb.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ouzilou L., Caliot E., Pelletier I., Prevost M.C., Pringault E., Colbere-Garapin F. Poliovirus transcytosis through M-like cells. J. Gen. Virol. 2002;83 Pt 9:2177–2182. doi: 10.1099/0022-1317-83-9-2177. [DOI] [PubMed] [Google Scholar]
- 67.Renz H., Brandtzaeg P., Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 68.Van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 69.Maranduba C.M., De Castro S.B., de Souza G.T., Rossato C., da Guia F.C., Valente M.A., Rettore J.V., Maranduba C.P., de Souza C.M., do Carmo A.M., et al. Intestinal microbiota as modulators of the immune system and neuroimmune system: Impact on the host health and homeostasis. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/931574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shu Z., Ma J., Tuerhong D., Yang C., Upur H. How intestinal bacteria can promote HIV replication. AIDS Rev. 2013;15:32–37. [PubMed] [Google Scholar]
- 71.Kane M., Case L.K., Kopaskie K., Kozlova A., MacDearmid C., Chervonsky A.V., Golovkina T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandez L., Langa S., Martin V., Jimenez E., Martin R., Rodriguez J.M. The microbiota of human milk in healthy women. Cell Mol. Biol. 2013;59:31–42. [PubMed] [Google Scholar]