Abstract
Antiretroviral therapy (ART) has led to dramatic improvements in the lives of HIV-infected persons. However, residual immune activation, which persists despite ART, is associated with increased risk of non-AIDS morbidities. Accumulating evidence shows that disruption of the gut mucosal epithelium during SIV/HIV infections allows translocation of microbial products into the circulation, triggering immune activation. This disruption is due to immune, structural and microbial alterations. In this review, we highlighted the key findings of gut mucosa studies of SIV-infected macaques and HIV-infected humans that have revealed virus-induced changes of intestinal CD4, CD8 T cells, innate lymphoid cells, myeloid cells, and of the local cytokine/chemokine network in addition to epithelial injuries. We review the interplay between the host immune response and the intestinal microbiota, which also impacts disease progression. Collectively, these studies have instructed clinical research on early ART initiation, modifiers of microbiota composition, and recombinant cytokines for restoring gut barrier integrity.
Keywords: HIV, SIV, Gut barrier integrity, Microbial translocation, Interleukin-7, Interleukin-21
Highlights
-
•
SIV/HIV-induced multiple gut damage contributes to impaired containment of gut luminal microbiota.
-
•
Early antiretroviral therapy (ART) does not fully restore HIV-induced gut damage.
-
•
Novel therapies that restore gut barrier integrity are needed to reduce microbial translocation and inflammation.
1. Introduction
The human gastrointestinal (GI) tract has coevolved with billions of commensal microorganisms that are considered by many immunologists to be an “extended self” (Reynolds et al., 2015). This symbiotic relationship between microorganisms and the host requires efficient barrier and tolerance mechanisms that preserve intestinal immune balance and tissue integrity. HIV infection leads to disruption of both immune balance and epithelial barrier integrity, contributing to residual immune activation (Younas et al., 2015). Such immune activation persists despite ART and is associated with an increased risk of non-AIDS morbidities, such as cardiovascular diseases and neurocognitive disorders. This persistent immune activation is thought to originate from latently infected cells contributing to residual HIV expression, and gut barrier damage. Cumulative evidence indicates that multiple events occur within the GI tract of pathogenically Simian Immunodeficiency Virus (SIV)-infected Asian macaques and HIV-infected individuals (Estes et al., 2010, Brenchley, 2013). These events cause increased intestinal permeability, resulting in the translocation of bacterial products of the GI microflora through the epithelial barrier and into the systemic circulation. This microbial translocation is considered to be a major driver of chronic immune activation and disease progression, even in patients receiving ART (Brenchley et al., 2006, Vyboh et al., 2015).
Our understanding of SIV- and HIV-induced immune gut damage was primarily based on CD4 T cell studies, with changes in CD8 T cells and myeloid populations being relatively less studied. Herein, we highlight the key findings of gut mucosa studies of SIV-infected rhesus macaques (RMs) and HIV-infected subjects that have revealed changes in the distribution of Th17, Treg, Th22, CD8 T cells, innate lymphoid cells and myeloid cells, in addition to the disruption of the local cytokine/chemokine network. We also review studies describing SIV/HIV-induced injuries to the intestinal epithelium, changes in the composition of the gut microbiota, and/or translocation of gut microbial products. Our findings underline the importance of designing novel therapies that target microbial composition and gut barrier integrity to further control immune activation that in turn will improve the quality of life of HIV-infected individuals, while potentially reducing the size of the HIV reservoir.
2. Gut Damage in Conditions Other Than HIV: Learning From “Outside the Box”
Early in the HIV pandemic, GI dysfunctions were reported in patients progressing to AIDS. These dysfunctions included nutrient malabsorption, diarrhea and weight loss, all of which pointed to a link between the disruption of immune homeostasis and gut damage (Kotler et al., 1984). Initial descriptions of the pathological changes observed in patients with advanced HIV infection evoked GI manifestations of Crohn's disease and ulcerative colitis, two major forms of inflammatory bowel disease (IBD), (Ho et al., 2014). Both IBD and HIV infections can be considered as conditions involving a breakage in the mutualism between the host and the gut microbiota. Graft-versus-host disease (GVHD) is also characterized by features of gut damage including immunosuppression, alteration of the composition of the intestinal microbiota, and microbial translocation, all of which lead to systemic inflammation (Jenq et al., 2012).
These findings raise questions regarding the determinants of immune tolerance towards the intestinal microbiota. Due to limitations in assessing mucosal immune responses in patients, non-human primate (NHP) models of SIV-related gut damage have played a critical role in understanding the interplay between microbiota, gut barrier integrity and systemic immune responses. In this review, we synthesize the studies of mucosal immunity in NHP models and discuss their relevance to HIV pathogenesis.
3. Gut Damage in SIV Infection: A Mucosal Tragedy
3.1. Non-human Primate Models of HIV/AIDS
Macaques that develop an AIDS-like disease following experimental SIV infection include rhesus (Macaca mulatta), cynomolgus (Macaca fascicularis), and pigtail (Macaca nemestrina) macaques. The RM model is the most extensively studied and includes the Indian and Chinese subtypes; the Indian subtype experiences faster disease progression compared to the Chinese subspecie (reviewed in Zhou et al., 2013). In contrast to progressive SIV infection of Asian macaques, over 40 known species of African non-human primates are endemically infected (Chahroudi et al., 2012). These natural hosts of SIV, which remain asymptomatic, are characterized by a low level of immune activation despite highly replicating virus. Among these species, sooty mangabeys (SMs) and African green monkeys (AGMs) have been extensively studied as nonpathogenic models. Progressive and nonpathogenic models have contributed to our understanding of mucosal immunity and immune activation during SIV infection.
The gut mucosal immune system of NHP and humans is divided into inducible and effector compartments. The inducible sites are composed of organized lymphoid structures such as Peyer's patches and isolated lymphoid follicles (Brandtzaeg et al., 2008). The effector sites, comprised of the epithelium lining the gut lumen and the subjacent lamina propria (LP), contain diffusely distributed myeloid and lymphoid cells.
3.2. CD4 T Cell Depletion: Targeting the “Helpers”
Mucosal CD4 T cell depletion was first reported in AIDS patients; this depletion was predominant in the duodenum (Rodgers et al., 1986), suggesting differential impact of HIV infection along the intestine. CD4 T cell changes in early SIV infection were subsequently investigated in NHP models; key study findings are summarized in Table 1. In Indian RMs, enteropathies were reported by Veazey et al. who observed the depletion of 80% to 90% of activated CD4 T cells in the gut mucosa at day 14 post-infection (Veazey et al., 2000). In Chinese RMs, rectal CD4+ T cells were depleted by 30% at day 14 post-infection, although no changes were observed in the small intestine (Couëdel-Courteille et al., 2003). In these studies, mucosal helper T cell depletion was assessed by measuring CD4 T cell frequencies, which is influenced by the size of other cell populations. Therefore, immunostaining was performed in subsequent studies. The colon of Indian RMs showed up to 50% depletion of CD4 T cells (Li et al., 2005); in newly HIV-infected individuals, CD4 T cell depletion was detected in the effector sites of the rectal mucosa although the authors reported that it was difficult to precisely quantify T cell numbers in small biopsies (Mehandru et al., 2004). In contrast, no change was observed in the duodenum during acute HIV infection (Allers et al., 2015) and in the ileum of acutely SIV-infected Chinese RMs (Ponte, 2014), suggesting that a reduction in the absolute numbers of CD4 T cells may be less dramatic during very early HIV infection and less pathogenic SIV model. The key findings that examined the effect of HIV on gut CD4 T cell numbers are summarized in Table 2.
Table 1.
Studies of intestinal CD4 T cell, CD8 T cell, and myeloid cell distribution during SIV infection of rhesus macaques.
| Gut segment | Methods | Time post-infection |
CD4 T cells | CD8 T cells | Myeloid cells | References |
|---|---|---|---|---|---|---|
| Jejunum | FC | Day 14 to 63 | 80–90% depletion of CD4+ CCR5+ T cells | NA | NA | Veazey et al., 2000 |
| Rectum S.I. |
FC, IHC | Day 14 | 30% depletion of LP CD4+ T cells No depletion |
NA | NA | Couëdel-Courteille et al., 2003 (Ch) |
| Colon | IHF | Day 14 | 50% depletion of CD4 T cells |
NA | NA | Li et al., 2005 |
| Jejunum | FC | Chronic | Th17+ Th22+ depletion | NA | NA | Xu et al., 2014 (In) |
| Jejunum | FC | Acute and chronic | CD4 T cell depletion | NA | NA | Glavan et al., 2015 |
| Jejunum, ileum, colon |
FC | Day 7 Days 14, 21, 50 |
30–50% depletion of LP CD4+ CD25+ T cells 80–90% depletion of LP CD4+ CD25+ T cells |
10–20% increase (jejunum and colon) 10–50% increase (greatest in the ileum) |
NA | Veazey et al., 1998 |
| Jejunum | FC | Days 3, 7, 10, 14 Days 14, 17 |
80% depletion of CD4+ CD45RA− CCR5+ T cells |
Increased frequency of total CD8 T cells |
NA | Mattapallil et al., 2005 |
| Rectum S.I., colon |
FC FC |
Day 14 to week 6 Chronic |
Decrease in CD4 Treg NA |
Expansion of total CD8 and CD8+ FOXP3+ T cells Increased frequency of CD8+ FOXP3+ T cells |
NA | Nigam et al., 2010 |
| Colorectal biopsies | FC | Acute Chronic |
NA |
Maintenance of Tc17 cells Loss of Tc17 cells |
NA | Nigam et al., 2011 |
| Jejunum | FC IHC |
Day 21 Chronic |
NA NA |
Increase of ILC-17 cells Decreased numbers of Tc17 and ILC-17 cells |
NA NA |
Xu et al., 2012 |
| Jejunum | FC | Day 8, 10 Day 13 |
Depletion of LP BrdU+ CD4 T cells |
Increased numbers of LPL BrdU+ CD8 T cells |
NA | Wang et al., 2013 |
| Colon | IHC | Chronic | NA | NA | Neutrophil infiltration (myeloperoxidase+) |
Estes et al., 2010 (In) |
| Colon | FC | Chronic | Loss of Th17 cells | Decreased frequency of Tc17 cells | Loss of CD103+ DCs; No change in the frequency of mDCs or pDCs |
Klatt et al., 2012 |
| Colon, rectum | FC, IHF | Chronic | NA | NA | 4-fold increase in the number of CD123+ pDCs No change in the number of mDCs |
Reeves et al., 2012 (In) |
| Rectum | FC IHC |
Day 14 Week 4 |
Decrease in CD4+ IL-17+ T cells |
NA |
Neutrophil infiltration (myeloperoxidase+) |
Pallikkuth et al., 2013 |
| Ileum | IHF | Days 3, 7, 10, 14 | NA | Increased numbers of LP CD8 T cells | Changes of macrophage counts |
Ponte, 2014 (Ch) |
S.I.: small intestine; FC: flow cytometry; IHC: immunohistochemistry; IHF: immunohistofluorescence; LP: lamina propria; DCs: dendritic cells; mDCs: myeloid-derived dendritic cells; pDCs: plasmacytoid dendritic cells; Tc17: IL-17 producing CD8 T cells; (Ch): rhesus macaque of Chinese origin and (In): rhesus macaque of Indian origin, when applicable; NA: not applicable. Studies are classified chronologically and according to the cell type.
Table 2.
Studies that assessed changes in immune-cell distribution in the gut of HIV-infected persons.
| Gut segment | Methods | Estimated time post-infection | ART status | Changes | References |
|---|---|---|---|---|---|
| Rectum | IHC | Early | Naïve | Decreased CD4/CD8 ratio | Mehandru et al. (2004) |
| Duodenum | FC | Chronic | Naïve | Increased number and frequency of Treg | Epple et al. (2006) |
| Rectum | FC | Chronic | Untreated | Depletion of Th17 cells | Shaw et al. (2011) |
| Increased Treg frequency | |||||
| Duodenum | IHC | Chronic | Naïve | Accumulation of CD68+ and CD163+ macrophages | Allers et al. (2014) |
| Duodenum | IHC | Hyper acute | Naïve | No depletion of CD4 T cells | Allers et al. (2015) |
| Higher CD8 T cell numbers | |||||
| Chronic | Naïve | Depletion of CD4 T cells | |||
| Increased number of CD8 T cells | |||||
| Accumulation of monocytes | |||||
| Colon | IHC | AIDS | Naïve | Accumulation of CD14+ macrophages | Cassol et al. (2015) |
| Colorectal tissue | IHC | Chronic | ART-treated and untreated | Neutrophil infiltration | Somsouk et al. (2015) |
IHC: immunohistochemistry; FC: flow cytometry; ART: antiretroviral therapy.
Most of the CD4 T cells depleted by SIV/HIV are memory cells that express CCR5, one of the main co-receptors for SIV/HIV entry (Mattapallil et al., 2005). Functional studies have shown the impairment of Th17 and Th22 subsets involved in the maintenance of gut barrier integrity in both acute (Pallikkuth et al., 2013) and chronic SIV/HIV infections (Klatt et al., 2012, DaFonseca et al., 2015, Xu, 2014). The loss of intestinal CD4 T cells was also reported in nonpathogenic SIV infections, although Th17 subsets in the LP were preserved (Chahroudi et al., 2012). In addition, a transient loss of mucosal Foxp3+ Treg was observed in the pathogenic SIV infection of pigtail macaques (Favre et al., 2009) and RMs (Nigam et al., 2010); these cells were not lost in AGMs, which are natural SIV hosts, demonstrating that an altered Th17/Treg ratio characterizes pathogenic SIV infection. In chronic HIV infection, Th17 cells were massively depleted while Treg frequency increased in the gut mucosa (Shaw et al., 2011, Epple et al., 2006). Overall, these results show that impairment of intestinal Th17/Th22 cells is associated with systemic immune activation, shedding light on the importance of the gut damage that occurs in early HIV infection.
3.3. The CD8 T Cell Compartment: Under the Shadow of the Almighty CD4 T Cells?
Most studies of SIV/HIV-induced gut damage have focused on T helper cells. Increasing evidence highlights the importance of the CD8 T cell compartment in SIV/HIV disease progression (Table 1, Table 2). An increased frequency of persistent, circulating CD8 T cells is associated with a higher risk of non-AIDS events (Mudd and Lederman, 2014). Several studies have demonstrated that CD8 T cell infiltration of the intestine is linked to memory CD4 T cell depletion in acute SIV infection of RMs (Veazey et al., 1998), (Mattapallil et al., 2005), (Wang et al., 2013). During acute SIV infection of Chinese RMs, we observed in the ileum LP an increase of CD8 T cell numbers concomitant with enhanced expression of T cell attracting chemokines, associated with a transient decrease in circulating CD8 T cells (Ponte, 2014). These results suggest that a shift in circulating CD8 T cell homing to the gut mucosa had occurred. In addition, the colon of acutely SIV-infected RMs was characterized by perforin overexpression, suggesting a strong cytotoxic response during early infection (Quigley et al., 2006). In chronic SIV infection, the presence of specific cytotoxic T cells in the intestine correlated with preserved CD4 T cell counts (Schultheiss and Stahl-Hennig, 2011), highlighting the role of the CD8 T cell specific immune response in maintaining mucosal integrity.
An expansion of total CD8 T cells was also reported in the rectal mucosa of SIV-infected RMs; upon further investigation, it was demonstrated that increased numbers of CD8 Treg (CD8+ FOXP3+) persisted during chronic SIV infection (Nigam et al., 2010). Although CD4 Th17 cells are considered to be the main source of interleukin (IL)-17 and IL-22, recent studies have revealed that Tc17 cells, a subset of CD8 T cells that produce IL-17, are maintained in the gut of RMs during acute SIV infection and are progressively depleted during the chronic stage (Nigam et al., 2011), (Xu et al., 2012). These results indicate that, in addition to the CD4 Th17/Treg ratio, an altered balance between Tc17 cells and CD8 Treg characterizes progressive SIV and HIV infections.
3.4. Intestinal Innate Immune Cell Dysfunction in SIV/HIV Infection: Disturbed Crosstalk Between Innate and Adaptive Immunity
3.4.1. Innate Lymphoid Cells: New Players in the Field of Gut Damage
Innate lymphoid cells (ILCs) are a growing group of immune cells enriched at mucosal surfaces that mirror the phenotypes and functions of T cells (Eberl et al., 2015). Conversely to T cells, ILCs do not express acquired antigen receptors or undergo clonal selection and expansion upon stimulation. Instead, ILCs react promptly to signals from infected tissues by secreting cytokines. ILCs have been classified into three main types based upon their cytokine responses and transcription factors that regulate their function. Type 1 ILCs (ILC1) secrete Th1-related cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor alpha (TNF-α) and promote immunity against intracellular pathogens. ILC2 produce type 2 cytokines (IL-4, IL-5) and play a significant role in response to helminth infection. ILC3 produce IL-17 and IL-22 and express the transcription factor RORγt, (Spits et al., 2013). Thus, ILC3 share effector functions with Th17 cells, although performing their functions in the absence of antigen specificity (Artis and Spits, 2015). Convergent study findings of Indian RM have shown that IL-17-producing ILCs are rapidly and persistently depleted in the GI tract following SIV infection (Xu et al., 2012, Li et al., 2014, Xu et al., 2015). Xu et al. identified translocated microbial products as a driver of ILC apoptosis through TLR-mediated signaling. Furthermore, both colonic and circulating blood ILC3 are decreased in HIV-infected individuals and predict disease progression (Zhang et al., 2015). The identification of ILCs represents a paradigm shift in mucosal immunology as these cells established a bridge between microenvironment and adaptive immunity and are severely damaged during SIV/HIV infections.
3.4.2. Intestinal Macrophages: Supplanting the Silent Sentinels
Intestinal macrophages are strategically located in the LP lining the epithelium. These cells can efficiently perform phagocytic and bactericidal functions without inducing inflammation (Smith et al., 2005). In healthy gut mucosa, macrophages express toll like receptor-4 (TLR-4), do not express CD14, the co-receptor for gram-negative bacteria lipopolysaccharide (LPS), and do not produce inflammatory cytokines such as TNF-α, IL-6 or CXCL10 (Smith et al., 2010). Therefore, gut macrophages are critical for protecting epithelium integrity. These cells also express low levels of CD4 and of the HIV co-receptors CCR5 and CXCR4, thereby reducing their susceptibility to HIV infection, and do not seem to support HIV replication (Shen et al., 2009).
Numerous studies have observed that SIV and HIV induce intestinal myeloid cell dysfunction, depicted in Table 1, Table 2, respectively. Gut macrophages with defective phagocytic function were observed by Estes et al. in chronically, but not acutely, SIV-infected Indian RMs (Estes et al., 2010). This observation could explain the limited microbial translocation observed in acute SIV/HIV infections during which time gut macrophages are still capable of effectively phagocytosing microbial products.
As intestinal resident macrophages have reduced proliferative capacity, the recruitment of circulating monocytes is likely the main source for their renewal. While the pro-inflammatory CD16+ monocyte subset is expanded in SIV-infected RMs, the number of these cells remains stable in nonpathogenic infection of SMs. These observations suggest that CD16+ monocytes contribute to SIV/HIV pathogenesis. In chronically HIV-infected individuals, inflammatory monocytes accumulate in the LP of the duodenum in response to increased mucosal levels of the CCL2 chemokine (Allers et al., 2014). Furthermore, CD14+ macrophages accumulate in the colon of AIDS patients and correlate with the degree of microbial translocation (Cassol et al., 2015).
Collectively, these observations indicate that the infiltration of pro-inflammatory monocytes into the intestinal mucosa during SIV/HIV infections, which results in the development of tissue-resident macrophages, promotes local inflammation and tissue injury; this process results in further recruitment of circulating monocytes. Moreover, the low phagocytic activity of the resulting macrophages prevents efficient purging of microbial products, resulting in increased microbial translocation across the injured intestinal barrier.
3.4.3. Neutrophil and Dendritic Cell Contribution to Gut Damage
Among the other immune cells in the gut mucosa are polymorphonuclear neutrophils (PMNs) and dendritic cells (DCs), whose contributions to gut damage in SIV/HIV infections have not been well studied. The results of studies that have been performed are summarized in Table 1, Table 2.
PMNs contribute to the first line of defense against bacterial and fungal pathogens. These cells rapidly migrate to the inflamed tissues where their activation triggers the release of antimicrobial peptides and proteolytic enzymes such as myeloperoxidase. During chronic SIV infection of RMs, neutrophils, which were shown to have infiltrated the colonic mucosa, were linked to epithelial barrier damage (Estes et al., 2010). Neutrophils were also reported to have invaded the rectal mucosa of RM four weeks after SIV infection (Pallikkuth et al., 2013). In contrast, Hensley-McBain et al. did not report increased PMN counts within the first 28 days following SIV infection of RMs, which was likely due to a lack of PMN-supporting cytokines (Hensley-McBain et al., 2014). Furthermore, circulating neutrophils are more prone to undergo apoptosis during pathogenic SIV infection as compared to nonpathogenic models (Elbim et al., 2008). Therefore, while the virus itself may not induce PMN accumulation during early infection, some lines of evidence point to PMN infiltration during chronic SIV/HIV infections, suggesting that PMNs might contribute to inflammation and gut barrier damage during the chronic phase.
In contrast to neutrophils, which are recruited by the inflamed mucosa, DCs are resident cells that are diffusely located throughout the LP, within Peyer's patches and in isolated lymphoid follicles. Intestinal DCs include CD123+ plasmacytoid DCs (pDCs) and CD11c+ myeloid-derived DCs (mDCs). pDCs are a major source of IFN-α, providing a first line of defense against HIV (Bruel et al., 2014). Studies of SIV infection have demonstrated that IFN-α and TNF-α producing pDCs accumulate in the gut mucosa and participate in the ongoing immune activation instead of controlling initial SIV dissemination (Li et al., 2015, Reeves et al., 2012). In another study, a subset of DCs that expressed CD103 (integrin αE), was found to be depleted in the colon, while no change was observed in mDC or pDC frequencies (Klatt et al., 2012). These contrasting results highlight the need to clarify the contribution of DCs to the gut mucosal inflammation that occurs during SIV infection.
3.5. Altered Chemokine/Cytokine Microenvironment: Do Recruited Soldiers Become Prey for SIV/HIV?
Numerous studies have shown that intestinal tissues exhibit altered chemokine and cytokine networks due to SIV infection; the results of these studies are summarized in Table 3. CCL20 and CCL21 chemokines associated with the trafficking of DCs, B and T cells are unchanged during the course of SIV infection (Choi et al., 2003), while CCL19 and CXCL9 are up-regulated in the small intestine of RMs during acute infection and are associated with the recruitment of CD4 T cells (Clay et al., 2005), potentially feeding viral replication. Tissue CD8 T cell counts increase during SIV infection and these cells secrete CCL5 (a CCR5 ligand) and GM-CSF into the small intestine (Kenway-Lynch et al., 2013), suggesting that DCs, monocytes/macrophages, T cells, B cells, as well as neutrophils, could be rapidly recruited to the gut. Importantly, increased expression of CCL5 could be implicated in the exacerbation of HIV pathogenesis (Zhou et al., 2011). We have also evidenced enhanced expression of gut-expressed CCL25, which recruits CCR9+ effector T cells, and IFN-γ induced CXCL10, which has the potential to attract monocytes/macrophages, DCs, and T and B cells, in acute SIV infection of RMs (Ponte, 2014).
Table 3.
Studies of early changes in intestinal cytokine/chemokine networks in SIV-infected rhesus macaques.
| Gut segment | Time post-infection | Cytokine/chemokine | Change | Source | References |
|---|---|---|---|---|---|
| Small intestine | Acute | CCL19 (MIP-3β), CXCL9 | Increase | LPLa | Clay et al., 2005 |
| Jejunum | Day 21 | Th1 and Th2 cytokines IL-17, IFN-γ, GM-CSF, MIF |
Decrease Increase |
CD4 T cellsa CD8 T cellsa |
Kenway-Lynch et al., 2013 |
| Ileum | Day 2–3 | IL-1β | Increase | Paneth cellsa | Hirao et al., 2014 |
| Jejunum, colon |
Day 28 | IL-10 TNF-α IFN-γ |
Increase Increase Increase |
T cells, B cells, monocytes/macrophages, DCs, ECsa T cells, B cells, monocytes/macrophages, NK, mast cells, endothelial cells, ECsb Th1 and CD8 T cells, B cells, macrophages, NKT, NK cells, DCsb |
Pan et al., 2014 (In) |
| Jejunum | Acute | IFN-α CXCL8 (IL-8) IL-12 IL-17, IL-22 IL-23 |
Increase Increase Increase Increase Increase |
Tcells, monocytes/macrophages, DCs, pDCs, NK cells, fibroblastsb T cells, monocytes/macrophages, endothelial cells, ECsb Monocytes/macrophages, DCsb Th17 cells, Th22 cells, ILCsb Macrophages, DC |
Glavan et al., 2015 |
| Small intestine |
Days 3 to 10 Days 7 to 14 |
IL-7 CCL5 (RANTES) CCL25 (TECK) CXCL10 (IP-10) |
Increase Increase Increase Increase |
ECsb T cellsb ECsb Monocytes/macrophages, endothelial cells, fibroblastsb |
Ponte, 2014. (Ch) |
MIP-3β: macrophage inflammatory protein-3beta; LPL: lamina propria lymphocytes; IL: interleukin; IFN: interferon; GM-CSF: granulocyte macrophage colony stimulating factor; MIF: macrophage migration inhibitory factor; TNF: tumor necrosis factor; DCs: dendritic cells; ECs: epithelial cells; NK: natural killer; NKT: natural killer T cells; pDCs: plasmacytoïd dendritic cells; RANTES: regulated on activation, normal T cell expressed and secreted; TECK: thymus-expressed chemokine; IP-10: interferon gamma-induced protein 10.; (Ch): rhesus macaque of Chinese origin and (In): rhesus macaque of Indian origin, when applicable.
As indicated in the study
As indicated in the literature.
Furthermore, several studies have demonstrated increased levels of pro-inflammatory cytokines such as IL-1β, TNF-α, IFN-α, IFN-γ, IL-8 and IL-17 in the gut mucosa following SIV infection. Increased levels of immunomodulatory IL-10 (Pan et al., 2014) and transforming growth factor beta (TGF-β), (Reeves et al., 2011) are also observed in SIV infection, suggesting that these cytokines were not able to induce anti-inflammatory responses. Importantly, in experimental SIV infection of SM and AGM natural hosts, the evaluation of plasma cytokine levels in early infection showed that the absence of immune activation was due to a significant downregulation of inflammatory cytokines rather than a lack of early inflammation (Pandrea and Apetrei, 2010).
3.6. Structural Gut Damage Causes Leakage of a Modified Gut Microbiota
3.6.1. Epithelial Barrier Disruption: Creating Breaches for Microbial Invaders
The intestinal epithelium, made of a single layer of enterocytes together with a luminal mucus layer, provides a physical and chemical barrier between the host and the so-called “extended-self” luminal microbiome. The chemical barrier includes soluble factors such as IgA and defensins. Epithelial cells establish close contact at their lateral cell membranes by dynamic structures named tight junctions, which interconnect the cells and restrict the passage of bacterial products through the paracellular space. Intestinal epithelial cells express the IL-22 receptor, which plays an important role in the renewal of the gut barrier (Aujla and Kolls, 2009).
Damage to the gut barrier involves the apoptosis of enterocytes during the acute stage of SIV infection, which persists in the chronic phase (Li et al., 2008, Pan et al., 2014). Li et al. reported massive SIV-induced epithelial cell apoptosis as an underlying mechanism of the regenerative enteropathy that characterizes early SIV infection. Consistently, another study described enhanced epithelial cell proliferation during SIV infection, as a repair mechanism triggered in response to massive enterocyte death (Mohan et al., 2013). In contrast, impaired epithelial proliferation, which was detected in the colorectal tissues of HIV-infected patients, was linked to increased apoptosis (Somsouk et al., 2015). Therefore, it remains unclear whether mucosal apoptosis in ART-treated individuals is a cause or a consequence of epithelial proliferative changes. Moreover, the rapid, SIV-induced secretion of IL-1β correlated with decreased expression and with mislocalization of tight junction proteins (Hirao et al., 2014).
Thus, during SIV/HIV infections, damage to the physical integrity of the epithelium causes breaches in the gut barrier that increases intestinal tract permeability, resulting in the passage of luminal microbiota products through the mucosa into the systemic circulation (Estes et al., 2010).
3.6.2. Microbiota Changes and Inflammation Throughout the Gut Barrier: A Two-way Street
Studies of untreated HIV-infected individuals have revealed altered composition of the fecal microbiota in which pathogenic bacteria such as proteobacteria species were found at higher levels than probiotics such as Lactobacilli (Gori et al., 2008); this flora modification persisted during short-term ART (Nowak et al., 2015). Furthermore, several groups have reported an altered Bacteroides/Prevotella ratio in untreated HIV-infected persons (Lozupone et al., 2013), detected in gut epithelium adherent microbiota (Dillon et al., 2014). Dysbiosis of the gut microbiota and changes in their dietary tryptophan catabolite by-products were associated with mucosal and systemic immune activation, contributing to disease progression (Vujkovic-Cvijin et al., 2013, Dillon et al., 2014, Routy et al., 2015), suggesting that gut-resident microbial communities influence HIV pathogenesis. NHP models, which have permitted the longitudinal sampling of feces throughout SIV infection, have diminished the bias attributed to differences in diet. In contrast to findings in human studies, SIV infection did not cause significant alterations of the fecal and gut-associated bacteriome (Handley et al., 2012), with dysbiosis, characterized by an enrichment of proteobacteria that may preferentially translocate, observed only in ART-treated macaques (Klase et al., 2015). However, a recent study of untreated acute SIV infection of RMs demonstrated specific depletion of Lactobacilli, supporting previous findings in HIV-infected persons (Vujkovic-Cvijin et al., 2015). This diminution of Lactobacilli was associated with increased activity of the tryptophan-catabolizing immunosuppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1) and with Th17 loss, suggesting that Lactobacillus species inhibit IDO1 activity as a mechanism to maintain mucosal immune homeostasis. Importantly, these results highlight the need for new therapeutic strategies to regulate both metabolic activity and composition of the intestinal flora.
3.6.3. Microbial Translocation and Biomarkers of Gut Damage
SIV-induced gut damage and subsequent microbial translocation have been linked for the first time to systemic immune activation in chronically SIV-infected Indian RMs (Brenchley et al., 2006, Estes et al., 2010). In these seminal studies, plasmatic bacterial LPS levels correlated with activation markers such as T cell CD38 and HLA-DR, and with plasma soluble CD14 (sCD14), which is released by activated monocytes. The effect of gut damage and microbial translocation on pathogenic immune activation was demonstrated in uninfected pigtail macaques (Klatt et al., 2010) and in experimentally-induced colitis in RMs (Hao et al., 2015). This latter study demonstrated the value of a non-invasive clinical monitoring technique to assess intestinal microvasculature leakage in IBD and HIV patients. Furthermore, early HIV infection has been associated with higher levels of LPS-binding protein (LBP) and with intestinal fatty acid binding protein (I-FABP), a marker of enterocyte damage (Marchetti et al., 2013).
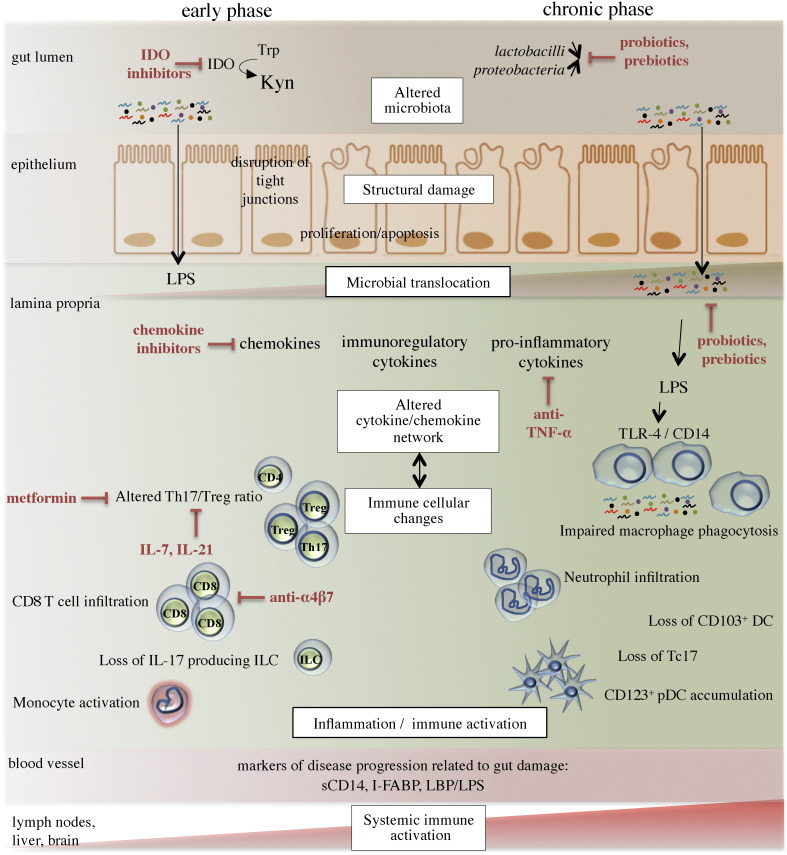
Clearly, numerous complex mechanisms contribute to gut damage that occurs following SIV/HIV infections (summarized in Fig. 1). We propose that therapies targeting the composition and metabolic activity of the GI microbiota, which are altered in HIV-infected individuals, could benefit ART-treated individuals.
Fig. 1.
Schematic representation of the multiple mechanisms that induce gut damage following SIV/HIV infections and strategies to reduce mucosal inflammation. Gut damage includes alterations to the microbiota, injuries to the gut epithelium, and changes to the immune mucosal landscape, all of which lead to persistent inflammation and systemic immune activation. Lymphoid cellular damage, which occurs in early infection, consists of CD4 T cell depletion, an altered Th17/Treg ratio, the loss of IL-17 producing innate lymphoid cells (ILCs), accumulation of CD8 T cells, and monocyte activation. These changes, which persist during disease progression, are accompanied by myeloid changes such as macrophage accumulation and defective phagocytosis, neutrophil and pDC infiltration, and the loss of CD103+ DCs. The loss of IL-17-producing CD8 T cells (Tc17 cells) also characterizes the chronic phase of infection. Overproduction of kynurenine (Kyn) metabolites from dietary tryptophan (Trp) by the immunosuppresive enzyme indoleamine 2,3-dioxygenase (IDO) contributes to an altered Th17/Treg ratio. Strategies to reduce mucosal inflammation are depicted in red.
4. Strategies to Restore the Integrity of the Gut Barrier
4.1. Early ART is Not Sufficient to Fully Restore Gut Damage
We have previously reviewed the results of several clinical trials that investigated the effect of early ART on gut dysfunction (Cao et al., 2015b). Importantly, early ART initiation preserved Th17 function and reversed immune activation, as defined by the expression of the T cell activation markers CD38 and HLA-DR. However, gut damage, which was assessed by measuring plasma I-FABP and LPS levels, was not reversed by early ART, suggesting ongoing enterocyte damage and subsequent bacterial translocation (Jenabian et al., 2015). We and others have reported that initiation of ART during acute infection preserved mucosal CD4 T cells without reducing CD8 T cell infiltration (Allers et al., 2015, Cao et al., 2015a). These data indicate that mucosal damage was not completely prevented or reversed by early ART initiation, implying that damage to other cells in addition to CD4 T cells in the gut is needed to lead to a significant augmentation of bacterial translocation.
4.2. Targeting Gut Microbiota and Repairing the Breaches: Rebuilding Harmony
The combination of sulfasalazine, an anti-inflammatory molecule, and the antibiotic rifaximin, significantly reduced immune activation in SIV-infected pigtail macaques (Marchetti et al., 2013). However, long-term antibiotic therapies may result in the emergence of resistant microbes and in further injury to the gut barrier. Another therapeutic option that has been investigated in a fast-progressing, SIV-infected pigtailed macaque model, consists of neutralizing translocated gut microbial products using the LPS-binding drug sevelamer (Kristoff et al., 2014). This treatment was associated with reduction of SIV-induced inflammation, immune activation and coagulation markers, providing an important causal link between microbial translocation and deleterious immune activation. However, in individuals with untreated early HIV infection, sevelamer administration did not influence microbial translocation and inflammation, possibly reflecting low LPS translocation during this period of infection (Sandler et al., 2014).
The use of ART highly effective in suppressing SIV replication in macaques has permitted the assessment of microbiota modulating strategies, involving probiotic bacteria and/or prebiotic nutrients. Remarkably, following treatment with a combination of probiotics, GI tract CD4 T cells were reconstituted in ART-treated, SIV-infected RMs (Klatt et al., 2013). Moreover, administration of commensal Lactobacillus plantarum directly into the intestine of SIV-infected macaques led to the decreased secretion of IL-1β by Paneth cells, in addition to epithelial tight junction repair (Hirao et al., 2014), suggesting that reduced inflammation is associated with gut barrier restoration. Encouragingly, probiotic/prebiotic therapies were also associated with immune benefits in ART-treated patients; however large, placebo-controlled clinical trials are necessary to assess the long-term efficacy of these therapies (reviewed in Brenchley, 2013). Finally, a more holistic picture may be required to treat SIV/HIV-induced gut damage given that certain microbes cohabit in a reciprocal relationship with parasitic helminths within the mammalian GI tract (Reynolds et al., 2015).
4.3. Immune-based Therapies for the Treatment of SIV/HIV-induced Gut Damage
Immune-based therapies designed to reduce intestinal inflammation and subsequent systemic immune activation have been investigated in RMs and in humans. While anti-TNF-α treatment successfully decreased the expression of proinflammatory genes and lowered the infiltration of PMNs into lymphoid tissues during early SIV infection, no change in viral load was reported (Tabb et al., 2013), further arguing for the need to combine such anti-inflammatory strategies with ART. The common use of serum-derived bovine immunoglobulin to efficiently reduce intestinal inflammation in animal models was the rationale to implement a pilot clinical trial in ART-treated adults. These individuals experienced intestinal immune reconstitution and epithelial repair in the setting of HIV enteropathies (Asmuth et al., 2013). These early findings highlight the value of therapy designed to repair both immune and epithelial GI tract compartments.
The immune inhibitory receptor programmed death-1 (PD-1) regulates the functional exhaustion of virus-specific CD8 T cells during SIV/HIV infections (Trautmann et al., 2006). PD-1 blockade in SIV-infected RMs resulted in a lower plasma viral load and in reduced expression of transcripts associated with IFN signaling in the blood and colorectal tissue (Dyavar Shetty et al., 2012). Furthermore, these findings were associated with enhanced expression of gut-junction associated genes in colorectal tissue along with lower plasma LPS levels, suggesting immune improvement of both SIV-specific and mucosal immunity. However, blocking the PD-1 pathway in SIV-infected macaques conducted by another group showed limited clinical benefit, generating the hypothesis that blocking a single inhibitory pathway may not be sufficient to control SIV/HIV-induced exhaustion (reviewed in Velu et al., 2015).
The success of metformin treatment in attenuating IBD severity and in regulating the Th17/Treg ratio in mice (Lee et al., 2015), along with its routine use in diabetic patients, has led to the design of a clinical trial that will assess whether metformin can reduce inflammation in HIV-infected individuals. To modulate the Th17/Treg ratio, IDO inhibitors such as 1-methyl-D-tryptophan were also administered to ART-treated, SIV-infected RMs; this treatment reduced plasma viral load only in macaques that did not receive any immunological benefit from ART (Boasso et al., 2009). Therefore, more studies are required to determine whether such an approach may be beneficial for the treatment of HIV infection.
Given the significant changes that occur in the chemokine network and in cell migration within the gut mucosa following SIV infection, strategies that target the gut-homing α4β7 integrin expressed by T cells have been investigated in RMs (Byrareddy et al., 2014). In this study, administration of an anti-α4β7 monoclonal antibody reduced SIV transmission and protected gut mucosal CD4 T cells from infection. Encouragingly, SIV transmission was also reduced by the use of the CCL20 chemokine inhibitor glycerol monolaurate (Li et al., 2009).
Other immune-based treatments that have been investigated for the treatment of SIV/HIV-mediated gut damage involved common gamma chain cytokines such as IL-7 and IL-21. ART-treated, SIV-infected RMs that received IL-7 demonstrated increased numbers of peripheral CD4 T cells (Beq et al., 2006); a similar effect was observed in HIV-infected patients (Levy et al., 2012). This therapy was shown to trigger chemokine expression in intestinal tissues as well as chemokine receptors and α4β7 integrin expression by circulating cells, leading to massive T cell homing to the gut mucosa, and decreased colonic inflammation (Beq et al., 2009, Cimbro et al., 2012, Sereti et al., 2014).
IL-21 is a pleiotropic cytokine that induces Th17 cell differentiation and that promotes CD8 T cell cytotoxic function. Pallikkuth et al. have shown that IL-21 treatment of SIV-infected RMs increased Th17 cell frequency and enhanced the cytotoxic function of total and SIV-specific CD8 T cells (Pallikkuth et al., 2013). Such immune improvements were associated with reduced microbial translocation and with a long lasting reduction in immune activation in the blood and in the rectum. Similar results were obtained in another study in which IL-21 and probiotics were concurrently administered to ART-treated, SIV-infected pigtailed macaques (Ortiz et al., 2015). Importantly, this treatment led to increased gut CD4 T cell frequencies without altering viral suppression by ART. Interestingly, a reduction in the size of the viral reservoir was detected in ART-treated RMs receiving IL-21 (Micci et al., 2015). Currently, IL-21 is being investigated as an immunomodulatory cytokine in cancer patients and in HIV-infected persons.
5. Conclusion
SIV infection studies have contributed to a better understanding of the detrimental role of gut damage in HIV pathogenesis. HIV infection induces important damages in lymphoid, myeloid and stromal cell gut compartments, while causing dysbiosis of microbial communities. These significant pathological changes induce the loss of gut barrier integrity, creating an “immune scar” that persists during chronic infection and that contributes to microbial translocation and inflammation, while increasing the risk of non-AIDS morbidities. We believe that, in addition to early ART initiation, modulation of the microbiota with probiotics and repairing gut damage with immune-based therapies have produced promising results that warrant future investigations to control residual inflammation and reduce viral reservoirs in persons living with HIV.
6. Outstanding Questions
The proficient development of therapies for reversing gut damage in HIV-infected individuals will rely on the scientific and medical community to address major issues. First, the key to elucidate mechanisms of gut barrier integrity restoration may rely in our further understanding of the disparities between pathogenic and nonpathogenic SIV infections. Given the absence of microbial translocation in the latter, mucosal defense pathways are certainly preserved and more work is needed to assess the essential determinants for the persistence of gut integrity in a context of viral replication in natural hosts. Second, it becomes imperative to determine the role of the gut microbiota in HIV-induced gut damage and downstream inflammation. Future studies on gut microbial metabolites together with the development of metagenomic approaches may bridge the gap in our current knowledge, coupling gut microbiota composition, epithelial integrity and immune responses. On a clinical perspective, orchestrating the assessment of gut biopsies and their associated “local” microbiota as well as feces from large cohort of patients would certainly broaden our understanding of microbial-host interaction associated with epithelial and immune damage. Ultimately, a better characterization of the persistence of gut damage even after early ART initiation is warranted to identify the mechanisms responsible for this residual alteration. Although findings on antiretroviral pharmacodynamics in the GI tract were not discussed in this review, important data are missing in order to delineate whether incomplete gut epithelial restoration could be due to low drug concentration within this compartment. Notably, future studies should also address to what extent gut reconstitution is required for HIV-infected individuals to achieve full protection against co-morbidities.
7. Search Strategy and Selection Criteria
We conducted a comprehensive literature review of English language publications in PubMed electronic database in which we searched for references for relevant articles; the search terms used were “acute SIV/HIV infections”, “gut T cell”, “innate lymphoid cells”, “intestinal macrophages”, “dendritic cells”, “neutrophils”, “epithelial damage”, “microbiota”, “microbial translocation”, and “cytokine therapy”. Except for seminal studies, only articles published between 2005 and 2015 were included.
Funding
This review article was supported by the Canadian Institutes of Health Research (grant MOP #103230 and CTN #257), and by FRQ-S: Therapie cellulaire, Réseau SIDA/Maladies Infectieuses, Québec.
Acknowledgements
The authors acknowledge Angie Massicotte and Danica Albert for technical and administrative assistance, and Dr. Gina M. Graziani for critically reviewing the manuscript.
RP is a post-doctoral fellow supported by the H. Grenville Smith Fellowship, an award from the Research Institute of the McGill University Health Centre; RP is also supported by funding from the Canadian HIV Cure Enterprise (CanCure) and from the Canadian Institutes of Health Research. VM is a post-doctoral fellow supported by Le Fonds de Recherche du Québec-Santé (FRQS). RP, ACC and RC were supported by the ANRS (Agence Nationale de Recherche sur le SIDA et les Hépatites Virales), SIDACTION, INSERM, CNRS and by the Université Paris Descartes. JPR holds the Louis Lowenstein Chair in Hematology & Oncology in the Faculty of Medicine at McGill University.
References
- Allers K. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J. Infect. Dis. 2014;209(5):739–748. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- Allers K. The effect of timing of antiretroviral therapy on CD4 + T-cell reconstitution in the intestine of HIV-infected patients. Mucosal Immunol. 2015:1–10. doi: 10.1038/mi.2015.58. [DOI] [PubMed] [Google Scholar]
- Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Asmuth D.M. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS (London, England) 2013;27(14):2207–2217. doi: 10.1097/QAD.0b013e328362e54c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla S.J., Kolls J.K. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. (Berl., Ger.) 2009;87(5):451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- Beq S. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol. (Baltimore, Md.: 1950) 2006;176(2):914–922. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- Beq S. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114(4):816–825. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- Boasso A. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J. Immunol. 2009;182(7):4313–4320. doi: 10.4049/jimmunol.0803314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1(1):31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 2013;6(4):657–665. doi: 10.1038/mi.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bruel T. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog. 2014;10(1):e1003915. doi: 10.1371/journal.ppat.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrareddy S.N. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat. Med. 2014;20(12):1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Mehraj V., Trottier B. Early initiation rather than prolonged duration of antiretroviral therapy in HIV infection contributes to the normalization of CD8 T-cell counts. Clin. Infect. Dis. 2015 doi: 10.1093/cid/civ809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Mehraj V., Vyboh K. Antiretroviral therapy in primary HIV-1 infection: influences on immune activation and gut mucosal barrier dysfunction. AIDS Rev. 2015;17(3):135–146. [PubMed] [Google Scholar]
- Cassol E. CD14 + macrophages that accumulate in the colon of African AIDS patients express pro-inflammatory cytokines and are responsive to lipopolysaccharide. BMC Infect. Dis. 2015:1–13. doi: 10.1186/s12879-015-1176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A. Natural SIV hosts: showing AIDS the door. Science. 2012;335(6073):1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.K. Productive infection of dendritic cells by simian immunodeficiency virus in macaque intestinal tissues. J. Pathol. 2003;201(4):616–628. doi: 10.1002/path.1482. [DOI] [PubMed] [Google Scholar]
- Cimbro R. IL-7 induces expression and activation of integrin 4 7 promoting naive T-cell homing to the intestinal mucosa. Blood. 2012;120(13):2610–2619. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay C.C. Distinct chemokine triggers and in vivo migratory paths of fluorescein dye-labeled T lymphocytes in acutely simian immunodeficiency virus SIVmac251-infected and uninfected macaques. J. Virol. 2005;79(21):13759–13768. doi: 10.1128/JVI.79.21.13759-13768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couëdel-Courteille A. Delayed viral replication and CD4(+) T cell depletion in the rectosigmoid mucosa of macaques during primary rectal SIV infection. Virology. 2003;316(2):290–301. doi: 10.1016/j.virol.2003.08.021. [DOI] [PubMed] [Google Scholar]
- DaFonseca S. Impaired Th17 polarization of phenotypically naive CD4(+) T-cells during chronic HIV-1 infection and potential restoration with early ART. Retrovirology. 2015;12:38. doi: 10.1186/s12977-015-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S.M. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyavar Shetty R. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J. Clin. Investig. 2012;122(5):1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbim C. Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Immunol. 2008;181(12):8613–8623. doi: 10.4049/jimmunol.181.12.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple H.-J. Mucosal but not peripheral FOXP3 + regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108(9):3072–3078. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- Estes J.D. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. In: Trkola A., editor. 6(8) 2010. p. e1001052. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. In: Luban J., editor. 5(2) 2009. p. e1000295. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavan T.W. Gut immune dysfunction through impaired innate pattern recognition receptor expression and gut microbiota dysbiosis in chronic SIV infection. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori A. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J. Clin. Microbiol. 2008;46(2):757–758. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley S.A. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151(2):253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X.P. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley-McBain T. HIV Research for Prevention conference. 2014. Lack of neutrophil recruitment to mucosal sites after SIV infection is associated with inflammation and viremia; p. 12.05. [Google Scholar]
- Hirao L.A. Early mucosal sensing of SIV infection by paneth cells induces IL-1β production and initiates gut epithelial disruption. In: Silvestri G., editor. 10(8) 2014. p. e1004311. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.H. Review article: the intersection of mucosal pathophysiology in HIV and inflammatory bowel disease, and its implications for therapy. Aliment. Pharmacol. Ther. 2014;40(10):1171–1186. doi: 10.1111/apt.12976. [DOI] [PubMed] [Google Scholar]
- Jenabian M.-A. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J. Infect. Dis. 2015;212(3):355–366. doi: 10.1093/infdis/jiv037. [DOI] [PubMed] [Google Scholar]
- Jenq R.R. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012;209(5):903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenway-Lynch C.S. Dynamics of cytokine/chemokine responses in intestinal CD4 + and CD8 + T cells during acute simian immunodeficiency virus infection. J. Virol. 2013;87(21):11916–11923. doi: 10.1128/JVI.01750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klase Z. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8(5):1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt N.R. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3(4):387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt N.R. Loss of mucosal CD103 + DCs and IL-17 + and IL-22 + lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5(6):646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt N.R. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J. Clin. Investig. 2013;123(2):903–907. doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler D.P. Enteropathy associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1984;101(4):421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- Kristoff J. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J. Clin. Investig. 2014;124(6):2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-Y. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. In: Unutmaz D., editor. 10(9) 2015. p. e0135858. (PLoS ONE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 2012;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Peak SIV replication in resting memory CD4 + T cells depletes gut lamina propria CD4 + T cells. Nature. 2005;434(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Li Q. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J. Infect. Dis. 2008;197(3):420–429. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- Li Q. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Hypercytotoxicity and rapid loss of NKp44 + innate lymphoid cells during acute SIV infection. In: Silvestri G., editor. 10(12) 2014. p. e1004551. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Bone marrow-imprinted gut-homing of plasmacytoid dendritic cells (pDCs) in acute simian immunodeficiency virus infection results in massive accumulation of hyperfunctional CD4 + pDCs in the mucosae. J. Infect. Dis. 2015;211(11):1717–1725. doi: 10.1093/infdis/jiu671. [DOI] [PubMed] [Google Scholar]
- Lozupone C.A. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14(3):329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G., Tincati C., Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013;26(1):2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil J.J. Massive infection and loss of memory CD4 + T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Mehandru S. Primary HIV-1 infection is associated with preferential depletion of CD4 + T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micci L. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J. Clin. Investig. 2015 doi: 10.1172/JCI81400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M. Focused examination of the intestinal epithelium reveals transcriptional signatures consistent with disturbances in enterocyte maturation and differentiation during the course of SIV infection. PLoS ONE. 2013;8(4):e60122. doi: 10.1371/journal.pone.0060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J.C., Lederman M.M. CD8 T cell persistence in treated HIV infection. Curr. Opin. HIV AIDS. 2014;9(5):500–505. doi: 10.1097/COH.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam P. Expansion of FOXP3 + CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J. Immunol. 2010;184(4):1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- Nigam P. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J. Immunol. 2011;186(2):745–753. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- Nowak P. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS (London, England) 2015 doi: 10.1097/QAD.0000000000000869. [DOI] [PubMed] [Google Scholar]
- Ortiz A.M. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. In: Douek D.C., editor. 9(7) 2013. p. e1003471. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection. J. Virol. 2014;88(22):13015–13028. doi: 10.1128/JVI.01757-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I., Apetrei C. Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts. Curr. HIV/AIDS Rep. 2010;7(1):28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte R. Paris Descartes University of Medicine; 2014. Perturbations de l'homéostasie lymphocytaire T chez le macaque rhésus chinois en phase aiguë d'infection par le SIVmac251. (Ph.D thesis) (Available from: Theses.fr. [9 october 2013]) [Google Scholar]
- Quigley M.F. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J. Virol. 2006;80(6):3083–3087. doi: 10.1128/JVI.80.6.3083-3087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R.K. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44 + mucosal NK cells during SIV infection. Blood. 2011;118(12):3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R.K. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J. Infect. Dis. 2012;206(9):1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L.A., Finlay B.B., Maizels R.M. Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J. Immunol. 2015;195(9):4059–4066. doi: 10.4049/jimmunol.1501432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers V.D., Fassett R., Kagnoff M.F. Abnormalities in intestinal mucosal T cells in homosexual populations including those with the lymphadenopathy syndrome and acquired immunodeficiency syndrome. Gastroenterology. 1986;90(3):552–558. doi: 10.1016/0016-5085(86)91108-x. [DOI] [PubMed] [Google Scholar]
- Routy J.-P. Clinical relevance of kynurenine pathway in HIV/AIDS: an immune checkpoint at the crossroads of metabolism and inflammation. AIDS Rev. 2015;17(2):96–106. [PubMed] [Google Scholar]
- Sandler N.G. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J. Infect. Dis. 2014;210(10):1549–1554. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss T., Stahl-Hennig C. Correlation between CD4 + T-cell loss and gag-specific T cells in different intestinal sites of chronically SIV-infected rhesus monkeys. AIDS (London, England) 2011;25(4):429–433. doi: 10.1097/QAD.0b013e3283430034. [DOI] [PubMed] [Google Scholar]
- Sereti I. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. In: Silvestri G., editor. 10(1) 2014. p. e1003890. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J.M. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J. Virol. 2011;85(21):11422–11434. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 2009;83(7):3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.D., Ochsenbauer-Jambor C., Smythies L.E. Intestinal macrophages: unique effector cells of the innate immune system. Immunol. Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Smith P.D. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2010;4(1):31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsouk M. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS (London, England) 2015;29(1):43–51. doi: 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Tabb B. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J. Infect. Dis. 2013;207(6):880–892. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat. Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Veazey R.S. et al., Gastrointestinal tract as a major site of CD4 + T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Veazey R.S. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 2000;74(23):11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. 2015;12:14. doi: 10.1186/s12977-015-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013;5(193):193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I. Gut-resident Lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected macaques. Cell Rep. 2015:1–10. doi: 10.1016/j.celrep.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyboh K. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J. Immunol. Res. 2015:1–9. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Divergent kinetics of proliferating T cell subsets in simian immunodeficiency virus (SIV) infection: SIV eliminates the “first responder” CD4 + T cells in primary infection. J. Virol. 2013;87(12):7032–7038. doi: 10.1128/JVI.00027-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang X., Veazey R.S. Th17 cells coordinate with Th22 cells in maintaining homeostasis of intestinal tissues and both are depleted in SIV-infected macaques. J. AIDS Clin. Res. 2014;05(05) doi: 10.4172/2155-6113.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5(6):658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus-infected macaques. FASEB J. 2015;29(12):5072–5080. doi: 10.1096/fj.15-276477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younas M. Immune activation in the course of HIV-1 infection: causes, phenotypes and persistence under therapy. HIV Med. 2015 doi: 10.1111/hiv.12310. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Plasmacytoid dendritic cells promote HIV-1–induced group 3 innate lymphoid cell depletion. J. Clin. Investig. 2015;125(9):3692–3703. doi: 10.1172/JCI82124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. In: Silvestri G., editor. 7(6) 2011. p. e1002100. (PLoS Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans. Retrovirology. 2013;10(1):1-1. doi: 10.1186/1742-4690-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]