Abstract
A hybridization-based point-of-care (POC) assay for HIV-1 drug resistance would be useful in low- and middle-income countries (LMICs) where resistance testing is not routinely available. The major obstacle in developing such an assay is the extreme genetic variability of HIV-1. We analyzed 27,203 reverse transcriptase (RT) sequences from the Stanford HIV Drug Resistance Database originating from six LMIC regions. We characterized the variability in a 27-nucleotide window surrounding six clinically important drug resistance mutations (DRMs) at positions 65, 103, 106, 181, 184, and 190. The number of distinct codons at each DRM position ranged from four at position 184 to 11 at position 190. Depending on the mutation, between 11 and 15 of the 24 flanking nucleotide positions were variable. Nonetheless, most flanking sequences differed from a core set of 10 flanking sequences by just one or two nucleotides. Flanking sequence variability was also lower in each LMIC region compared with overall variability in all regions. We also describe an online program that we developed to perform similar analyses for mutations at any position in RT, protease, or integrase.
Keywords: HIV-1, drug resistance mutation, variability, point-of-care
1. Introduction
The increasing prevalence of acquired and transmitted HIV-1 drug resistance is an obstacle to successful antiretroviral (ARV) therapy in the low- and middle-income countries (LMICs) hardest hit by the HIV-1 pandemic [1]. Genotypic drug resistance testing could facilitate the choice of initial ARV therapy in areas with rising transmitted drug resistance (TDR) and enable care-providers to determine which individuals with virological failure on a first- or second-line ARV regimen require a treatment change. Despite the decreasing costs of standard genotypic resistance testing and next-generation sequencing (NGS), these assays remain prohibitively complex and costly for many LMICs [2,3]. Additionally, the dependency on batching samples to reduce the cost of NGS is a disadvantage when timeliness is desired [4]. An inexpensive point-of-care (POC) genotypic resistance test would be useful in settings where the resources, capacity, and infrastructure to perform standard genotypic drug resistance testing or NGS are limited. A POC genotypic resistance test would be particularly useful in conjunction with the POC HIV-1 viral load tests that are currently being introduced in LMICs [5,6,7].
A POC genotypic resistance test is likely to involve the use of a hybridization-based point mutation assay for detecting the most clinically significant drug-resistance mutations (DRMs) [8,9,10,11]. Preliminary data suggests that a set of six reverse transcriptase (RT) DRMs—the nucleoside reverse transcriptase inhibitor (NRTI)-associated DRMs K65R and M184V and the non-nucleoside reverse transcriptase inhibitor (NNRTI)-associated DRMs K103N, V106M, Y181C and G190A—are about 60% sensitive for detecting intermediate or high-level TDR and 99% sensitive for detecting intermediate or high-level acquired drug resistance (ADR) in patients with virological failure on a first-line WHO recommended NRTI/NNRTI containing regimen [12]. The major obstacle to the development of a hybridization-based assay is the extreme genetic variability of HIV-1 [11,13]. Here we characterize the genetic variability at and surrounding each of the six DRMs mentioned above and introduce a web-based program that allows researchers to perform analyses similar to those we present here.
2. Materials and Methods
2.1. Sequence Selection
We analyzed group M HIV-1 plasma RT sequences from the Stanford HIV Drug Resistance Database (HIVDB) [14]. Sequences were characterized by the country of origin and year of collection. Sequences were assigned to one of the following six LMIC regions: Southern Africa, Central Africa, Eastern Africa, Western Africa, India, and the LMICs of South and Southeast Asia [15]. Isolates were assigned a subtype using the Rega Subtyping tool and the annotation provided by authors.
2.2. Analysis of Codons
Codon variability was characterized by the proportions of distinct nucleotide triplets encoding either wild type or mutant residues at each DRM position. Because there are well known examples of inter-subtype differences in the proportions of codons at several drug-resistance positions [16], we examined codon variability within each of the seven most common subtypes: A, B, C, D, G, CRF01_AE, and CRF02_AG. Codons that included electrophoretic nucleotide mixtures were not included.
2.3. Analysis of Flanking Segments
We examined a span of 27 nucleotides encompassing each drug-resistance position as well as 12 upstream and 12 downstream nucleotides. These flanking nucleotides are important for hybridization strategies relying on a terminal 3’ mismatch for either positive or negative-stranded cDNA and for those that rely on a central mismatch [11,17,18].
We defined the positional variability of flanking segments—the 12 upstream and downstream nucleotides—as the proportions of nucleotides at each of the 24 flanking nucleic acid positions. To represent positional variability, we generated sequence logos with heights proportional to the information content at each nucleotide position [19].
We defined the segmental variability of flanking segments as the distribution of distinct haplotypes flanking each DRM position. For this analysis, we determined how many distinct haplotypes were present in the complete dataset and the extent to which haplotype diversity segregated with geographic region. The 10, 25, and 100 most common haplotypes were referred to as universal if they were from the complete set of sequences from the six LMIC regions or regional if there were from one of the six LMIC regions.
3. Results
3.1. Sequences
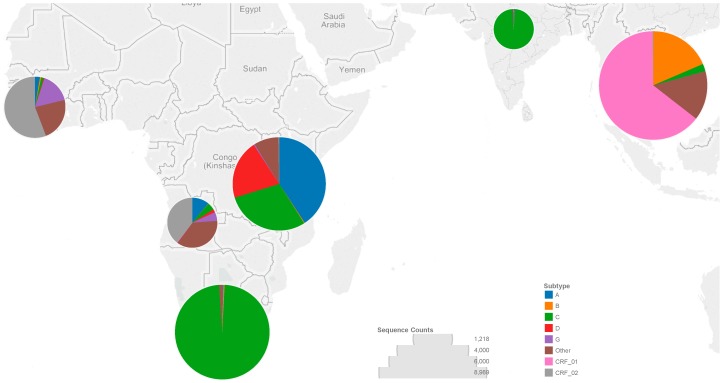
We analyzed 27,203 HIV-1 RT sequences from as many individuals from six LMIC regions. Overall, 32% of sequences were from the LMICs of South and Southeast Asia, 22% from Southern Africa, 25% from Eastern Africa, 10% from Western Africa, 7% from Central Africa, and 4% from India (Figure 1). The most common subtypes were subtype C (35%), CRF01_AE (21%), A (11%), CRF02_AG (9%), B (6%), D (5%), and G (2%). Less common subtypes or circulating recombinant forms (CRFs) comprised 11% of sequences. Sequences were from 18,564 (68%) untreated individuals, 7551 (28%) treated individuals and 1088 (4%) individuals with unknown treatment status.
Figure 1.
The number of sequences from each low- and middle-income country (LMIC) region corresponds to the diameter of the circle overlying each region, as indicated by the circle diameters in the “Sequence Counts” legend. The colors making up each circle correspond to the proportion of each subtype or circulating recombinant form (CRF) in that region, as indicated in the “Subtype” legend.
3.2. Codons
Table 1 shows the proportions of distinct wild type and mutant codons at each DRM position present in ≥1% of sequences for any of the seven most common subtypes or CRFs. In addition to K65R, K103N, V106M, Y181C, M184V, and G190A, these six positions also encode the following less common DRMs: K65N, K103S, V106A, Y181I/V, M184I, and G190S/E/Q and two polymorphic mutations, K103R and V106I, that do not confer significant drug resistance. The total number of distinct wild type and mutant codons at each DRM position ranged from four for position 184 to 11 for position 190.
Table 1.
Wild type and mutant codon frequency by subtype.
| DRM Position | Codon | AA | A n = 2968 | B n = 1725 | C n = 9405 | D n = 1355 | G n = 597 | CRF_01 n = 5590 | CRF_02 n = 2342 |
|---|---|---|---|---|---|---|---|---|---|
| 65 | WT (23,365; 98.1) | ||||||||
| AAA | K | 96.7 | 97.8 | 0.9 | 97.6 | 98.8 | 98.9 | 97.7 | |
| AAG | K | 3.3 | 2.2 | 99.1 | 2.4 | 1.2 | 1.1 | 2.4 | |
| Mutant (446; 1.9) | |||||||||
| AGA | R | 84.6 | 100 | 3.7 | 100 | 100 | 85.9 | 93.1 | |
| AGG | R | 7.7 | 0 | 95.6 | 0 | 0 | 1.9 | 3.5 | |
| AAT | N | 7.7 | 0 | 0.7 | 0 | 0 | 2.8 | 0 | |
| AAC | N | 0 | 0 | 0 | 0 | 0 | 9.4 | 3.5 | |
| Total coverage | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ||
| 103 | WT (20,748; 89.8) | ||||||||
| AAA | K | 95.6 | 95.7 | 91.6 | 96.5 | 92.2 | 95.7 | 98.0 | |
| AAG | K | 4.2 | 2.1 | 6.4 | 3.2 | 6.8 | 3.8 | 1.7 | |
| AGA | R | 0.3 | 2.3 | 2.0 | 0.2 | 1.0 | 0.6 | 0.2 | |
| Mutant (2369; 10.3) | |||||||||
| AAC | N | 84.1 | 77.8 | 77.3 | 75.5 | 80.8 | 77.6 | 82.6 | |
| AAT | N | 11.2 | 17.8 | 18.5 | 20.4 | 19.2 | 19.2 | 16.9 | |
| AGC | S | 4.7 | 4.3 | 4.3 | 2.0 | 0 | 2.5 | 0.5 | |
| ACA | T | 0 | 0 | 0 | 2.0 | 0 | 0.7 | 0 | |
| Total coverage | 99.83 | 99.58 | 99.65 | 99.92 | 99.28 | 99.64 | 99.96 | ||
| 106 | WT (22,427; 96.0) | ||||||||
| GTA | V | 97.51 | 90.1 | 13.3 | 95.4 | 96.2 | 86.4 | 97.4 | |
| GTG | V | 1.7 | 2.6 | 86.6 | 4.0 | 1.2 | 8.5 | 1.9 | |
| ATA | I | 0.8 | 7.4 | 0.2 | 0.6 | 2.6 | 5.2 | 0.7 | |
| Mutant (926; 3.4) | |||||||||
| GCA | A | 85.7 | 70.8 | 0.4 | 80.0 | 90.9 | 37.5 | 75.0 | |
| GCG | A | 0 | 0 | 2.4 | 0 | 0 | 0 | 0 | |
| ATG | M | 14.3 | 29.2 | 97.2 | 20.0 | 9.1 | 62.5 | 25.0 | |
| Total coverage | 99.49 | 99.64 | 99.34 | 99.55 | 100 | 99.48 | 99.66 | ||
| 181 | WT (21,972; 93.5) | ||||||||
| TAT | Y | 95.7 | 97.5 | 96.3 | 95.5 | 10.0 | 98.4 | 8.6 | |
| TAC | Y | 4.3 | 2.5 | 3.8 | 4.5 | 90.0 | 1.6 | 91.4 | |
| Mutant (1541; 6.6) | |||||||||
| TGT | C | 81.8 | 96.4 | 88.3 | 88.4 | 9.4 | 86.5 | 8.5 | |
| TGC | C | 7.3 | 0.9 | 4.3 | 4.7 | 87.1 | 3.0 | 87.3 | |
| ATT | I | 5.5 | 0.9 | 3.9 | 0 | 0 | 4.8 | 0 | |
| ATC | I | 0 | 0.9 | 0.2 | 0 | 2.4 | 0.2 | 2.1 | |
| GTT | V | 5.5 | 0.9 | 3.2 | 7.0 | 0 | 5.5 | 0.7 | |
| GTC | V | 0 | 0 | 0 | 0 | 1.2 | 0.2 | 1.4 | |
| Total coverage | 100.0 | 99.9 | 100.0 | 100.0 | 99.8 | 100.0 | 100.0 | ||
| 184 | WT (19,231; 81.0) | ||||||||
| ATG | M | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Mutant (4498; 19.0) | |||||||||
| GTG | V | 90.3 | 78.1 | 90.2 | 89.4 | 83.3 | 81.5 | 87.6 | |
| GTA | V | 9.7 | 7.3 | 6.9 | 9.6 | 15.4 | 14.7 | 10.4 | |
| ATA | I | 0 | 14.6 | 3.0 | 1.0 | 1.3 | 3.8 | 2.0 | |
| Total coverage | 100.0 | 99.5 | 99.9 | 99.9 | 99.8 | 99.8 | 99.9 | ||
| 190 | WT (22,097; 94.7) | ||||||||
| GGA | G | 95.2 | 95.3 | 95.4 | 96.4 | 90.1 | 94.6 | 92.8 | |
| GGC | G | 1.5 | 3.2 | 1.3 | 0.5 | 1.3 | 3.4 | 1.6 | |
| GGG | G | 3.3 | 1.5 | 3.3 | 3.1 | 8.6 | 2.0 | 5.7 | |
| Mutant (1243; 5.3) | |||||||||
| GCA | A | 92.9 | 70.7 | 83.4 | 89.7 | 92.3 | 87.9 | 89.0 | |
| GCG | A | 0 | 1.2 | 1.7 | 3.5 | 2.6 | 2.2 | 1.4 | |
| GCC | A | 0 | 2.4 | 0.8 | 0 | 0 | 1.2 | 0 | |
| AGC | S | 2.9 | 24.4 | 3.9 | 3.5 | 2.6 | 2.7 | 2.7 | |
| AGT | S | 0 | 1.2 | 1.9 | 0 | 2.6 | 1.9 | 2.7 | |
| TCA | S | 1.4 | 0 | 0.9 | 0 | 0 | 1.5 | 1.4 | |
| GAA | E | 2.9 | 0 | 4.5 | 0 | 0 | 1.7 | 2.7 | |
| CAA | Q | 0 | 0 | 3.0 | 3.5 | 0 | 1.0 | 0 | |
| Total coverage | 99.7 | 99.9 | 99.5 | 99.6 | 99.1 | 99.8 | 99.8 |
The frequency of all codons of at least 1% frequency in any of the seven most common subtypes or circulating recombinant forms (CRFs) are shown by subtype for both wild type and mutant codons. The 23,982 sequences from the most seven most common subtypes or CRFs were included in this analysis. Within the analysis of each drug resistance mutation (DRM position, sequences bearing mixtures in the codon of interest were excluded. The number and proportion of wild type and mutant sequences used in the analysis of each DRM are listed in the Codon columns (N; %). Total coverage represents the number of codons from all sequences in the database of that subtype that would match one of the codons listed here for that DRM position. Notable inter-subtype differences in codon frequencies appear in bold font. Abbreviations: AA, amino acid; WT, wild type.
At position 65, the wild type lysine (K) is encoded by AAG in 99% of subtype C sequences but by AAA in >95% of the sequences of the other subtypes. At position 106, the wild type valine (V) is encoded by GTG in 87% of subtype C sequences but by GTA in >85% of other subtype sequences. At position 181, the wild type tyrosine (Y) is encoded by TAC in >90% of subtypes G and CRF02_AG sequences but by TAT in >95% of other subtype sequences. Each of these silent nucleotide changes results in a predisposition for a different subtype-specific mutant variant. At position 106, this predisposition leads to an increased prevalence of the DRM V106M in subtype C viruses (Table 1; [20]). In most other subtypes the dominant mutation is V106A, which results in intermediate efavirenz and high-level nevirapine resistance, whereas V106M results in high-level resistance to both NNRTIs [14,21].
3.3. Flanking Segments
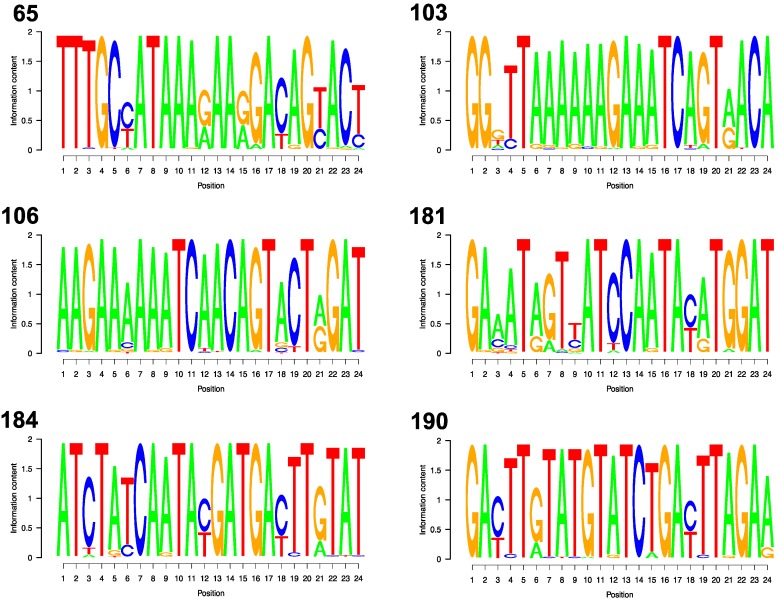
Figure 2 shows the sequence logos for each of the six DRM codons. The mean information content per position was 1.72 surrounding position 65, 1.77 for position 103, 1.82 for position 106, 1.68 for 181, 1.79 for 184, and 1.81 for 190. Depending on the DRM, between 11 and 15 of the 24 flanking nucleotide positions were variable, defined as having two or more nucleotides with ≥1% prevalence: codon 65 (13 variable positions), codon 103 (15 variable positions), codon 106 (14 variable positions), codon 181 (12 variable positions), codon 184 (11 variable positions), and codon 190 (13 variable positions). However, at most variable nucleotide positions (58% to 85% depending on the DRM) variability resulted only from transitions (the presence of A and G or C and T) which would result in mismatched base pairs (A:C and G:T) that do not cause the most severe disruption of hybridization [22,23].
Figure 2.
For each DRM Position, the variability at each of the 24 nucleotide positions making up the flanking segments are shown. The letter(s) shown at each nucleotide position indicate which of the four nucleotides are found at that position among all low and middle income country sequences. The relative sizes of the letters indicate their frequency at the position that was weighted by the information content of the position. Therefore, the combined height of the letter(s) in each nucleotide position corresponds to the information content of the position.
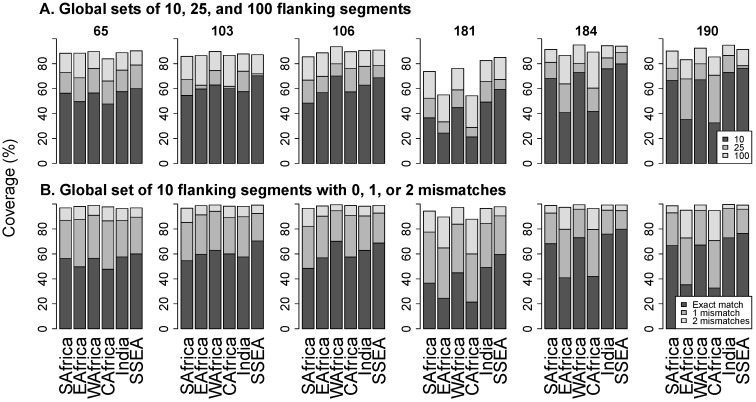
Figure 3A contains stacked bar plots that show the proportions of flanking segments that exactly match the 10, 25, and 100 most common universal flanking segments pooled from all LMIC regions. The figure shows that the universal set of 10 flanking segments exactly match from a mean of 39.3% sequences at position 181 to 63.2% at position 184. Even the 100 most common flanking segments exactly match from just 71% of sequences at position 181 to 92% at position 184.
Figure 3.
Panel (A) shows the proportion of sequences that exactly match the 10 (black bar), 25 (dark grey), and 100 (light grey) most common universal flanking segments overall. Panel (B) shows the proportion of sequences that exactly match (black bar), differ by one nucleotide (dark grey), or differ by two nucleotides (light grey) from the 10 most common universal flanking sequences overall. Abbreviations: SAfrica, Southern Africa; EAfrica, East Africa; WAfrica, West Africa; CAfrica, Central Africa; SSEA, South and Southeast Asia.
Figure 3B contains stacked bar plots that show the proportions of flanking segments that exactly match, differ by one nucleotide, or differ by two nucleotides from a universal set of 10 flanking segments. The proportion of sequences with up to one mismatch with any member of the universal set ranged from a mean of 77% at position 181 to 90% at position 103. The proportion of sequences with up to two mismatches with the set ranged from a mean of 94% at position 181 to 98% at position 184.
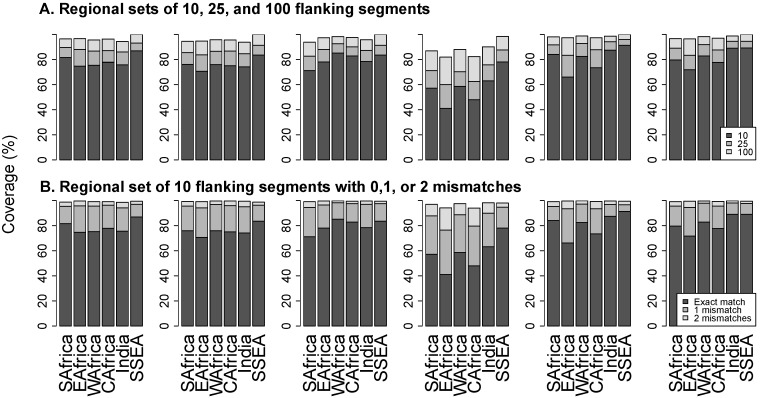
Figure 4A contains stacked bar plots that show the proportions of flanking segments that exactly match the 10, 25, and 100 most common regional flanking segments. The proportion of exact matches for the regional set of 10 flanking segments ranged from a mean of 58% at position 181 to 82% at position 190. The proportion of exact matches for the 100 most common flanking sequences ranged from a mean of 88% at position 181 to 98% at position 184.
Figure 4.
Panel (A) shows the proportion of sequences that exactly match the 10 (black bar), 25 (dark grey), and 100 (light grey) most common regional flanking segments. Panel (B) shows the proportion of sequences that exactly match (black bar), differ by one nucleotide (dark grey), or differ by two nucleotides (light grey) from the 10 most common regional flanking sequences. Abbreviations: SAfrica, Southern Africa; EAfrica, East Africa; WAfrica, West Africa; CAfrica, Central Africa; SSEA, South and Southeast Asia.
Figure 4B contains stacked bar plots that show the proportion of sequences that exactly match, differ by one nucleotide, or differ by two nucleotides from regional sets of 10 flanking segments. The proportion of sequences with up to one mismatch with the set ranged from a mean of 86% at position 181 to 97% at position 106. The proportion of sequences with up to two mismatches with the set ranged from a mean of 97% at position 181 to 99% at position 106.
3.4. Online Program
The set of 27,203 RT sequences used for our analysis is available at [24]. An online program that allows users to retrieve: (1) the proportions of codons at a specified position in protease, RT, and integrase according to geographic region and/or subtype; and (2) the proportions of 5' and 3' flanking sequence segments according to segment size, geographic region, and/or subtype is also available at the URL above.
4. Discussion
The main challenge in developing hybridization-based point mutation assays for detecting HIV-1 drug resistance mutations is the sequence variability at and surrounding each DRM [11,13]. This genetic variability interfered with the clinical uptake of two previously developed hybridization-based assays: The Affymetrix GeneChip HIV PRT 440 and the Innogenetics INNO-LiPA HIV-1 RT assays [25,26]. However, there has been renewed interest in developing a low cost point-mutation assay for detecting key drug-resistance mutations in LMIC settings [8,9,10,11].
Our analysis characterizes the extent and nature of the sequence variability at and surrounding six candidate POC DRMs by position, subtype, region, and nature of hybridization mismatches. Overall 42 codons at positions 65, 103, 106, 181, 184, and 190 occur in 1% or more sequences of the seven most common subtypes; 13 of these encode the six major DRMs proposed to be most useful for a POC mutation assay. Although the phenotypic effect of these DRMs is likely similar between subtypes, the inter-subtype differences in the surrounding sequences may lead to subtle variations in ARV therapy susceptibilities [27]. Additionally, important differences in codon preference were noted by subtype, including those with clinical implications, and these should be considered in assay development [14,21].
Although the sequence variability surrounding each drug-resistance position may present a more formidable challenge than the variability at the codons of interest, our analysis suggests that most of this variability results from haplotypes that differ from a core set of haplotypes at just one or two positions. Therefore, if the stringency for DRM discrimination can be preserved while allowing for one or two flanking segment mismatches, sensitivity can be increased while maintaining specificity. Our analysis and online program may also identify positions at which degenerate and/or universal bases would be most useful [28,29]. Our analyses also suggest that assays with a flexible design, in that they enabled the use of different probe sets in different regions, would also have increased sensitivity.
5. Conclusions
We have described the sequence variability at and surrounding six clinically important HIV-1 DRM positions in a way that identifies several potentially useful strategies for hybridization-based assay development. Additionally, our publicly available online program will allow researchers to perform similar customized analyses to target any HIV-1 DRM position.
Acknowledgments
Dana S. Clutter was supported by a training grant (T32 AI052073) from the NIH and a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083 AND NIH UL1 TR 001085). Soo-Yon Rhee and Robert W. Shafer were supported in part by a grant from the Bill and Melinda Gates Foundation and from the NIH (AI068581). Patricia Rojas Sánchez was supported by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) 2015 Mobility Program.
Author Contributions
Dana S. Clutter contributed to analysis plan, analyzed data and wrote the manuscript, Patricia Rojas Sánchez developed the online program, and reviewed the manuscript, Soo-Yon Rhee contributed to the online program development, and reviewed the manuscript, Robert W. Shafer conceived the analysis plan, analyzed the data, and contributed to the writing of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Rhee S.Y., Blanco J.L., Jordan M.R., Taylor J., Lemey P., Varghese V., Hamers R.L., Bertagnolio S., de Wit T.F., Aghokeng A.F., et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: An individual-patient- and sequence-level meta-analysis. PLoS Med. 2015;12:48. doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronze M., Aitken S.C., Wallis C.L., Steegen K., Stuyver L.J., de Wit T.F., Stevens W. Evaluation of an affordable HIV-1 virological failure assay and antiretroviral drug resistance genotyping protocol. J. Virol. Methods. 2013;194:300–307. doi: 10.1016/j.jviromet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Aitken S.C., Bronze M., Wallis C.L., Stuyver L., Steegen K., Balinda S., Kityo C., Stevens W., Rinke de Wit T.F., Schuurman R. A pragmatic approach to HIV-1 drug resistance determination in resource-limited settings by use of a novel genotyping assay targeting the reverse transcriptase-encoding region only. J. Clin. Microbiol. 2013;51:1757–1761. doi: 10.1128/JCM.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapointe H.R., Dong W., Lee G.Q., Bangsberg D.R., Martin J.N., Mocello A.R., Boum Y., Karakas A., Kirkby D., Poon A.F., et al. HIV drug resistance testing by high-multiplex “wide” sequencing on the MiSeq instrument. Antimicrob. Agents Chemother. 2015;59:6824–6833. doi: 10.1128/AAC.01490-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie A.V., Ushiro-Lumb I., Edemaga D., Joshi H.A., de Ruiter A., Szumilin E., Jendrulek I., McGuire M., Goel N., Sharma P.I., et al. Samba HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J. Clin. Microbiol. 2014;52:3377–3383. doi: 10.1128/JCM.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott L., Gous N., Carmona S., Stevens W. Laboratory evaluation of the liat HIV Quant (IQuum) whole-blood and plasma HIV-1 viral load assays for point-of-care testing in South Africa. J. Clin. Microbiol. 2015;53:1616–1621. doi: 10.1128/JCM.03325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haleyur Giri Setty M.K., Hewlett I.K. Point of care technologies for HIV. AIDS Res. Treat. 2014;2014 doi: 10.1155/2014/497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panpradist N., Beck I., Chung M., Kiarie J., Frenkel L.M., Lutz B.R. Simplified paper format for detecting HIV drug resistance in clinical specimines by oligonucleotide ligation assay (OLA); Proceedings of the 2015 International HIV Drug Resistance Workshop; Seattle, WA, USA. 21–22 February 2015; Seattle, WA, USA: IHL Press; 2015. [Google Scholar]
- 9.MacLeod I.J., Rowley C.F., Essex M. Pan degenerate amplification and adaptation for highly sensitive detection of ARV drug resistance; Proceedings of the 2014 Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 3–6 March 2014; Boston, MA, USA: IAS-USA; 2014. Abstract Number 606. [Google Scholar]
- 10.Morabito K., Kantor R., Tai W., Schreier L., Tripathi A. Detection of HIV-1 minority variants containing the K103N drug-resistance mutation using a simple method to amplify RNA targets (SMART) J. Mol. Diagn. 2013;15:401–412. doi: 10.1016/j.jmoldx.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G., Cai F., Zhou Z., DeVos J., Wagar N., Diallo K., Zulu I., Wadonda-Kabondo N., Stringer J.S., Weidle P.J., et al. Simultaneous detection of major drug resistance mutations in the protease and reverse transcriptase genes for HIV-1 subtype C by use of a multiplex allele-specific assay. J. Clin. Microbiol. 2013;51:3666–3674. doi: 10.1128/JCM.01669-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee S.Y., Jordan M.R., Raizes E., Chua A., Parkin N., Kantor R., van Zyl G.U., Mukui I., Hosseinipour M.C., Frenkel L.M., et al. HIV-1 drug resistance mutations: Potential applications for point-of-care genotypic resistance testing. PLoS ONE. 2015;10:48. doi: 10.1371/journal.pone.0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal R.B., Chakrabarti S., Yang C. Impact of genetic diversity of HIV-1 on diagnosis, antiretroviral therapy & vaccine development. Indian J. Med. Res. 2005;121:287–314. [PubMed] [Google Scholar]
- 14.Rhee S.Y., Gonzales M.J., Kantor R., Betts B.J., Ravela J., Shafer R.W. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31:298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations Geoscheme. [(accessed on 30 September 2015)]. Available online: https://en.wikipedia.org/wiki/United_Nations_geoscheme.
- 16.Martinez-Cajas J.L., Pai N.P., Klein M.B., Wainberg M.A. Differences in resistance mutations among HIV-1 non-subtype B infections: A systematic review of evidence (1996–2008) J. Int. AIDS Soc. 2009;12 doi: 10.1186/1758-2652-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck I.A., Crowell C., Kittoe R., Bredell H., Machaba M., Willamson C., Janssens W., Jallow S., van der Groen G., Shao Y., et al. Optimization of the oligonucleotide ligation assay, a rapid and inexpensive test for detection of HIV-1 drug resistance mutations, for non-north american variants. J. Acquir. Immune Defic. Syndr. 2008;48:418–427. doi: 10.1097/QAI.0b013e31817ed7d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadiwikarta W.W., van Dorst B., Hollanders K., Stuyver L., Carlon E., Hooyberghs J. Targeted resequencing of HIV variants by microarray thermodynamics. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider T.D., Stephens R.M. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner B., Turner D., Oliveira M., Moisi D., Detorio M., Carobene M., Marlink R.G., Schapiro J., Roger M., Wainberg M.A. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:F1–F5. doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Melikian G.L., Rhee S.Y., Varghese V., Porter D., White K., Taylor J., Towner W., Troia P., Burack J., Dejesus E., et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: Implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J. Antimicrob. Chemother. 2014;69:12–20. doi: 10.1093/jac/dkt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piao X., Sun L., Zhang T., Gan Y., Guan Y. Effects of mismatches and insertions on discrimination accuracy of nucleic acid probes. Acta Biochim. Pol. 2008;55:713–720. [PubMed] [Google Scholar]
- 23.Stadhouders R., Pas S.D., Anber J., Voermans J., Mes T.H., Schutten M. The effect of primer-template mismatches on the detection and quantification of nucleic acids using the 5' nuclease assay. J. Mol. Diagn. 2010;12:109–117. doi: 10.2353/jmoldx.2010.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clutter D.S., Sánchez P.R., Rhee S.Y., Shafer R.W. Variability in HIV-1 Sequences from Low & Middle Income Countries (LMICs) [(accessed on 9 February 2016)]. Available online: http://hivdb.stanford.edu/cgi-bin/Probe.cgi.
- 25.Servais J., Lambert C., Fontaine E., Plesseria J.M., Robert I., Arendt V., Staub T., Schneider F., Hemmer R., Burtonboy G., et al. Comparison of DNA sequencing and a line probe assay for detection of human immunodeficiency virus type 1 drug resistance mutations in patients failing highly active antiretroviral therapy. J. Clin. Microbiol. 2001;39:454–459. doi: 10.1128/JCM.39.2.454-459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahey M., Nau M.E., Barrick S., Cooley J.D., Sawyer R., Sleeker A.A., Vickerman P., Bloor S., Larder B., Michael N.L., et al. Performance of the Affymetrix GeneChip HIV PRT 440 platform for antiretroviral drug resistance genotyping of human immunodeficiency virus type 1 clades and viral isolates with length polymorphisms. J. Clin. Microbiol. 1999;37:2533–2537. doi: 10.1128/jcm.37.8.2533-2537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basson A.E., Rhee S.Y., Parry C.M., El-Khatib Z., Charalambous S., de Oliveira T., Pillay D., Hoffmann C., Katzenstein D., Shafer R.W., et al. Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2015;59:960–971. doi: 10.1128/AAC.04215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orum H., Wengel J. Locked nucleic acids: A promising molecular family for gene-function analysis and antisense drug development. Cur. Opin. Mol. Ther. 2001;3:239–243. [PubMed] [Google Scholar]
- 29.Kumar T.S., Myznikova A., Samokhina E., Astakhova I.K. Rapid genotyping using pyrene-perylene locked nucleic acid complexes. Artif. DNA PNA XNA. 2013;4:58–68. doi: 10.4161/adna.25903. [DOI] [PMC free article] [PubMed] [Google Scholar]