Abstract
Objective
To determine the impact of poor glycaemic control on the prevalence of erectile dysfunction among men with type 2 Diabetics aged 27 to 85 years.
Design
The databases Embase classic+Embase, Global health, Ovid Medline and PsychINFO, were searched for relevant studies in June 2014 using the keywords: (Diabetes Mellitus OR diabetes mellitus type2 OR DM2 OR T2DM OR insulin resistance) AND (erectile dysfunction OR sexual dysfunction OR impotence) AND glycaemic control.
Setting
All study settings were considered (primary care, secondary care and tertiary care setting).
Participants
Type 2 Diabetic Patients with erectile dysfunction.
Main outcome measures
Included studies must include one of the following outcomes: (1) HBA1c for assess the level of glycaemic control; (2) Erectile dysfunction (any stage: IIEF-5 = 21 or less).
Results
Five cross-sectional studies involving 3299 patients were included. The findings pointed to a positive association between erectile dysfunction and glycaemic control. Three studies showed a significant positive association, while one study showed only a weak correlation and one study showed borderline significance. Patients’ age, diabetes mellitus duration, peripheral neuropathy and body mass index had positive association with erectile dysfunction. However, smoking and hypertension were not associated with erectile dysfunction in most included studies. Physical activity had a protective effect against erectile dysfunction.
Conclusion
We may conclude that the risk of erectile dysfunction is higher in type 2 diabetic men with poor glycaemic control than those with good control.
Keywords: diabetes, endocrinology, clinical, sexual health
Introduction
Erectile dysfunction (ED) is defined as the inability to achieve and/or maintain penile erection sufficient for satisfactory sexual intercourse.1 ED is a common problem in men with a history of diabetes mellitus (DM).2 The prevalence of ED among patients with history of type 1 and/or type 2 DM in the literature varies from 35% to 90%.3–12 Literature including patients with history of type 2 DM only shows the prevalence of ED severity, by international index of erectile function (IIEF), as 73.10%,10 86.10%11 and 90%.12
Diabetic men have almost a threefold higher probability to develop ED compared with non-diabetics;13 they are also prone for the onset of ED to occur 10 to 15 years earlier than in non-diabetic men.13 ED in diabetic men has also been shown to be more severe and associated with a poorer quality of life.14 It is less responsive to medical treatment compared with ED in non-diabetic men.15 However, it is still unclear whether ED in diabetic men is a consequence only of hyperglycaemia and microvascular complications or a collection of risk factors, as the patients often present with other ED risk factors, such as cardiovascular diseases, hypertension, smoking and obesity at the same time.16
The importance of poor glycaemic control as an indicator of reduced erectile function in diabetic men is still unclear. Several studies have demonstrated a significant correlation between the two;11,17–21 however, some studies have been mixed as to whether there is a statistically significant correlation between ED and poor glycaemic control, showing only a borderline correlation8,22,23 or no correlation at all.24–26 The inconsistency in the literature means that further studies are needed to clarify a causal link between prolonged hyperglycaemia and ED. This disparity between studies may be the result of the sample sizes used and multivariate strategies used to analyse the data.
In our review, we aim to clearly determine the impact of poor glycaemic control on the prevalence of ED in men with type 2 DM, as well as the impact of other possible risk factors, such as duration of DM, patients’ age, hypertension and cigarette smoking on the prevalence of ED.
Methods
The databases Embase classic + Embase from 1947, Global health from 1973, Ovid Medline from 1946 and PsychINFO from 1967 were searched for relevant studies in June 2014 using the keywords: (Diabetes Mellitus OR diabetes mellitus type 2 OR DM2 OR T2DM OR insulin resistance) AND (erectile dysfunction OR sexual dysfunction OR impotence) AND glycaemic control.
In consultation with the research team, we considered any observational study at any clinical settings that explored the impact of glycaemic control level on the prevalence of ED in men with type 2 DM. The inclusion criteria for the participants were any patient with type 2 DM, aged between 27 and 85 years. The primary outcome must include: glycaemic control which was measured by glycosylated haemoglobin (HBA1c) and diagnosis of ED was done by using the international index of erectile function (IIEF-5). We defined poor glycaemic control as HBA1c more than 7% (53 mmol/mol) and ED, if IIEF-5 is equal to or less than 21.27 Our secondary outcomes were the impact of other possible risk factors on the prevalence of ED for men with T2DM, e.g. duration of DM, patients’ age, hypertension and smoking. Searching was restricted to articles in the English language.
Two reviewers (TB and SH) performed the search and reviewed the results. The duplicate studies were removed using EndNote. During the initial review for titles and abstracts, studies that did not meet our criteria were excluded. If the reviewers were uncertain about certain studies during the initial review, then the full text article was assessed. Independently, two reviewers (TB and SH) assessed all relevant studies. Disagreement had been resolved by discussion and external opinion had been requested if needed.
Two reviewers (TB and SS) independently assessed the included studies for quality. Full critical appraisal was done for each study, by using Newcastle Ottawa quality assessment tool for cohort studies; checklists were adapted to be applied for cross-sectional studies.28 Items reviewed included representativeness of the sample; sample size; response rate; validity of measurement tool, if validated and if non-validated; study controls for the most important factor and additional factors; assessment of the outcome; and statistical test used.
After the data extraction form was developed, two reviewers (TB and SS) independently extracted the data from included studies on the prevalence of ED among type 2 DM and the correlation between glycaemic control and other risk factors with ED. p values were used for the magnitude of the effect.
Results
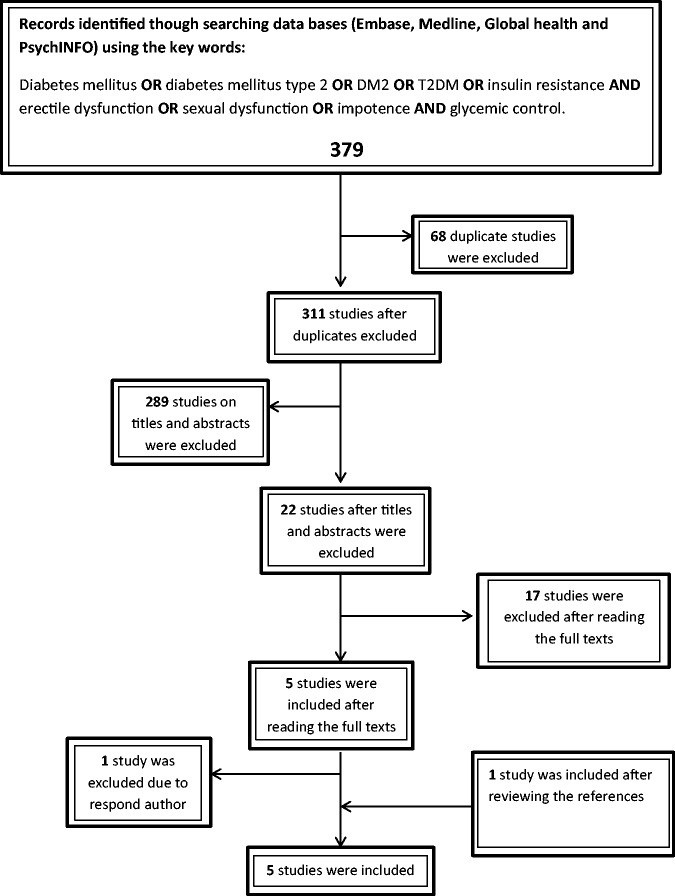
Our electronic search identified 379 studies (Figure 1, PRISMA flow chart), of which 68 duplicated studies were excluded. An additional 289 studies were excluded after title and abstract review as they did not meet our inclusion criteria, leaving 22 studies; of these 22 studies, 17 studies were further excluded on reviewing their full text. The main reasons for exclusion were that type 1 diabetic patients were included and some studies did not measure the association between the ED prevalence and glycaemic control.
Figure 1.
Flow chart showing search findings.
We found one additional study11 through bibliography hand searches; also one study29 was excluded because the author did not respond to our query about the assessment of DM control. Five studies were finally included in this systematic review;8,11,17,20,23 they were all of cross-sectional design. Table 1 summarises the characteristics and the main findings of the five included studies.
Table 1.
Data extracted from articles.
| Study | Objective of study | Sample characteristics | Outcomes | Confounders included |
|---|---|---|---|---|
| Sexual function in men with diabetes type 2: association with glycaemic control Romeo et al.17 Cross-sectional study, Ohio | To evaluate the association of glycaemic control with ED in men with type 2 DM | – Total study population: 78 – Mean age: 62.0 ± 12.3 years (38–82) – Mean HBA1c: 8.1% ± 1.9% (5.2–15.6) – Mean EF score: 16.6 ± 5.9 (5–23) | – After EF scores were stratified by the level of glycaemic control: – Mean EF score decreased as HBA1c increased (analysis of variance p = 0.002) – After bivariate analysis, to examine the correlation of ED with subject characteristics: There was a significant correlation of HBA1c with neuropathy but not with participant age, duration of DM or some medication use (data not shown) – Multivariate analysis showed that HBA1c was an independent predictor of EF score (p < 0.001) even after adjusting for peripheral neuropathy, which was also an independent predictor (p = 0.023) – When subject age and DM duration were included in multivariate models, only HBA1c and neuropathy were significant independent predictors of EF score | – HBA1c – Age – DM duration – Peripheral neuropathy – Some medications |
| Determinants of erectile dysfunction in type 2 diabetes F Giugliano et al.20 Cross-sectional study, Naples (Italy) | To evaluate the prevalence and correlates of ED in a population of diabetic men | – Total study population: 555 – All ED: 333 (60%) – Mild: 9% – Mild to moderate: 11.2% – Moderate: 16.9% – Severe: 22.9% – Mean age: 57.9 ± 6.9 years (35–70) – Mean HBA1c 8.4% ± 1.3% – Mean DM duration: 4.9 ± 1.5 years | Contribution of different risk factors to risk of ED in the diabetic population (based on multivariate logistic regression): 1-Age (OR 1.10) 95% CI 1.05–1.15 (p 0.001) 2-DM duration (OR 1.05) 95% CI 1.01–1.10 (p 0.01) 3-HBA1c (OR 1.18) 95% CI 1.02–1.37 (p 0.03) 4-MS (OR 2.08) 95% CI 1.17–3.26 (p 0.01) 5-BMI (OR 1.03) 95% CI 1.00–1.07 (p 0.04) 6-WHR^ (OR 1.04) 95% CI 1.01–1.08 (p 0.03) 7-HTN (OR 1.34) 95% CI 1.08-2.03 (p 0.02) 8-DLD (OR 1.23) 95% CI 1.04–1.49 (p 0.01) 9-Cigarette smoking: a-past (OR 1.15) 95% CI 0.86–1.98 (p 0.56) not significant b-current (OR 1.36) 95% CI 0.81–2.09 (p 0.35) not significant 10-Physical activity (OR 0.90) 95% CI 0.77–0.98 (p 0.04) protective of ED 11-Depression (OR 1.09) 95% CI 1.02–1.19 (p 0.03) The mean HBA1c level was significantly higher in diabetic patients with ED than those without ED (8.7 ± 1.0% vs 7.9 ± 0.9%, p = 0.01). | – HBA1c – Age – DM duration – Metabolic syndrome – BMI – WHR – HTN – DLD – Cigarette smoking – Physical activity – Depression |
| Prevalence of erectile dysfunction in Korean men with type 2 DM Cho et al.8 Cross-sectional study, May 2002 to March 2003, Korea | To investigate the prevalence and risk factors for developing ED in 1312 Korean men with diabetes | – Total study population: 1312 – All ED: 858 (65.4%) – Mild: 20.1% – Moderate: 19.5 – Complete: 25.8% – Mean age: 53.8 ± 6.65 years (40–64) – Mean HBA1c 7.9% ± 1.83% – Median DM duration 6 years (range 1–43) | When the subjects were stratified according to ED status (Normal, mild, moderate and complete), there were significant trends relating the severity of ED to: 1-Age (p < 0.001) 2-DM duration (p < 0.001) 3-Fasting glucose (p < 0.05) 4-HBA1c (p < 0.001) 5-Duration of alcohol consumption (p < 0.001) – No significant differences were observed in blood pressure or duration of smoking Other risk factors for ED were examined: 1-Subjects who exercised regularly had rate of complete ED 0.62 times those of alcohol abstainers or sedentary subjects (95% CI 0.44–0.89, p < 0.01) 2-Subjects who consumed alcohol had rate of complete ED 0.49 times the same comparison above (95% CI 0.36–0.66, p < 0.001) 3-Subjects who were on insulin treatment are 6.1 times more likely to have complete ED than non-insulin users (95% CI 3.2–11.4, p < 0.001) 4-Subjects who were on diet therapy alone had rates of complete ED only 0.59 times of those receiving the other treatments (95% CI 0.36–0.95, p < 0.001) 5-Subjects with either neuropathy or macrovascular disease were, respectively, 1.8 times (95% CI 1.11–2.9, p < 0.05) and 3.5 times (95% CI 1.14–10.6, p < 0.05) as likely to have complete ED as those subjects without such complications 6-Complete ED was not significantly related to either HTN or smoking status When multiple logistic regression analysis was used to identify significant independent risk factors for all types of ED: 1-Age (p < 0.001) 2-DM duration (p < 0.005) 3-Neuropathy (p < 0.05) 4-Use of insulin (p < 0.001) 5-Macrovascular complications (p 0.038) Were independent positive risk factors for all types of ED. But, alcohol consumption (p < 0.05) and exercise (p < 0.01) were independent negative risk factors. Moreover, HBA1c showed only weak (or no) independent relationship with the development of diabetic-related ED (p 0.092). – When we analyzed the data further using complete ED as the dependent variable, those variables also showed independent relationship with all types of ED with exception of neuropathy. | – HBA1c – Age – DM duration – HTN – Smoking – Neuropathy – Use of insulin – Macrovascular disease – Alcohol consumption – Exercise |
| Association of glycaemic control with risk of erectile dysfunction in men with type 2 diabetes Lu et al.20 Cross-sectional study, January 2004–May 2006, Taiwan | To evaluate the association of glycaemic control with risk of ED in type 2 diabetics | – Total study population: 792 – All ED: 662 (83.6%) – Mild: 123 (15.5%) – Mild to moderate: 133 (16.8%) – Moderate: 64 (8.1%) – Severe: 342 (43.2%) – Mean age: 65.6 ± 13.2 (27–85) – Mean duration of DM: 9.0 ± 7.5 (1–39) – Mean HBA1c: 8.2% ± 2.0% (4.3–17.5) | The prevalence of ED was positively correlated with subject's age and duration of diabetes (p 0.000) Higher HBA1c level was associated with a higher risk of ED with borderline significance (p = 0.059) – The ORs of ED for risk factors (HBA1c, HTN, DLD and cigarette smoking) after adjusting for age and DM duration: only HBA1c level was significantly associated with ED risk (p 0.034) – The prevalence of ED was 66.7% in younger group and 93.1% in the older group (p = 0.000) – Those with ED had a significantly higher mean HBA1c level than those without ED in younger group (8.8 ± 2.2 vs 7.9 ± 2.0%, p < 0.0009) – There was no significant difference in mean HBA1c level between those with or without ED in the older group (8.0 ± 1.8 vs 8.1 ± 2.0%, p = 0.63) – When multivariate logistic regression was used for the contribution of risk factors to risk of ED: (1) in young group ( < 60): a-Age OR 1.06 (95% CI 1.02–1.10) p 0.002 b-DM duration OR 1.06(95% CI 1.001–1.12) p 0.045 c-HBA1c OR 1.21 (95% CI 1.06–1.39) p 0.004 were significant independent risk actors for ED (2) in old group (>60): a-Age OR 1.07 (95% CI 1.02–1.13) p 0.009 b-DM duration OR 1.07 (95% CI 1.01–1.14) p 0.019 were significant independent risk factors for ED – The mean HBA1c level was significantly higher in those with severe ED than those without severe ED among the younger group (9.6 ± 2.3 vs 8.3 ± 2.1%, p = 0.0002) – The mean HBA1c level did not show significant difference between those with severe ED and those without among the older group (8.0 ± 1.9 vs 8.0 ± 1.7%, p = 0.99) – Contribution of HBA1c and other risk factors to risk of SEVERE ED based on multivariate logistic regression: (1) in young group: a-DM duration OR 1.09 (95% CI 1.03–1.16) p 0.003 b-HBA1c OR 1.27 (95% CI 1.09–1.49) p 0.003 c-HTN OR 2.68 (95% CI 0.64–1.53) p 0.015 were significantly independent risk factors for severe ED compared with normal, mild or moderate ED (2) in old group: only age OR 1.08 (95% CI 1.05–1.11) p 0.000 was significant independent risk factor for severe ED compared with normal, mild or moderate ED. | – HBA1c – Age – DM duration – HTN – DLD – Cigarette Smoking |
| Erectile dysfunction risk factors in non-insulin dependent diabetic Saudi patients El-Sakka et al.11 Cross-sectional study, Saudi Arabia | To assess the prevalence of and analyze risk factors for ED in patients with non-insulin dependent diabetes in Makkah, Saudi Arabia | – Total study population: 562 – All ED: 86.1% – Mild: 7.7% – Moderate: 29.4% – Severe: 49.1% – Mean age 53.7 ± 10.8 years (27–85) – Mean DM duration 10.8 ± 7.5 years (1–40) | – The prevalence of ED increased with age, in younger than 50 years the prevalence was 25% and in 50 years or older the prevalence was 75%. Men without ED 70% were younger and 30% were older than 50 years (p = 0.0001) – Patents with a greater than 10 years history of DM were 3 times as likely to report ED as those with a history of less than 5 years (p = 0.0001). – Patients with poor glycaemic control were 12.2 times as likely to report ED as those with good glycaemic control The prevalence of ED was significantly associated with: 1-poor glycaemic control (p = 0.0001). 2-increased body mass index (p = 0.0001). 3-a history of smoking (p = 0.0001). 4-the duration of smoking (p = 0.003). 5-the number of cigarettes daily (p = 0.0001). 6-Some DM treatment (p = 0.0001) | – HBA1c – Age – DM duration – BMI – Smoking – DM treatments |
ED: erectile dysfunction; DM: diabetes mellitus; EF: erectile function.
Studies included were published between 2000 and 2010. Total sample size was 3299 patients; they were conducted in the USA (78 participants), Italy (555 participants), Korea (1312 participants), Taiwan (792 participants) and Saudi Arabia (562 participants). Mean age ± SD were 62 ± 12.3 years,17 57.9 ± 6.9 years,20 53.8 ± 6.65 years,8 65.6 ± 13.2 years23 and 53.7 ± 10.8 years.11 Mean HBA1c ± SD were 8.1 ± 1.9%,17 8.4 ± 1.3%,20 7.9 ± 1.83%,8 8.2 ± 2.0%23 and there were no data from El-Sakka and Tayeb.11 Mean DM duration were 4.9 ± 1.5 years,20 9.0 ± 7.5 years,23 10.8 ± 7.5 years,11 median DM duration was six years8 and there were no data from Romeo et al.17 Regarding all degrees of ED, the prevalence were 60%,20 65.4%,8 83.6%,23 86.1%11 and data were not shown in one study.17
The highest score of quality assessment is 9 points and the lowest score is 7 points, which demonstrate a good quality of included studies.
The findings generally pointed to a positive association between ED and glycaemic control. Three studies showed a significant positive association,11,17,20 while one study showed only a weak correlation8 and one study showed a borderline significant association.23
In El-Sakka and Tayeb,11 there was a higher likelihood of 12.2 times of patients with poor glycaemic control to suffer ED as compared with their counterparts with good glycaemic control. In the study by Romeo et al.,17 the researchers showed that HBA1c was an independent predictor of EF score (p < 0.001). In Giugliano et al.,20 there was a higher average level of HBA1c in diabetic men with ED than in those who do not have ED (8.7 ± 1.0% vs. 7.9 ± 0.9%, p = 0.01). Meanwhile, in the largest study,8 the data from 1312 Korean men with type 2 DM, after using multivariate logistic regression to recognise independent risk factors for all types of ED, only a weak independent connection with the occurrence of diabetic-related ED was shown by HBA1c (p 0.092). In Lu et al.,23 men suffering from ED had significantly higher average HBA1c level compared with those not suffering from ED in the youthful age group (8.8 ± 2.2 vs. 7.9 ± 2.0%, p < 0.0009); however, no significant difference in mean HBA1c level between men with ED and those not suffering among the older age group (8.0 ± 1.8% vs. 8.1 ± 2.0%, p = 0.63). There was also a significant higher mean HBA1c level in those with severe ED than in those with no severe ED among the youth (9.6 ± 2.3 vs. 8.3 ± 2.1%, p = 0.0002), while mean HBA1c level did not show significant difference between those with severe ED and those who did not have it among the older generation (8.0 ± 1.9 vs. 8.0 ± 1.7%, p = 0.99).
Patients’ age, DM duration, peripheral neuropathy and body mass index had positive association with ED. However, smoking and hypertension were not associated with ED in most included studies. Physical activity had a protective effect against ED.
Discussion
Penile erection is defined as the result of smooth muscle relaxation in the cavernous body and associated blood vessels.30 Nitric oxide plays a major role in this process as it is one of the most important endogenous smooth muscle relaxants. For chronic hyperglycaemia and insulin resistance in diabetic patients, endothelial dysfunction is manifested as a decreased level of nitric oxide, leading to insufficient smooth muscle relaxation.
The correlation between glycaemic control and ED
In our systematic review, we identified five cross-sectional studies that examined the association between the glycaemic control (measured by HBA1c) and ED (measured by IIEF-5) among type 2 diabetic men. Sixty per cent of included studies11,17,20 suggested that poor glycaemic control is positively associated with ED in type 2 diabetics as the mean HBA1c was found to be higher among those with ED than those without ED. In the literature, other studies had also shown positive correlation between poor glycaemic control and ED among diabetic patients.18
Lu et al.23 showed a significant positive association between ED and glycaemic control in a younger age group (≤60 years), but not in an older age group (>60 years). Also in the same study, odds ratio of ED for different risk factors, after adjustment for duration of DM and age, showed that the HBA1c level was significantly associated with ED risk (p 0.034). However, Thomas et al.’s22 study has shown that patients diagnosed with ED are mostly older and the commonness of the ED condition increased with age. Cho et al.8 showed a weak relationship between HBA1c level and diabetes-related ED when using a multiple logistic regression analysis to identify risk factors for all types of ED. However, in the same study, classifying the patients based on the level of ED showed the connection between the severity of ED to HBA1c was significant (p < 0.001).
Several studies had demonstrated an insignificant correlation between glycaemic control and ED in diabetic men.24–26 In terms of severe (complete) ED, Cho et al.8 showed a significant positive correlation between complete ED with patients who were on insulin and patients with either macrovascular disease or neuropathy. However, complete ED was not significantly associated to either smoking status or hypertension. On the other hand, patients who were on diet only had rates of complete ED 0.59 times of those on other treatments, also patients who exercised regularly and those who consumed alcohol had a lower rate of complete ED than sedentary patients and those of alcohol abstainers, respectively.
Lu et al.23 showed a significant positive association between severe ED with HBA1c, DM duration and hypertension among a young age group (≤60 years), while only age was a significant independent risk factor for severe ED among an older age group (>60 years).
In summary, we may conclude that the risk of ED is higher in type 2 diabetic men with poor glycaemic control than those with good control, since three studies showed that there were positive associations between the two and the other two studies showed some correlations.
Risk factors for ED
Four of the included studies8,11,20,23 highlighted that the prevalence of ED was mainly attributable to patients’ age and the duration of diabetes. This positive association was confirmed by additional study.31 Only one study17 showed that both subjects' age and DM duration were not associated with ED prevalence.
Two studies8,17 examined the peripheral neuropathy and the correlation with ED; both studies showed significant positive association. This was consistent with previous reports.32,33
Two studies11,20 examined the correlation between body mass index and ED; both studies confirmed a significant association with ED. A similar finding was reported by Esposito et al.34
Giugliano et al.20 is the only study that examined metabolic syndrome, waist hip ratio and depression and their correlation with ED; all of these factors were positively associated with ED prevalence.
Hypertension was examined in three studies;8,20,23 only one study20 showed a positive association, which was supported by previous evidence,35 while the other two studies did not show any association with ED.
Cigarette smoking was examined in four studies;8,11,20,23 only one of these studies showed a significant correlation between smoking and prevalence of ED.11 A systematic review of observational studies came to a conclusion that ED risk is higher in current and former users of smoking than in those who never smoke, and smoking cessation may lead to lower risk of ED than current smoking.36
Dyslipidaemia was examined in Giugliano et al.20 and Lu et al.;23 one study20 showed a positive association and the other study23 did not show that.
In Cho et al.,8 stratifying of the patients according to ED status (normal, mild, moderate and complete) showed a significant trend connecting the severity of ED to the duration of alcohol consumption (p < 0.001), but similarly using multivariate regression analysis independent predictors for all types of ED: alcohol consumption (p < 0.05) and exercise (p < 0.01) were negative independent risk factors of ED. Additional study by Giugliano et al. showed that physical activity protected against ED. An assessment of the association between ED and physical activity was performed in population-based studies with meta-analysis, and higher physical activity was seen to lower the risk of ED.37 In Look AHEAD (action for health in diabetes),31 cardiorespiratory fitness was found to protect ED among the 373 men with diabetes aged 45–75 years. Further study by De Berardis et al.4 measured quality of life in diabetic men with ED and showed that exercise can help prevent ED.
A systematic review of the association between ED and cardiovascular disease38 has shown that ED could be a possible sign of systematic endothelial dysfunction. ED usually occurs before cardiovascular disease and could therefore be an early sign of symptomatic cardiovascular disease.
Limitation of studies
Included studies had some limitations; for example, there were considerable differences in study settings, sample size and in adjustment of confounding factors. Romeo et al.’s17 study had the lowest sample size (78 participants). The description of the sampling strategy was not mentioned in El-Sakka and Tayeb's study.11 In both studies, there were no descriptions of the response rate. However, there were similarities of included studies in terms of study design since all included studies are cross-sectional studies and they all used IIEF-5 for the determination of ED and HBA1c to evaluate the glycaemic control level.
Conclusion
We may conclude that the risk of ED is higher in type 2 diabetic men with poor glycaemic control than those with good control. Also, an increase in patients’ age, DM duration, BMI and peripheral neuropathy existence can increase the risk of ED among diabetic men.
This will raise the importance of early screening of ED among diabetic men and the importance of HBA1c control as there is supporting evidence for the reduction of DM complications. We therefore recommend the incorporation of early ED screening for all diabetic men alongside the screening of neuropathy, retinopathy and nephropathy which are already endorsed by all existing guidelines.
Acknowledgements
None
Declarations
Competing interests
None declared
Funding
None declared
Guarantor
SR
Ethical approval
Not applicable
Contributorship
TB and SH developed the concept for the research and the paper. TB collected data and analysis. TB and SS independently assessed the included studies for quality TB, SH, SR and AM finalised the text.
Provenance
Not commissioned; peer-reviewed by Aasem Saif
References
- 1.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA 1993; 270: 83–90. [PubMed] [Google Scholar]
- 2.Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, Girman CJ, et al. Diabetes and sexual dysfunction: results from the Olmsted County study of urinary symptoms and health status among men. J Urol 2007; 177: 1438–1442. [DOI] [PubMed] [Google Scholar]
- 3.McCulloch DK, Campbell IW, Wu FC, Prescott RJ, Clarke BF. The prevalence of diabetic impotence. Diabetologia 1980; 18: 279–283. [DOI] [PubMed] [Google Scholar]
- 4.De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 2002; 25: 284–291. [DOI] [PubMed] [Google Scholar]
- 5.Fedele D, Coscelli C, Santeusanio F, Bortolotti A, Chatenoud L, Colli E, et al. Erectile dysfunction in diabetic subjects in Italy. Gruppo Italiano Studio Deficit Erettile nei Diabetici. Diabetes Care 1998; 21: 1973–1977. [DOI] [PubMed] [Google Scholar]
- 6.Bacon CG, Hu FB, Giovannucci E, Glasser DB, Mittleman MA, Rimm EB. Association of type and duration of diabetes with erectile dysfunction in a large cohort of men. Diabetes Care 2002; 25: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 7.Siu SC, Lo SK, Wong KW, Ip KM, Wong YS. Prevalence of and risk factors for erectile dysfunction in Hong Kong diabetic patients. Diabet Med 2001; 18: 732–738. [DOI] [PubMed] [Google Scholar]
- 8.Cho NH, Ahn CW, Park JY, Ahn TY, Lee HW, Park TS, et al. Prevalence of erectile dysfunction in Korean men with type 2 diabetes mellitus. Diabet Med 2006; 23: 198–203. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano FA, Leriche A, Jaudinot EO, de Gendre AS. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology 2004; 64: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 10.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med 2008; 5: 2125–2134. [DOI] [PubMed] [Google Scholar]
- 11.El-Sakka AI, Tayeb KA. Erectile dysfunction risk factors in noninsulin dependent diabetic Saudi patients. J Urol 2003; 169: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki H, Yamasaki H, Ogawa K, Nanjo K, Kawamori R, Iwamoto Y, et al. Prevalence and risk factors for erectile dysfunction in Japanese diabetics. Diabetes Res Clin Pract 2005; 70: 81–89. [DOI] [PubMed] [Google Scholar]
- 13.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol 1994; 151: 54–61. [DOI] [PubMed] [Google Scholar]
- 14.Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF, et al. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care 2003; 26: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998; 338: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 16.Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010; 7: 1598–1607. [DOI] [PubMed] [Google Scholar]
- 17.Romeo JH, Seftel AD, Madhun ZT, Aron DC. Sexual function in men with diabetes type 2: association with glycemic control. J Urol 2000; 163: 788–791. [PubMed] [Google Scholar]
- 18.Roth A, Kalter-Leibovici O, Kerbis Y, Tenenbaum-Koren E, Chen J, Sobol T, et al. Prevalence and risk factors for erectile dysfunction in men with diabetes, hypertension, or both diseases: a community survey among 1412 Israeli men. Clin Cardiol 2003; 26: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matfin G, Jawa A, Fonseca VA. Erectile dysfunction: interrelationship with the metabolic syndrome. Curr Diab Rep 2005; 5: 64–69. [DOI] [PubMed] [Google Scholar]
- 20.Giugliano F, Maiorino M, Bellastella G, Gicchino M, Giugliano D, Esposito K. Determinants of erectile dysfunction in type 2 diabetes. Int J Impot Res 2010; 22: 204–209. [DOI] [PubMed] [Google Scholar]
- 21.De Angelis L, Marfella MA, Siniscalchi M, Marino L, Nappo F, Giugliano F, et al. Erectile and endothelial dysfunction in type II diabetes: a possible link. Diabetologia 2001; 44: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GN, Tomlinson B, Abdullah AS, Yeung VT, Chan JC, Wong KS. Association of erectile dysfunction with cardiovascular risk factors and increasing existing vascular disease in male Chinese type 2 diabetic patients. Diabetes Care 2005; 28: 2051–2053. [DOI] [PubMed] [Google Scholar]
- 23.Lu CC, Jiann BP, Sun CC, Lam HC, Chu CH, Lee JK. Association of glycemic control with risk of erectile dysfunction in men with type 2 diabetes. J Sex Med 2009; 6: 1719–1728. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hunayan A, Al-Mutar M, Kehinde EO, Thalib L, Al-Ghorory M. The prevalence and predictors of erectile dysfunction in men newly diagnosed with type 2 diabetes mellitus. BJU Int 2007; 99: 130–134. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Fan W, Li G, Tam T. Predictors for erectile dysfunction among diabetics. Diabetes Res Clin Pract 2006; 71: 313–319. [DOI] [PubMed] [Google Scholar]
- 26.Shiri R, Ansari M, Falah Hassani K. Association between comorbidity and erectile dysfunction in patients with diabetes. Int J Impot Res 2006; 18: 348–353. [DOI] [PubMed] [Google Scholar]
- 27.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999; 11: 319–326. [DOI] [PubMed] [Google Scholar]
- 28.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13: 154–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal A, Singh P, Ahuja A. Prevalence and severity of erectile dysfunction as assessed by IIEF-5 in north Indian type 2 diabetic males and its correlation with variables. J Clin Diagn Res 2013; 7: 2936–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 2010; 7: 445–475. [DOI] [PubMed] [Google Scholar]
- 31.Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano IN, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med 2010; 7: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecht MJ, Neundörfer B, Kiesewetter F, Hilz MJ. Neuropathy is a major contributing factor to diabetic erectile dysfunction. Neurol Res 2001; 23: 651–654. [DOI] [PubMed] [Google Scholar]
- 33.Ledda A. Diabetes, hypertension and erectile dysfunction. Curr Med Res Opin 2000; 16: s17–s20. [DOI] [PubMed] [Google Scholar]
- 34.Esposito K, Giugliano F, Ciotola M, De Sio M, D'Armiento M, Giugliano D. Obesity and sexual dysfunction, male and female. Int J Impot Res 2008; 20: 358–365. [DOI] [PubMed] [Google Scholar]
- 35.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol 2004; 171: 2341–2345. [DOI] [PubMed] [Google Scholar]
- 36.Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8: e60443–e60443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng JY, Ng EM, Ko JS, Chen RY. Physical activity and erectile dysfunction: meta-analysis of population-based studies. Int J Impot Res 2007; 19: 245–252. [DOI] [PubMed] [Google Scholar]
- 38.Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65: 968–978. [DOI] [PubMed] [Google Scholar]