Abstract
Formaldehyde is the simplest of all aldehydes and is highly cytotoxic. Its use and associated dangers from environmental exposure have been well documented. Detoxification systems for formaldehyde are found throughout the biological world and they are especially important in methylotrophic bacteria, which generate this compound as part of their metabolism of methanol. Formaldehyde metabolizing systems can be divided into those dependent upon pterin cofactors, sugar phosphates and those dependent upon glutathione. The more prevalent thiol-dependent formaldehyde detoxification system is found in many bacterial pathogens, almost all of which do not metabolize methane or methanol. This review describes the endogenous and exogenous sources of formaldehyde, its toxic effects and mechanisms of detoxification. The methods of formaldehyde sensing are also described with a focus on the formaldehyde responsive transcription factors HxlR, FrmR, and NmlR. Finally, the physiological relevance of detoxification systems for formaldehyde in bacterial pathogens is discussed.
Keywords: formaldehyde, glutathione, host–pathogen interactions, Neisseria, Haemophilus
Introduction
Formaldehyde (H2C = O), structurally the simplest of all aldehydes, is a major byproduct of the manufacturing industry (Heck et al., 1990), a common environmental hazard (Flyvholm and Andersen, 1993; Tang et al., 2009; International Agency for Research on Cancer, 2012), and a product of the cellular metabolism of many methylated compounds (see Potential Sources of Formaldehyde). In the scientific literature, studies relating to formaldehyde have focused almost exclusively on its toxicology in animals and humans. The carcinogenic properties and detrimental effects of formaldehyde exposure on growth and reproductive development have been described and summarized extensively (Golden et al., 2006; Tang et al., 2009; Zhang et al., 2009; Szende and Tyihak, 2010; Duong et al., 2011; Tulpule and Dringen, 2013). Formaldehyde is also highly toxic to microbes and it has widespread application as a disinfectant for sterilization. Of interest in this review are the adaptive responses to formaldehyde that occur in microbes, especially bacterial pathogens.
Although it is often considered in a toxicological context, formaldehyde is an important cellular metabolite. In the bacterial world, formaldehyde is generated by methanotrophs and methylotrophs during the oxidation of short-chain hydrocarbons such as methane or methanol. Thus, details of the metabolic reactions and physiological fate of this aldehyde are available mostly in the context of methane or methanol catabolism (Vorholt et al., 2000; Marx et al., 2003, 2004). However, recent discovery of inducible formaldehyde detoxification systems in bacteria that do not use methane or methanol as a carbon source highlights the significance of this aldehyde in the general physiology of prokaryotes. This review will summarize contemporary findings in this area and assess the potential role of formaldehyde during interactions between bacterial pathogens and their host.
Mechanisms of Formaldehyde Toxicity
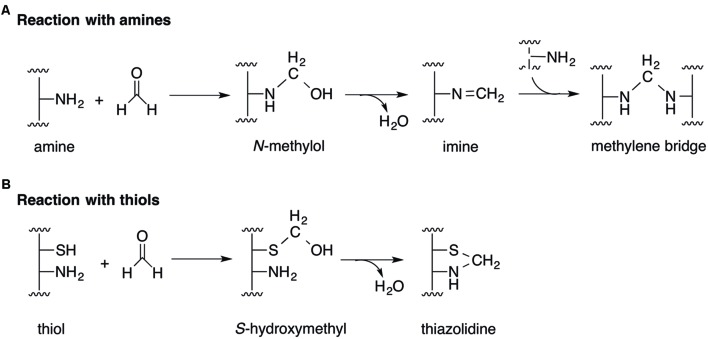
The toxicity of formaldehyde in cells arises primarily from its reactivity as an electrophile. It reacts rapidly with free thiol (-SH; Klatt and Lamas, 2000; Moran et al., 2001; Paget and Buttner, 2003) and amine (-NH2; Feldman, 1973; Conaway et al., 1996) groups on proteins and DNA. The nucleophilic addition of an amine to formaldehyde generates an N-methylol adduct that may subsequently condense to an imine (Figure 1A). This imine carbon is susceptible to further nucleophilic addition by a second amine, forming an irreversible cross-link composed of a methylene bridge (Figure 1A; Feldman, 1973; Conaway et al., 1996). In the reaction between formaldehyde and thiols, nucleophilic addition of the sulfur atom to the aldehyde forms a hemithioacetal (S-hydroxymethyl adduct), which may cyclize rapidly and irreversibly with a neighboring amine to generate a thiazolidine adduct (Figure 1B; Kallen, 1971; Higgins and Giedroc, 2014). Indeed, formaldehyde exposure has been shown to result in DNA and protein damage (Casanovaschmitz et al., 1984; Casanova et al., 1994; Yu et al., 2015), including formation of irreversible formaldehyde adducts (Heck et al., 1990) as well as formaldehyde-catalyzed DNA-DNA (Lu et al., 2010), DNA-protein (Solomon and Varshavsky, 1985; Loshon et al., 1999; Heck and Casanova, 2004), and protein-protein cross-links (Metz et al., 2004).
FIGURE 1.
Mechanisms of formaldehyde toxicity. (A) The reaction of formaldehyde with amines forms an imine adduct via an N-methylol intermediate. The imine can react further with other amines to form methylene bridges between protein and DNA molecules. (B) The reaction between formaldehyde and thiols forms S-hydroxymethyl and thiazolidine adducts.
Biochemical Strategies for Formaldehyde Tolerance in Bacteria
Mechanisms of Formaldehyde Detoxification and Assimilation
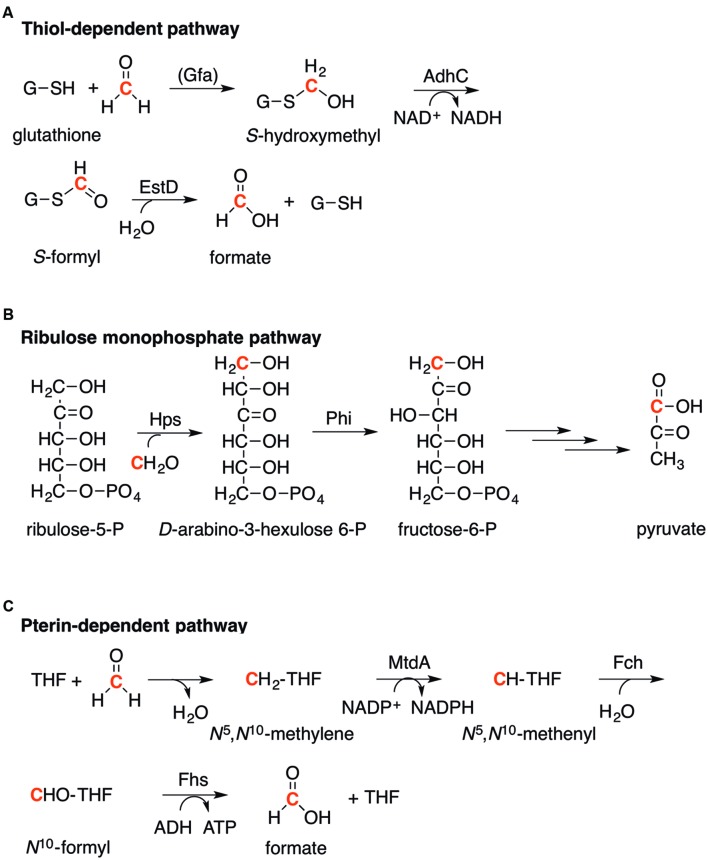
Three major bacterial pathways for formaldehyde detoxification have been identified: thiol-dependent, ribulose monophosphate (RuMP)-dependent, and pterin-dependent. The stepwise transformations of formaldehyde within these pathways are well understood (Figure 2). Each proceeds by initial capture of formaldehyde as a less reactive derivative, which is assimilated subsequently into the usual pathways for carbon metabolism (in the case of the RuMP pathway), or is detoxified to formate (in the pterin- and thiol-dependent pathways).
FIGURE 2.
Pathways for the detoxification of formaldehyde. (A). Thiol-dependent pathway, as exemplified by glutathione (GSH). (B). Ribulose monophosphate (RuMP) pathway. (C). Pterin-dependent pathway, as exemplified by tetrahydrofolate (THF). (A–C). The formaldehyde carbon is highlighted in red.
Thiol-Dependent
The most widespread and perhaps the best characterized pathway for formaldehyde detoxification employs reactive thiols as the initial formaldehyde acceptor. In most microorganisms, this thiol is the tripeptide glutathione (GSH, L-γ-glutamyl-L-cysteinylglycine). The first step is the nucleophilic addition of GSH to formaldehyde to form S-hydroxymethylglutathione (HMGS; Figure 2A; Mason et al., 1986). This reaction occurs spontaneously but in certain bacterial species, such as Paracoccus denitrificans and Rhodobacter sphaeroides, it is catalyzed by a formaldehyde-activating enzyme (Gfa, EC 4.4.1.22; Goenrich et al., 2002; Wilson et al., 2008). The HMGS adduct is oxidized subsequently by a zinc-containing, NAD+-dependent alcohol dehydrogenase (AdhC, EC 1.1.1.284) to generate the thioester S-formylglutathione (SFG; Uotila and Koivusal, 1974a). Formate is produced and GSH is regenerated finally upon hydrolysis of SFG (Figure 2A). This final step is catalyzed by an esterase or S-formylglutathione hydrolase (EstD, EC 3.1.2.12; Uotila and Koivusal, 1974b).
GSH-dependent systems for formaldehyde detoxification were first thought to exist exclusively in environmental bacteria such as Gram-negative methylotrophs but they have now been identified in a diverse range of microorganisms, many of which do not oxidize methanol (Uotila and Koivusal, 1974a; Kaulfers and Marquardt, 1991; Fernandez et al., 1993; Gutheil et al., 1997). These include the FrmAB system in Escherichia coli (Herring and Blattner, 2004; Gonzalez et al., 2006), the NmlR regulons in the human pathogens Haemophilus influenzae and Neisseria meningitidis (Kidd et al., 2012; Chen et al., 2013), and the AdhR regulon in Bacillus subtilis (Huyen et al., 2009), each of which is described further in section “Organization and Functional Regulation of Genes in the Glutathione-Dependent Pathways.”
In bacteria that do not use glutathione, the alternative thiol mycothiol (MSH) or bacillithiol (BSH) is used as the formaldehyde carrier (Sakuda et al., 1994; Newton et al., 2009). MSH and BSH contain glycoside linkages between N-acetylated cysteine, D-glucosamine, and myo-inositol moieties (MSH), or between L-cysteine, D-glucosamine, and malic acid (BSH). A MSH-dependent homolog of AdhC has been described in Mycobacterium smegmatis (AdhE2, EC 1.2.1.66) and in the actinomycete Corynebacterium glutamicum (FadH; Norin et al., 1997; Vogt et al., 2003; Lessmeier et al., 2013). However, an S-formylmycothione hydrolase has not been identified in these organisms. Nevertheless, formate and MSH have been detected as the final products of formaldehyde oxidation in M. smegmatis, presumably as a consequence of the spontaneous degradation of S-formylmycothione (Vogt et al., 2003). Similarly, a BSH-dependent homolog of AdhC has been identified in B. subtilis (AdhA, EC 1.1.1.-) (Huyen et al., 2009) but the corresponding S-formylbacillithione hydrolase has not been identified. Whether formate is generated as the final oxidation product is yet to be determined.
RuMP Pathway
The RuMP pathway comprises two enzymes, 3-hexulose-6-phosphate synthase (Hps, EC 4.1.2.43) and 6-phospho-3-hexuloisomerase (Phi, EC 5.3.1.27) [Figure 2B; reviewed in (Kato et al., 2006)]. This pathway was first described in methylotrophic bacteria and archaea that use formaldehyde as a sole carbon source but it has now been identified in non-methylotrophs such as B. subtilis [annotated as HxlA (Hps) and HxlB (Phi)] and Burkholderia cepacia (Mitsui et al., 2003; Yurimoto et al., 2005; Huyen et al., 2009). In this pathway, formaldehyde is captured initially by Hps-catalyzed condensation with the C1 carbon of ribulose-5-phosphate to form D-arabino-3-hexulose-6-phosphate. This product is isomerized by Phi to generate fructose-6-phosphate, which is shuttled subsequently into the bacterium’s glycolytic pathways. The initial formaldehyde acceptor ribulose-5-phosphate is regenerated from fructose-6-phosphate and glyceraldehyde-3-phosphate via a series of transketolase, transaldolase, and isomerization reactions (Kato et al., 2006). The use of sugar phosphates in formaldehyde detoxification has also been identified in eukaryotic microbes such as Candida sp. However, xylylose-5-phosphate is used as the initial formaldehyde acceptor (Veenhuis et al., 1983).
Pterin-Dependent
The pterin-dependent pathway for formaldehyde detoxification takes advantage of the reactivity of formaldehyde with amines, such as that present in the pterin moiety of tetrahydrofolate (THF; Figure 2C). The spontaneous condensation between formaldehyde and a secondary amine in the pterin forms N5,N10-methylene-THF, which is in turn oxidized to N5,N10-methenyl-THF. The latter is catalyzed by a dehydrogenase (MtdA, EC 1.5.1.5) using NADP+ as the electron acceptor. N5,N10-methenyl-THF is hydrolyzed further to N10-formyl-THF by a cyclohydrolase (Fch, EC 3.5.4.9). In the final step, N10-formyl-THF is hydrolyzed by formate THF ligase (or formyl THF synthetase, Fhs, EC 6.3.4.3) to generate formate as the final product and regenerate THF (Figure 2C; Vorholt, 2002).
The three enzymes depicted in Figure 2C – MtdA, Fch, and Fhs – constitute the central pathway for methyl transfer, which is required for the synthesis of purines and amino acids, and for the initiation of protein translation. As such, this pathway is widely distributed in the bacterial world. In species that already possess the thiol- or RuMP-linked pathways, the THF-dependent pathway for methyl transfer may still act as a secondary or auxiliary system for the removal of formaldehyde. In methanotrophs and methylotrophs such as Methylobacterium sp. and Hyphomicrobium sp., this THF-linked pathway is upregulated in the presence of methane or methanol, presumably to cope with the production of formaldehyde during methane or methanol oxidation (Vorholt, 2002). Certain methanogenic archaea and methylotrophic proteobacteria use tetrahydromethanopterin (THMP) in place of THF (Maden, 2000; Vorholt, 2002). The two pterins are structurally related and molecular details of the THF- and THMP-linked pathways are analogous.
Direct Oxidation of Formaldehyde to Formate
In several bacterial species such as Pseudomonas putida, P. aeruginosa, and Burkholderia fungorum, the oxidation of formaldehyde to formate occurs in a single step that is independent of thiol, pterin, or RuMP (Ando et al., 1979; Marx et al., 2004; Liao et al., 2013). This process is catalyzed by a zinc-dependent formaldehyde dehydrogenase (EC 1.2.1.46) using NAD+ as the electron acceptor (Liao et al., 2013).
Mechanisms of Formaldehyde Sensing
The various pathways for formaldehyde detoxification operate under the control of formaldehyde-responsive transcriptional factors but the biochemical mechanisms for formaldehyde sensing remain poorly understood. Transcriptional response to formaldehyde relies typically on the presence of one or more conserved cysteine thiols. Mutation of this cysteine leads invariably to a failure to respond to exogenous formaldehyde or formaldehyde generators, but how this cysteine detects formaldehyde remains unknown. Based on current understanding of other families of cysteine-based transcriptional sensors, it has been speculated that this conserved cysteine may be S-alkylated. The mechanism for S-alkylation of cysteine by formaldehyde would be analogous to that described earlier for the reaction between formaldehyde and glutathione (see Figure 1B). Alternatively, this cysteine may also be S-alkylated by a downstream product of formaldehyde such as HMGS or SFG (see Figure 2A) or by a product of the toxic reactions between formaldehyde and a cellular target. To date, there has been no evidence of any such S-modification in vitro or in vivo. The available knowledge of formaldehyde sensing in bacteria is outlined below.
HxlR
HxlR from B. subtilis controls the expression of hxlAB, which encodes for the RuMP pathway for formaldehyde assimilation (Yurimoto et al., 2005). It is a member of the MarR/DUF24 family of repressors that sense reactive oxygen (ROS) and electrophilic species (RES; Antelmann and Helmann, 2011; Hillion and Antelmann, 2015), as exemplified by OhrR from B. subtilis (Fuangthong and Helmann, 2002) and Xanthomonas campestris (Panmanee et al., 2006). In vitro, exposure of OhrR to ROS such as organic peroxides was shown to result in the oxidation of the conserved cysteine (Cys15) to a sulfenic acid (-SOH; Fuangthong and Helmann, 2002). This sulfenic acid reacts further with a second thiol, either from BSH or from a cysteine on a neighboring OhrR monomer, to form a disulfide (-S-S-), which in turn leads to dissociation of OhrR from DNA and thus derepression of gene expression (Panmanee et al., 2006; Lee et al., 2007; Newberry et al., 2007). Although HxlR also contains a conserved cysteine (Cys11) near the N-terminus, the reaction between formaldehyde and a cysteine thiol is not likely to generate a sulfenic acid intermediate (cf. see Mechanisms of Formaldehyde Toxicity and Figure 1B).
FrmR
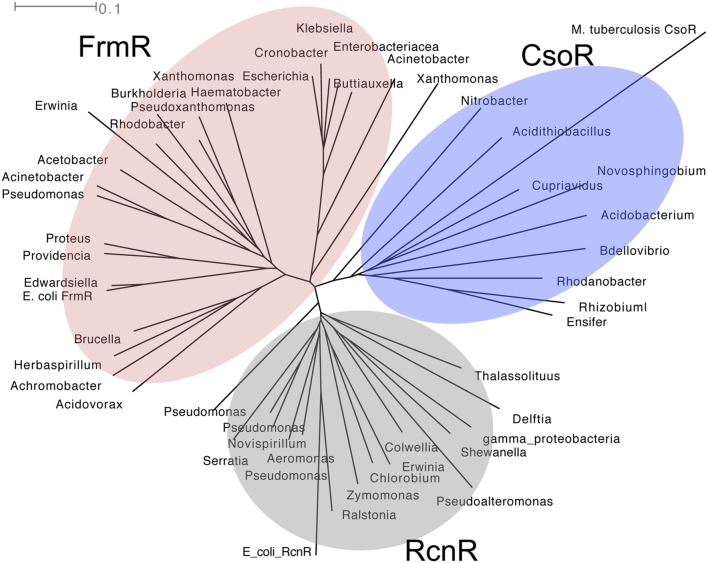
FrmR regulates the expression of frmAB, the GSH-dependent pathway for formaldehyde detoxification in E. coli (Herring and Blattner, 2004). Its homologs, along with the complete FrmAB pathway, have also been identified in pathogens such as P. aeruginosa and Klebsiella pneumoniae (Figure 3). FrmR is a member of the CsoR/RcnR family of metal ion-sensing transcriptional repressors (Figure 3). Prototypes of this family possess a conserved cysteine within X-Cys-His-Cys or His-Cys-His-His motifs for binding the cognate metal ion (Liu et al., 2007; Iwig et al., 2008). The conserved cysteine in FrmR from Salmonella enterica sv. Typhimurium (Cys35) was found to bind Co(II) and Zn(II) in vitro [KCo(II) = 7.6 × 10-6 M; KZn(II) = 1.7 × 10-10 M] but this protein was unable to compete with dedicated metal sensors such as RcnR [KCo(II) = 5.1 × 10-10 M] and ZntR [KZn(II) = 3.2 × 10-12 M; Osman et al., 2015). To date, the relevance of metal ion binding to formaldehyde sensing by FrmR remains undefined. Instead, it has been hypothesized that Cys35 reacts with formaldehyde directly to form an S-hydroxymethyl adduct and, in the presence of a neighboring primary amine, a thiazolidine-like adduct (see Figure 1B; Higgins and Giedroc, 2014). Only one CsoR/RcnR homolog has been demonstrated to detect non-metals using Cys35 (Luebke et al., 2014). This is CstR, a persulfide sensor that controls sulfide homeostasis in Staphylococcus aureus (Luebke et al., 2014).
FIGURE 3.
Phylogenetic tree of CsoR (shaded in blue), RcnR (gray), and FrmR (red) family of regulators. Amino acid sequences were aligned using ClustalX 2.1 (Larkin et al., 2007) and analyzed using SplitsTree4 (Huson and Bryant, 2006). The tree shown was drawn using the ConsensusTree function and 500 bootstrap cycles.
NmlR
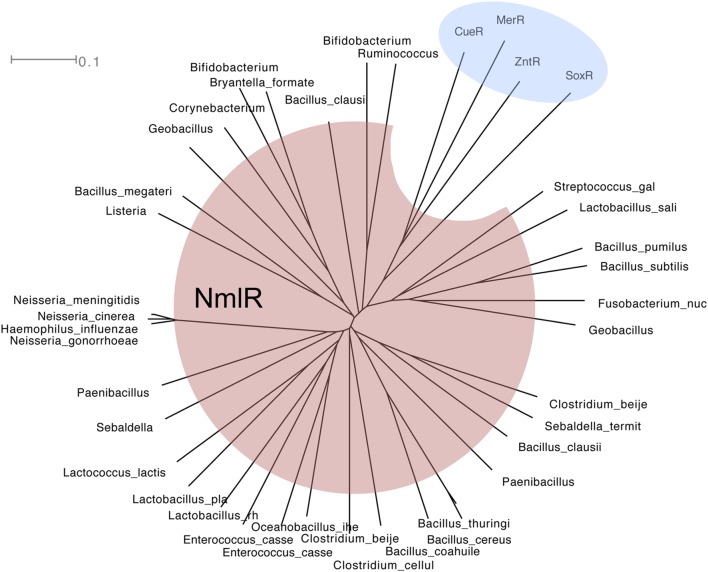
NmlR controls the expression of the GSH-dependent pathway for formaldehyde detoxification. It was first identified in pathogenic Neisseria species but its homologs have now been found in several medically significant human pathogens, including H. influenzae, Streptococcus pneumoniae, Lactobacillus sp., and Clostridium sp. (Kidd et al., 2005, 2012; Stroeher et al., 2007; McEwan et al., 2011; Chen et al., 2013). NmlR homologs form a clade within the diverse family of MerR repressor-activators that respond to a wide range of molecules, including soft transition metal ions, the superoxide anion, and drug-like compounds (Figure 4; Ahmed et al., 1994, 1995; Hidalgo and Demple, 1994; Brown et al., 2003; McEwan et al., 2011). Members of the NmlR clade are thought to sense oxidative and/or carbonyl stressors (Kidd et al., 2005; Stroeher et al., 2007; Huyen et al., 2009).
FIGURE 4.
Phylogenetic tree of MerR (shaded in blue) and NmlR (red) family of regulators. Amino acid sequences were aligned using ClustalX 2.1 (Larkin et al., 2007) and analyzed using SplitsTree4 (Huson and Bryant, 2006). The tree shown was drawn using the ConsensusTree function and 500 bootstrap cycles.
As is the norm for all known formaldehyde sensors, there is absolute conservation of a cysteine within the NmlR clade [Cys54 in NmlR from H. influenzae or Cys52 in NmlR (AdhR) from B. subtilis]. Mutation of Cys54 to an Ala in the homolog from H. influenzae led to an enhanced sensitivity to growth inhibition and a failure to activate the expression of AdhC in the presence of formaldehyde (manuscript submitted). Likewise, a mutant strain of B. subtilis carrying the C52A variant of AdhR was unable to generate an adhA (adhC) transcript in response to challenge with formaldehyde (Huyen et al., 2009). For both NmlR and AdhR, no evidence of S-alkylation by formaldehyde, formaldehyde generators, or downstream formaldehyde detoxification products has been reported thus far.
Genetic and Functional Basis for Formaldehyde Detoxification via Glutathione-Dependent Pathways
Amongst the three pathways for the detoxification of formaldehyde, the GSH-dependent pathway is the most widely distributed in the biological world, with examples from bacteria, plants, and mammals (Uotila and Koivusal, 1974a; Harms et al., 1996; Gutheil et al., 1997; Barber and Donohue, 1998b; Cummins et al., 2006; Chen et al., 2013). As outlined briefly in section “Mechanisms of Formaldehyde Detoxification and Assimilation,” three separate enzymes catalyze the consecutive steps of the oxidation of formaldehyde to formate. These are the formaldehyde-activating enzyme Gfa, the alcohol dehydrogenase AdhC, and the thioesterase EstD (Figure 2A). The biochemical properties of each of these enzymes have been fairly well characterized and are summarized in this section. Although this core pathway is conserved, the organization and regulation of the encoding genes are varied, and are reviewed below.
Enzymes of the GSH-Dependent Pathway
Formaldehyde-Activating Enzyme (Gfa, EC 4.4.1.22)
Gfa is a zinc-dependent enzyme that accelerates the spontaneous the condensation of GSH with formaldehyde to form HMGS. It was first described in P. denitrificans but it has also been identified in Sinorhizobium meliloti and R. sphaeroides (Goenrich et al., 2002; Neculai et al., 2005; Wilson et al., 2008). The pseudo first-order rate constant for the formation of HMGS as catalyzed by Gfa has been estimated to be 10-fold higher than that for the spontaneous formation of HMGS (Goenrich et al., 2002). However, a recent study has suggested that this enzyme does not catalyze the formation of HMGS, but instead it may act as a GSH carrier to promote co-localization with formaldehyde within the cell (Hopkinson et al., 2015). Nevertheless, Gfa is notably absent from the GSH-dependent pathway for formaldehyde tolerance in non-methanotrophs such as pathogenic Neisseria and H. influenzae. Thus it is likely that the rate of spontaneous condensation with GSH is sufficient for the initial capture of formaldehyde in these organisms.
Alcohol Dehydrogenase (AdhC, EC 1.1.284)
The class III, zinc-dependent enzyme AdhC catalyzes the oxidation of HMGS to S-formylglutathione using NAD+ as the electron acceptor (Figure 2A). The human AdhC homolog ADH3 is particularly well characterized. ADH3 displays a wide range of specific activity in the presence of HMGS as a substrate (kcat/Km values between 50 and 1000 μM-1 min-1; Hedberg et al., 2003; Hoog et al., 2006; Sanghani et al., 2006; Staab et al., 2008a). Intriguingly, recent biochemical studies of AdhC homologs from human, Saccharomyces cerevisiae and E. coli demonstrated that AdhC may participate in the defense against nitrosative (nitric oxide) stress, as it also catalyzes the reduction of S-nitrosoglutathione (GS-NO) to generate glutathione sulfinamide (GS-ONH2) using NADH as the electron donor (Jensen et al., 1998; Liu et al., 2001; Hedberg et al., 2003). While the relevance to formaldehyde detoxification is unclear, it has been proposed that AdhC may function as a GS-NO reductase by NAD+/NADH cofactor recycling by using the HMGSH oxidase pathway to regenerate NADH (Staab et al., 2008b, 2009).
S-formylglutathione Hydrolase (EstD, EC 3.1.2.12)
EstD is a Ser-His-Asp esterase. Homologs from human (ESD), Arabidopsis thaliana (AtSFGH), E. coli (FrmB), and N. meningitidis (EstD) hydrolyze a range of synthetic esters, including p-nitrophenyl acetate, 4-methylumbelliferyl acetate, and naphthyl acetate, but each displays a high specific activity (up to 10-fold higher) toward the predicted physiological substrate S-formylglutathione (kcat/Km values between 0.015 and 2 × 106 M-1 s-1; Uotila and Koivusal, 1974b; Cummins et al., 2006; Gonzalez et al., 2006; Chen et al., 2013). A second homolog of EstD, annotated as YeiG, is present in E. coli. Compared to FrmB, YeiG displayed a 20-fold higher specific activity for S-lactoylglutathione. S-lactoylglutathione itself is an intermediate in the pathway for the detoxification of methylglyoxal via the glyoxalase system (Gonzalez et al., 2006). Therefore, YeiG was hypothesized to participate in the removal of methylglyoxal. As methylglyoxal is a potential source of formaldehyde in cells (see Potential Sources of Formaldehyde), YeiG may also contribute indirectly to formaldehyde tolerance.
All EstD homologs possess a conserved cysteine that is situated in close proximity to the active site pocket but is not essential for enzyme activity (Cummins et al., 2006; Gonzalez et al., 2006; Chen et al., 2013). Recent biochemical studies with EstD from N. meningitidis and A. thaliana suggested that this cysteine (Cys54 in N. meningitidis) acts as a site of post-translational regulation of enzyme activity (Cummins et al., 2006; Chen et al., 2013). Cys54 is readily alkylated with agents such as iodoacetamide (Cummins et al., 2006; Gonzalez et al., 2006; Chen et al., 2013). This S-modification was thought to physically block substrate access to the catalytic site (Chen et al., 2013). Indeed, treatment with iodoacetamide abolished the activity of EstD completely (Cummins et al., 2006; Gonzalez et al., 2006; Chen et al., 2013). The physiological significance for these in vitro observations is yet to be established.
Organization and Functional Regulation of Genes in the Glutathione-Dependent Pathways
AfdRS and RfdRS Regulons
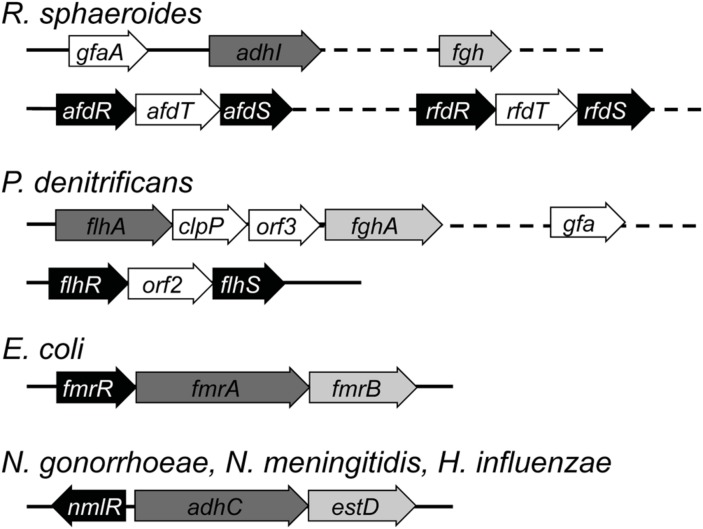
The purple non-sulfur photosynthetic bacterium R. sphaeroides produces formaldehyde during methanol utilization. The gfa, adhC (adhI), and estD (fgh) genes in this bacterium are not organized in an operon (Figure 5). While gfa is adjacent to adhI and this arrangement is clustered with genes that encode for other metabolic enzymes such as formate dehydrogenase (Wilson et al., 2008). An adhI mutant strain of R. sphaeroides failed to oxidize formaldehyde, as demonstrated using whole-cell NMR studies in the presence of 13C-formaldehyde. This mutant was also unable to grow in the presence of methanol as a sole carbon source (Barber and Donohue, 1998b; Hickman et al., 2004), presumably as a consequence of the buildup of formaldehyde during methanol oxidation. The direct effect of excess formaldehyde on the growth of the adhI mutant is not known.
FIGURE 5.
The genetic organization of glutathione-dependent pathways for formaldehyde detoxification. Genes encoding for the transcriptional regulators are shaded in black. Genes encoding for the glutathione-dependent alcohol dehydrogenase and the S-formylglutathione hydrolase are shaded in dark gray and light gray respectively. Genes that are located on different parts of the chromosome are connected using dashed lines.
Transcriptional regulation of gfa and fgh expression in response to formaldehyde or methanol has not yet been described. In the case of adhI, its promoter was shown to be activated by both formaldehyde and methanol, although induction by formaldehyde occurs more rapidly and at a lower concentration (Barber and Donohue, 1998a). Expression of adhI is controlled by two separate, two-component regulatory systems: (i) AfdRS, which activates transcription in the presence of formaldehyde, and (ii) RfdRS, which is thought to act as a repressor, although the signal for derepression is unknown (Hickman et al., 2004). Unlike the other regulators of formaldehyde detoxification, both these systems do not seem to harbor any conserved cysteines (see Mechanisms of Formaldehyde Sensing) and thus it is unknown how they sense formaldehyde.
afdR and afdS are organized within an operon, as are rfdR and rfdS. Inserted between each pair of genes is a predicted open reading frame, afdT or rfdT (Figure 5), with both genes displaying high sequence identity to each other. To date, their role in the detoxification of formaldehyde is unclear, as an rfdT mutant of R. sphaeroides did not affect the expression of adhI in the presence of formaldehyde (Hickman et al., 2004).
FlhRS Regulon
The adhC (flhA) and estD (fghA) genes in P. denitrificans are arranged in an operon, along with two genes of unknown function, clpP and orf3 (Figure 5; Harms et al., 1996). Expression of flhA and fghA is activated by FlhRS, a two-component regulator that displays high sequence similarity (>50%) to AfdRS and RfdRS from R. sphaeroides (Hickman et al., 2004). The flhRS operon is located away from flhA-fghA. Between flhR and flhS is an open reading frame, orf2 (Figure 5), which shows sequence similarity to both afdT and rfdT from R. sphaeroides. gfa is also present in the genome of P. denitrificans but it is not part of the flhA-fghA operon (Goenrich et al., 2002). Whether its expression is controlled by FlhRS is yet to be defined.
A flhRS mutant strain of P. denitrificans failed to activate the expression of flhA and fghA in the presence of choline, a formaldehyde-generating substrate, as the sole carbon source (Harms et al., 2001). Inactivation of flhRS, flhA, or fghA each led to an inability to grow in the presence of methanol or methylamine as the sole carbon source, indicating that these genes are required for methanotrophic growth (Ras et al., 1995; Harms et al., 1996, 2001). This growth defect was not unexpected, as catabolism of methanol and methylamine both generate formaldehyde as a byproduct.
FrmR Regulon
The genes encoding for AdhC (frmA) and EstD (frmB) from E. coli are arranged in an operon, along with the gene that codes for their transcriptional regulator (frmR; Figure 5; Herring and Blattner, 2004). Exposure to formaldehyde was shown to induce robust expression of frmAB (over 100-fold; Herring and Blattner, 2004) and increase the activity of FrmA in whole cell extracts (Gutheil et al., 1997). Expression of frmB was not induced upon treatment with GSNO, hydrogen peroxide, or methyl viologen, indicating that the regulon did not respond to general oxidative or nitrosative stress (Herring and Blattner, 2004; Gonzalez et al., 2006).
NmlR Regulons
The adhC-estD operon in N. meningitidis (meningococcus), N. gonorrhoeae (gonococcus), and H. influenzae is located adjacent but divergent to nmlR, which encodes for their transcriptional regulator (Figure 5; Kidd et al., 2005; Potter et al., 2007; Chen et al., 2013). Meningococcal mutant strains of adhC and estD displayed an enhanced sensitivity to growth inhibition by exogenous formaldehyde but not other aldehydes or carbonyl compounds such as methylglyoxal (Chen et al., 2013). The growth defect was more pronounced for the estD mutant when compared with the adhC single mutant or the adhC-estD double mutant. It was thus speculated that accumulation of S-formylglutathione, the substrate for EstD, is more toxic than that of HMGS, the substrate for AdhC, or than formaldehyde itself (Chen et al., 2013).
In the case of H. influenzae, growth of an adhC mutant strain was inhibited by formaldehyde, methylglyoxal, and glycolaldehyde (Kidd et al., 2012). An nmlR mutant strain displayed increased growth sensitivity toward formaldehyde but not methylglyoxal or glycolaldehyde when compared to the wild-type organism (manuscript submitted). AdhC activity in this pathogen is upregulated in response to both formaldehyde exposure and high oxygen tension (Gutheil et al., 1997; Kidd et al., 2012). Conversely, growth of the adhC mutant was suppressed by high oxygen tension in the presence of glucose as a sole carbon source but in the absence of added formaldehyde (Kidd et al., 2012). These growth conditions are known to promote the generation of dicarbonyls such as methylglyoxal (Okado-Matsumoto and Fridovich, 2000; Kidd et al., 2012), a precursor for the production of formaldehyde (see Potential Sources of Formaldehyde).
This nmlR-adhC-estD arrangement is not universal (McEwan et al., 2011). estD is absent in the human pathogen S. pneumoniae and there is no evidence of a formaldehyde-related phenotype in the pneumococcal nmlR mutant (Stroeher et al., 2007). Likewise, estD is not present in B. subtilis. Instead, NmlR in B. subtilis, annotated as AdhR, was found to upregulate three genes in response to methylglyoxal or formaldehyde exposure. These are adhA, yraC, and yraA, which encode for an AdhC homolog, a γ-carboxymuconolactone decarboxylase, and a cysteine proteinase, respectively (Huyen et al., 2009). YraC is proposed to be a component of protocateculate metabolism and a homolog of YraC from Legionella pneumophila has been shown to display peroxidase activity (Huyen et al., 2009; Chen et al., 2015). How YraC contributes to the defense against formaldehyde toxicity remains to be defined. Likewise, the role for yraA is not understood, although it has been hypothesized to function in the repair of formaldehyde-induced protein damage (Huyen et al., 2009).
A Role for Formaldehyde Detoxification in Bacterial Pathogenesis
Evidence for Horizontal Transfer of nmlR-adhC-estD Genes Between Pathogenic Species
The presence of inducible formaldehyde detoxification systems in non-methylotrophs, including those that cause human diseases, hints at the significance and role of this toxic aldehyde in bacterial physiology. In some pathogens, the loss or mutation of these detoxification genes led to phenotypic defects even in the absence of added formaldehyde. As already mentioned earlier, growth of the adhC mutant strain of H. influenzae was suppressed when cultured under high oxygen tension and in the presence of glucose as the sole carbon source (Kidd et al., 2012). Although a growth defect in the absence of formaldehyde was not reported for the equivalent mutant of N. meningitidis, the adhC, as well as the nmlR and estD mutant strains of this bacterium were shown to be non-viable or “aged” within mature biofilm communities (Chen et al., 2013). Together, these studies provided strong evidence, albeit indirect, that formaldehyde accumulates endogenously.
The majority of NmlR regulators identified by phylogenetic analysis are found in Gram-positive bacteria (Figure 4). The only examples in Gram-negative bacteria are from the Neisseria genus and a few Pasteurellaceae species. Within the Neisseria genus, the nmlR-adhC-estD locus is identified only in the lineage of meningococcal-related species (Guibourdenche et al., 1986), namely N. meningitidis, N. gonorrhoeae, N. lactamica, N. cinerea, and N. polysaccharea. Within the Pasteurellaceae family, the same nmlR-adhC-estD locus is found in H. influenzae and two other species, Aggregatibacter actinomycetemcomitans, a bacterium from the buccal normal flora that is often associated with periodontitis, and the rumen bacterium Mannheimia succiniciproducens. This in silico analysis raises the possibility that the presence of the nmlR operon in Gram-negative bacteria is a consequence of a gene transfer event from Gram-positive bacteria that occupy the same environmental niche.
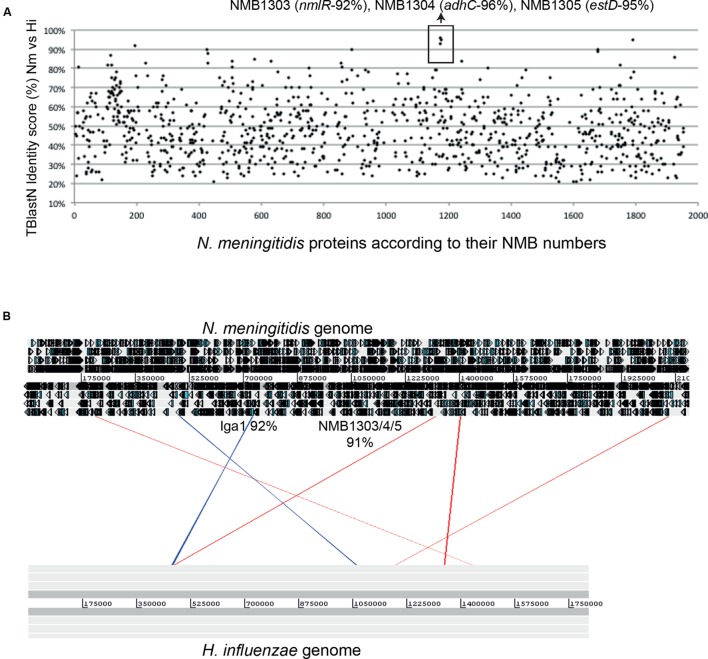
It has been proposed that H. influenzae received the gene coding for an IgA protease (IgaB), a well-defined bacterial virulence determinant, by horizontal gene transfer from N. meningitidis (Murphy et al., 2011). It must be noted that the nmlR-adhC-estD locus in H. influenzae is found adjacent to igaB. Interestingly, comparison of the identity score for all proteins from N. meningitidis MC58 BLAST against H. influenzae PittEE, revealed that the NmlR, AdhC, EstD protein sequences share an abnormally high percentage of identity (Figure 6A). The same is true for their DNA sequences (Figure 6B). In addition, the surrounding regions of the nmlR-adhC-estD locus are conserved in other Haemophillus species (Figure 6B). Considering all the evidence presented here, these in silico analyses suggest recent transfer of nmlR, adhC, and estD from pathogenic Neisseria to pathogenic Haemophilus species.
FIGURE 6.
Horizontal transfer of the nmlR-adhC-estD locus between Haemophilus and Neisseria. (A) Graphical representation of the TBlastN identity scores of N. meningitidis MC58 proteins against Haemophilus influenzae genomes. (B) ACT image of BlastN genome comparison of Neisseria meningitidis MC58 and H. influenzae genomes. The strongest hits potentially representing exchange of DNA are represented.
It is notable that the nmlR-adhC-estD operon does not appear to play a role in formaldehyde detoxification in N. gonorrhoeae (see Organization and Functional Regulation of Genes in the Glutathione-Dependent Pathways). The gonococcal adhC gene is inactive as a consequence of a frameshift mutation (Potter et al., 2007). A link between nmlR, adhC, or estD to formaldehyde detoxification in N. gonorrhoeae has not been reported (Kidd et al., 2005; Potter et al., 2007, 2009). Instead, the NmlR regulon in this bacterium has been linked to the response to general thiol/disulfide stress. Mutants of the estD gene were sensitive to killing by agents that induce nitrosative stress, such as nitrite and GS-NO (Potter et al., 2009). An nmlR mutant also displayed a growth defect in the presence generators of oxidative stress such as cumene hydroperoxide and the thiol oxidant diamide (Kidd et al., 2005).
It is tempting to hypothesize that the apparent divergence of function between two closely related pathogens may relate to their different infection niches. While N. gonorrhoeae colonizes the mucosal surfaces of the genitourinary tract, N. meningitidis and H. influenzae both colonize the nasopharynx, and they are able to cause invasive disease including meningitis and septicemia. The loss of adhC in N. gonorrhoeae and the conservation of a fully functional nmlR-adhC-estD locus in the meningococcus and H. influenzae may be an example of a positive selective pressure for this locus during bacteria-host interaction within the nasopharynx. This selection pressure may arise as an indirect consequence of conditions that predispose the invading pathogen to the production of endogenous formaldehyde. Additionally, the potential existence of formaldehyde in the host tissue at the site of infection must also be considered.
Potential Sources of Formaldehyde
Bacterial-Derived Sources
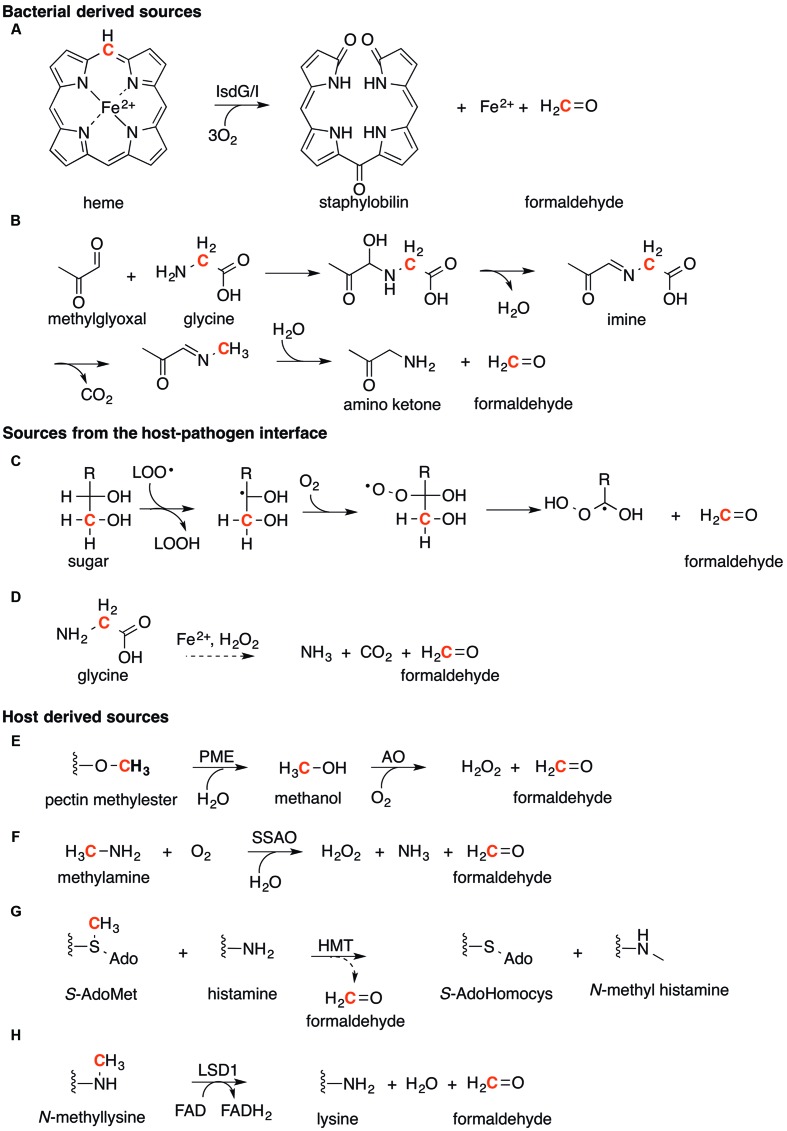
Methylglyoxal, a byproduct of glycolysis, represents a major source of endogenous formaldehyde in bacteria (Schonberg and Moubacher, 1952; Thornalley, 1993; Okado-Matsumoto and Fridovich, 2000). This diketone is produced from the degradation of two triose sugar phosphates, namely glyceraldehyde-3-phosphate and dihydroxyacetone-phosphate (Thornalley, 1993). Methylglyoxal is also formed by the enolization and oxidation of glyceraldehyde, a short-chain sugar of the pentose-phosphate cycle (Okado-Matsumoto and Fridovich, 2000). Generation of formaldehyde from methylglyoxal occurs during Strecker degradation of glycine (Figure 7A; Schonberg and Moubacher, 1952). Nucleophilic addition of the amino terminus in glycine to the terminal carbonyl in methylglyoxal reaction creates an imine intermediate, which is subsequently hydrolyzed to generate formaldehyde as the final product.
FIGURE 7.
Sources of formaldehyde. The generation of formaldehyde can be organized into three main categories. (1) Bacterial derived sources. (A) The degradation of heme (substituents omitted for clarity) by IsdG/I, and (B) Strecker degradation of glycine with methylglyoxal. (2). Sources from the host-pathogen interface. (C) The lipid peroxidation of sugar molecules and (D) degradation of glycine by Fenton chemistry. (3) Host derived sources. (E) Formaldehyde formed from the oxidation of methanol derived from pectin in fruit. (F) Oxidative deamination of methylamine by semicarbazide amine oxidase (SSAO). (G) The methyl transfer of histamine by histamine-N-methyltransferase (HMT). (H) The demethylation of histones by lysine specific demethylase (LSD1). For all reactions, the carbon atom which formaldehyde is generated from is highlighted in Red.
Production of formaldehyde from methylglyoxal may explain the growth defect of the adhC mutant strain of H. influenzae under high oxygen tension and in the presence of glucose as the sole carbon source (Kidd et al., 2012). In silico analysis of H. influenzae has suggested that carbon utilization occurs primarily via the pentose phosphate pathway under these conditions (Edwards and Palsson, 1999), leading to the production of methylglyoxal and presumably also formaldehyde.
In the pathogen S. aureus, formaldehyde is generated as a byproduct of the degradation of heme during iron acquisition. This process is catalyzed by two heme oxygenases IsdG and IsdI (EC 1.14.99.3, Figure 7B; Reniere et al., 2010; Matsui et al., 2013). This process is unique to certain Gram-positive bacteria, including B. anthracis, S. epidermidis, and Listeria monocytogenes (Skaar et al., 2004), and is distinct from the pathway for heme degradation in Gram-negative bacteria including H. influenzae, which generates carbon dioxide in place of formaldehyde (Tenhunen et al., 1969). Analysis of the S. aureus genome identified the presence of a complete RuMP pathway for formaldehyde detoxification. It is likely no coincidence that the most abundant source of heme in the human body is in hemoglobin contained in erythrocytes found in blood, the same environment that S. aureus can invade and cause disease. Their acquisition of iron from heme in blood would generate increasing amounts of formaldehyde, necessitating for the RuMP based detoxification system.
Formaldehyde Generators at the Host–Pathogen Interface
During inflammation, the generation of reactive oxygen species, including the superoxide anion (O2-•) and hydrogen peroxide (H2O2), during respiratory burst by macrophages and neutrophils can also produce formaldehyde as a toxic end product (Figure 7C). Superoxide and hydrogen peroxide have been demonstrated to damage bacterial iron-sulfur (Fe-S) clusters (Flint et al., 1993; Jang and Imlay, 2007) and mononuclear Fe enzymes (Anjem and Imlay, 2012; Gu and Imlay, 2013), causing the release of Fe as free or bioavailable ions. This bioavailable Fe may catalyze Fenton-like reactions with excess hydrogen peroxide to generate hydroxyl radicals (•OH). These radicals in turn may lead to the formation of lipid peroxyl radicals (LOO•), which can react with sugars (Figure 7C) such as glyceraldehyde in a process that has been shown to produce the toxic aldehydes malondialdehyde and formaldehyde (Cordis et al., 1994; Maboudou et al., 2002). In this process, (Figure 7C) initial attack of the lipid peroxide radical with a sugar molecule, followed by reaction with molecular oxygen forms a sugar peroxyl radical. Further rearrangement of this radical occurs to release formaldehyde (Thornalley et al., 1984; Spiteller, 2008). In addition Fenton-catalyzed degradation of L-glycine has been shown to generate formaldehyde (Figure 7D) although the precise mechanism is still unknown (Dakin, 1906).
Host-Derived Sources
The concentration of formaldehyde in healthy human blood has been measured at 0.1 mM (Heck et al., 1985). This aldehyde is produced by multiple metabolic processes in human and mammalian cells, as described below:
Oxidation of methanol by alcohol oxidases
Ingestion of fruits such as apples has been shown to lead to a 10-fold increase in methanol concentration in human breath (Lindinger et al., 1997). This methanol is produced by the hydrolysis of methyl esters in pectins as catalyzed by pectin methylesterases from gut bacteria (PME, EC 3.1.1.11, Figure 7E; Siragusa et al., 1988). Methanol is in turn oxidized by human alcohol oxidases (EC 1.1.3.13) to generate formaldehyde as the end product (Mani et al., 1970).
Oxidative deamination of primary amines by amine oxidases
Deamination of methylamine by semicarbazide-sensitive amine oxidase (SSAO, EC 1.4.3.6, Figure 7F) produces formaldehyde and hydrogen peroxide (Yu and Zuo, 1996). Methylamine itself is produced from deamination of adrenaline, an important hormone and neurotransmitter; sarcosine, a product of glycine biosynthesis; or creatinine, a product of muscle breakdown. This primary amine has been detected in the blood, urine, and brain tissue (Asatoor and Kerr, 1961; Zeisel et al., 1983; Yu et al., 2003). Similarly, the enzyme SSAO is found primarily in blood vessels, although it has also been detected in the meninges and the microvessels of the brain (Zuo and Yu, 1994).
Transfer of methyl groups by methyltransferases
Methylation of the neurotransmitter histamine using S-adenosylmethionine as the methyl donor is catalyzed by histamine-N-methyltransferase (HMT, EC 2.1.1.43, Figure 7G). N-methylhistamine is generated as the final product but formaldehyde is produced as an intermediate during catalysis (Meller et al., 1974; Huszti and Tyihak, 1986). Significantly, like SSAO, HMT activity has been detected in adult human brain (Nowak and Zelazowska, 1987). Formaldehyde is also generated as an end product of the demethylation of histones by histone lysine specific demethylase 1 (LSD1, EC 1.14.11.27, Figure 7H), a nuclear homolog of amine oxidases (Shi et al., 2004). This reaction is likely ubiquitous in all human tissues, as it is crucial for the DNA packing in the nucleus, DNA repair, general stress response, and aging (Greer and Shi, 2012),
Summary and Outlook
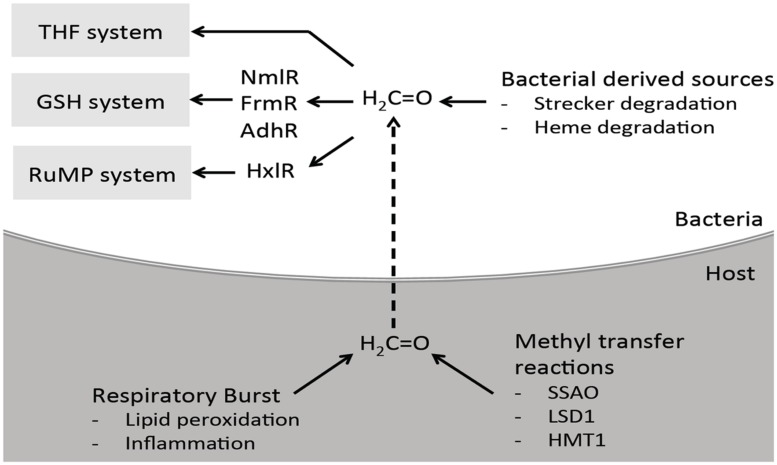
It is clear from this review that the ability to sense and detoxify formaldehyde is not limited to environmental organisms that use methane and methanol as a carbon source. It is likely significant that formaldehyde detoxification pathways are also present in host-adapted bacterial pathogens that were not previously expected to encounter formaldehyde during their physiology. However, it is now recognized that there is a variety of formaldehyde generators at the host-pathogen interface (Figure 8). This can be a consequence of the metabolism and growth of the pathogenic bacteria, the host innate immune response and respiratory burst, or the natural metabolic reactions of the infection sites.
FIGURE 8.
Proposed interaction and clearance of formaldehyde by bacteria at the host-pathogen interface. During infection, bacteria may encounter formaldehyde produced endogenously by themselves, and also by the host cells they are infecting. Bacterial endogenously derived sources include heme degradation and Strecker degradation of glycine. The immune system can indirectly release formaldehyde as a consequence of their respiratory burst leading to inflammation and lipid peroxidation. Methyl transfer reactions by host enzymes also contribute to the overall formaldehyde pool. To combat the formaldehyde, bacteria are able to sense and detoxify formaldehyde using the GSH and THF dependent or RuMP systems.
Some of these sources of formaldehyde are concentrated in the blood, brain, and surrounding tissues, placing them within the same approximate niche with N. meningitidis and H. influenzae during the later stages in their infection cycle. The function of NmlR, AdhC, and EstD in these pathogens may contribute to systemic dissemination from the nasopharynx into the blood stream and, ultimately, the brain, which is often associated with invasive disease. We have also shown evidence of the possible transfer of the formaldehyde sensitive nmlR regulon from pathogenic Neisseria to Haemophilus species. Whether the presence of formaldehyde within the nasopharynx directly influenced this transfer is still unknown.
In addition, E. coli, including pathogenic strains, P. aeruginosa, and K. pneumoniae, possess the FrmRAB regulon, while the RuMP pathway is present in L. monocytogenes and S. aureus, and co-factor independent formaldehyde dehygrogenases have been identified in the opportunistic pathogens P. aeruginosa and P. putida The formaldehyde detoxifications systems found in these medically significant pathogens are very likely required during pathogenesis to remove the endogenous and exogenously produced formaldehyde, however, this contribution still remains to be tested empirically.
The precise mechanism of how they sense formaldehyde requires further investigation, as does measurement of the intracellular formaldehyde in bacterial pathogens and at the host–pathogen interface. Additional further testing of mutants in these detoxification systems in host infection models and global transcriptome analysis would be useful to determine how great of an extent they are required for overall survival. Continued investigation into the role of formaldehyde during host-pathogen interactions will no doubt be useful to further understanding the already complex field of bacterial pathogenesis.
Author Contributions
AM conceived the manuscript. NC performed the literature review. NC, KD, FV and AM co-wrote the manuscript. NC, KD and AM performed the final review and editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Our work was supported by the Australian Research Council (ARC) grant DP0986578 to AM and the National Health & Medical Research Council (NHMRC) Program grant 565526 to AM.
References
- Ahmed M., Borsch C. M., Taylor S. S., Vazquezlaslop N., Neyfakh A. A. (1994). A protein that activates expression of a multidrug efflex transporter upon binding the transporter substrates. J. Biol. Chem. 269 28506–28513. [PubMed] [Google Scholar]
- Ahmed M., Lyass L., Markham P. N., Taylor S. S., Vazquezlaslop N., Neyfakh A. A. (1995). 2 highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J. Bacteriol. 177 3904–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Yoshimoto T., Ogushi S., Rikitake K., Shibata S., Tsuru D. (1979). Formaldehyde dehydrogenase from Pseudomonas putida purification and some properties. J. Biochem. 85 1165–1172. [PubMed] [Google Scholar]
- Anjem A., Imlay J. A. (2012). Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 287 15544–15556. 10.1074/jbc.M111.330365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelmann H., Helmann J. D. (2011). Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 14 1049–1063. 10.1089/ars.2010.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatoor A. M., Kerr D. N. S. (1961). Amines in blood and urine in relation to liver disease. Clin. Chim. Acta 6 149–156. 10.1016/0009-8981(61)90078-x [DOI] [PubMed] [Google Scholar]
- Barber R. D., Donohue T. J. (1998a). Function of a glutathione-dependent formaldehyde dehydrogenase in Rhodobacter sphaeroides formaldehyde oxidation and assimilation. Biochemistry 37 530–537. 10.1021/bi971463t [DOI] [PubMed] [Google Scholar]
- Barber R. D., Donohue T. J. (1998b). Pathways for transcriptional activation of a glutathione-dependent formaldehyde dehydrogenase gene. J. Mol. Biol. 280 775–784. 10.1006/jmbi.1998.1900 [DOI] [PubMed] [Google Scholar]
- Brown N. L., Stoyanov J. V., Kidd S. P., Hobman J. L. (2003). The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27 145–163. 10.1016/s0168-6445(03)00051-2 [DOI] [PubMed] [Google Scholar]
- Casanova M., Morgan K. T., Gross E. A., Moss O. R., Heck H. D. (1994). DNA-protein cross-links and cell replication at specific sites in the nose of F344 rats exposed subchronically to formaldehyde. Fundam. Appl. Toxicol. 23 525–536. 10.1006/faat.1994.1137 [DOI] [PubMed] [Google Scholar]
- Casanovaschmitz M., Starr T. B., Heck H. D. (1984). Differentiation between metabolic incorporation and covalent binding in the labeling of macromolecules in the rat nasal-mucosa and bone-marrow by inhaled C-14 formaldehyde and H-3 formaldehyde. Toxicol. Appl. Pharmacol. 76 26–44. 10.1016/0041-008x(84)90026-7 [DOI] [PubMed] [Google Scholar]
- Chen N. H., Counago R. M., Djoko K. Y., Jennings M. P., Apicella M. A., Kobe B., et al. (2013). A glutathione-dependent detoxification system Is required for formaldehyde resistance and optimal survival of Neisseria meningitidis in biofilms. Antioxid. Redox Signal. 18 743–755. 10.1089/ars.2012.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Hu Y., Yang B., Gong X., Zhang N., Niu L., et al. (2015). Structure of lpg0406, a carboxymuconolactone decarboxylase family protein possibly involved in antioxidative response from Legionella pneumophila. Protein Sci. 24 2070–2075. 10.1002/pro.2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway C. C., Whysner J., Verna L. K., Williams G. M. (1996). Formaldehyde mechanistic data and risk assessment: endogenous protection from DNA adduct formation. Pharmacol. Ther. 71 29–55. 10.1016/0163-7258(96)00061-7 [DOI] [PubMed] [Google Scholar]
- Cordis G. A., Bagchi D., Maulik N., Das D. K. (1994). High-performance liquid-chromatographic method for the simultaneous detection of malonaldehyde, acetaldehyde, formaldehyde, acetone and propionaldehyde to monitor the oxidative stress in heart. J. Chromatogr. 661 181–191. 10.1016/0021-9673(94)85189-1 [DOI] [PubMed] [Google Scholar]
- Cummins I., McAuley K., Fordham-Skelton A., Schwoerer R., Steel P. G., Davis B. G., et al. (2006). Unique regulation of the active site of the serine esterase S-formylglutathione hydrolase. J. Mol. Biol. 359 422–432. 10.1016/j.jmb.2006.03.048 [DOI] [PubMed] [Google Scholar]
- Dakin H. D. (1906). The oxidation of amido-acids with the production of substances of biological importance. J. Biol. Chem. 1 171–176. [Google Scholar]
- Duong A., Steinmaus C., McHale C. M., Vaughan C. P., Zhang L. P. (2011). Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat. Res. 728 118–138. 10.1016/j.mrrev.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. S., Palsson B. O. (1999). Systems properties of the Haemophilus influenzae Rd metabolic genotype. J. Biol. Chem. 274 17410–17416. 10.1074/jbc.274.25.17410 [DOI] [PubMed] [Google Scholar]
- Feldman M. Y. (1973). Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog. Nucleic Acid Res. Mol. Biol. 13 1–49. 10.1016/s0079-6603(08)60099-9 [DOI] [PubMed] [Google Scholar]
- Fernandez M. R., Jornvall H., Moreno A., Kaiser R., Pares X. (1993). Cephalopod alcohold-dehydrogenase – purification and enzymatic characterization. FEBS Lett. 328 235–238. 10.1016/0014-5793(93)80934-m [DOI] [PubMed] [Google Scholar]
- Flint D. H., Tuminello J. F., Emptage M. H. (1993). The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268 22369–22376. [PubMed] [Google Scholar]
- Flyvholm M. A., Andersen P. (1993). Identification of formaldehyde releasers and occurrence of formaldehyde and formaldehyde releasers in registered chemical-products. Am. J. Ind. Med. 24 533–552. 10.1002/ajim.4700240505 [DOI] [PubMed] [Google Scholar]
- Fuangthong M., Helmann J. D. (2002). The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc. Natl. Acad. Sci. U.S.A. 99 6690–6695. 10.1073/pnas.102483199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenrich M., Bartoschek S., Hagemeier C. H., Griesinger C., Vorholt J. A. (2002). A glutathione-dependent formaldehyde-activating enzyme (Gfa) from Paracoccus denitrificans detected and purified via two-dimensional proton exchange NMR spectroscopy. J. Biol. Chem. 277 3069–3072. 10.1074/jbc.C100579200 [DOI] [PubMed] [Google Scholar]
- Golden R., Pyatt D., Shields P. G. (2006). Formaldehyde as a potential human leukemogen: an assessment of biological plausibility. Crit. Rev. Toxicol. 36 135–153. 10.1080/10408440500533208 [DOI] [PubMed] [Google Scholar]
- Gonzalez C. F., Proudfoot M., Brown G., Korniyenko Y., Mori H., Savchenko A. V., et al. (2006). Molecular basis of formaldehyde detoxification – Characterization of two S-formylglutathione hydrolases from Echerichia coli, FrmB and YeiG. J. Biol. Chem. 281 14514–14522. 10.1074/jbc.M600996200 [DOI] [PubMed] [Google Scholar]
- Greer E. L., Shi Y. (2012). Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13 343–357. 10.1038/nrg3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Imlay J. A. (2013). Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol. 89 123–134. 10.1111/mmi.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibourdenche M., Popoff M. Y., Riou J. Y. (1986). Deoxyribonucleic-acid relatedness among Neisseria gonorrhoeae, Neisseria meningitidis, Neisseria lactamica, Neisseria cinerea and Neisseria polysaccharea. Ann. Inst. Pasteur Microbiol. 137B 177–185. 10.1016/s0769-2609(86)80106-5 [DOI] [PubMed] [Google Scholar]
- Gutheil W. G., Kasimoglu E., Nicholson P. C. (1997). Induction of glutathione-dependent formaldehyde dehydrogenase activity in Escherichia coli and Hemophilus influenzae. Biochem. Biophys. Res. Commun. 238 693–696. 10.1016/s0006-291x(00)90000-7 [DOI] [PubMed] [Google Scholar]
- Harms N., Ras J., Reijnders W. N. M., vanSpanning R. J. M., Stouthamer A. H. (1996). S-formylglutathione hydrolase of Paracoccus denitrificans is homologous to human esterase D: a universal pathway for formaldehyde detoxification? J. Bacteriol. 178 6296–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms N., Reijnders W. N. M., Koning S., van Spanning R. J. M. (2001). Two-component system that regulates methanol and formaldehyde oxidation in Paracoccus denitrificans. J. Bacteriol. 183 664–670. 10.1128/jb.183.2.664-670.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck H. D., Casanova M. (2004). The implausibility of leukemia induction by formaldehyde: a critical review of the biological evidence on distant-site toxicity. Regul. Toxicol. Pharmacol. 40 92–106. 10.1016/j.yrtph.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Heck H. D., Casanova M., Starr T. B. (1990). Formaldehyde toxicity – new understanding. Crit. Rev. Toxicol. 20 397–426. 10.3109/10408449009029329 [DOI] [PubMed] [Google Scholar]
- Heck H. D., Casanova-Schmitz M., Dodd P. B., Schachter E. N., Witek T. J., Tosun T. (1985). Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am. Ind. Hyg. Assoc. J. 46 1–3. 10.1080/15298668591394275 [DOI] [PubMed] [Google Scholar]
- Hedberg J. J., Griffiths W. J., Nilsson S. J. F., Hoog J. O. (2003). Reduction of S-nitrosoglutathione by human alcohol dehydrogenase 3 is an irreversible reaction as analysed by electrospray mass spectrometry. Eur. J. Biochem. 270 1249–1256. 10.1046/j.1432-1033.2003.03486.x [DOI] [PubMed] [Google Scholar]
- Herring C. D., Blattner F. R. (2004). Global transcriptional effects of a suppressor tRNA and the inactivation of the regulator frmR. J. Bacteriol. 186 6714–6720. 10.1128/jb.186.20.6714-6720.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. W., Witthuhn V. C., Dominguez M., Donohue T. J. (2004). Positive and negative transcriptional regulators of glutathione-dependent formaldehyde metabolism. J. Bacteriol. 186 7914–7925. 10.1128/jb.186.23.7914-7925.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. (1994). An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 13 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins K. A., Giedroc D. (2014). Insights into protein allostery in the CsoR/RcnR family of transcriptional repressors. Chem. Lett. 43 20–25. 10.1246/cl.130965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion M., Antelmann H. (2015). Thiol-based redox switches in prokaryotes. Biol. Chem. 396 415–444. 10.1515/hsz-2015-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog J. O., Staab C. A., Hedberg J. J., Grafstrom R. C. (2006). “Mammalian alcohol dehydrogenase 3 (ADH3) has several essential functions,” in Enzymology and Molecular Biology of Carbonyl Metabolism, eds Weiner H., Plapp B., Lindahl R., Maser E. (New York, NY: Springer; ), 154–160. [Google Scholar]
- Hopkinson R. J., Leung I. K., Smart T. J., Rose N. R., Henry L., Claridge T. D., et al. (2015). Studies on the glutathione-dependent formaldehyde-activating enzyme from Paracoccus denitrificans. PLoS ONE 10:e0145085 10.1371/journal.pone.0145085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H., Bryant D. (2006). Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23 254–267. 10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- Huszti Z., Tyihak E. (1986). Formation of formaldehyde from S-adenosyl-L-methyl-H-3 methionine during enzymatic transmethylation of histamine. FEBS Lett. 209 362–366. 10.1016/0014-5793(86)81143-7 [DOI] [PubMed] [Google Scholar]
- Huyen N. T. T., Eiamphungporn W., Mader U., Liebeke M., Lalk M., Hecker M., et al. (2009). Genome-wide responses to carbonyl electrophiles in Bacillus subtilis: control of the thiol-dependent formaldehyde dehydrogenase AdhA and cysteine proteinase YraA by the MerR-family regulator YraB (AdhR). Mol. Microbiol. 71 876–894. 10.1111/j.1365-2958.2008.06568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2012). “Formaldehyde,” in Chemical Agents and Related Compounds: A Review of Human Carcinogens, ed. Galichet L. (Lyon: International Agency for Research on Cancer; ). [Google Scholar]
- Iwig J. S., Leitch S., Herbst R. W., Maroney M. J., Chivers P. T. (2008). Ni(II) and Co(II) sensing by Escherichia coli RcnR. J. Am. Chem. Soc. 130 7592–7606. 10.1021/ja710067d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Imlay J. A. (2007). Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282 929–937. 10.1074/jbc.M607646200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. E., Belka G. K., Du Bois G. C. (1998). S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 331 659–668. 10.1042/bj3310659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen R. G. (1971). Mechanism of reactions involving Schiff base intermediates – thiazolidine formation from L-cysteine and formaldehyde. J. Am. Chem. Soc. 93 6236–6248. 10.1021/ja00752a040 [DOI] [PubMed] [Google Scholar]
- Kato N., Yurimoto H., Thauer R. K. (2006). The physiological role of the ribulose monophosphate pathway in bacteria and archaea. Biosci. Biotechnol. Biochem. 70 10–21. 10.1271/bbb.70.10 [DOI] [PubMed] [Google Scholar]
- Kaulfers P. M., Marquardt A. (1991). Demonstration of formaldehyde dehydrogenase activity in formaldehyde-resistant Enterobacteriaceae. FEMS Microbiol. Lett. 79 335–338. 10.1111/j.1574-6968.1991.tb04551.x [DOI] [PubMed] [Google Scholar]
- Kidd S. P., Jiang D., Tikhomirova A., Jennings M. P., McEwan A. G. (2012). A glutathione-based system for defense against carbonyl stress in Haemophilus influenzae. BMC Microbiol. 12:6 10.1186/1471-2180-12-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S. P., Potter A. J., Apicella M. A., Jennings M. P., McEwan A. G. (2005). NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol. Microbiol. 57 1676–1689. 10.1111/j.1365-2958.2005.047773.x [DOI] [PubMed] [Google Scholar]
- Klatt P., Lamas S. (2000). Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 267 4928–4944. 10.1046/j.1432-1327.2000.01601.x [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lee J. W., Soonsanga S., Helmann J. D. (2007). A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. U.S.A. 104 8743–8748. 10.1073/pnas.0702081104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmeier L., Hoefener M., Wendisch V. F. (2013). Formaldehyde degradation in Corynebacterium glutamicum involves acetaldehyde dehydrogenase and mycothiol-dependent formaldehyde dehydrogenase. Microbiology 159 2651–2662. 10.1099/mic.0.072413-0 [DOI] [PubMed] [Google Scholar]
- Liao Y., Chen S., Wang D., Zhang W., Wang S., Ding J., et al. (2013). Structure of formaldehyde dehydrogenase from Pseudomonas aeruginosa: the binary complex with the cofactor NAD(+). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69 967–972. 10.1107/S174430911302160X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger W., Taucher J., Jordan A., Hansel A., Vogel W. (1997). Endogenous production of methanol after the consumption of fruit. Alcohol. Clin. Exp. Res. 21 939–943. 10.1111/j.1530-0277.1997.tb03862.x [DOI] [PubMed] [Google Scholar]
- Liu L. M., Hausladen A., Zeng M., Que L., Heitman J., Stamler J. S. (2001). A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410 490–494. 10.1038/35068596 [DOI] [PubMed] [Google Scholar]
- Liu T., Ramesh A., Ma Z., Ward S. K., Zhang L. M., George G. N., et al. (2007). CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 3 60–68. 10.1038/nchembio844 [DOI] [PubMed] [Google Scholar]
- Loshon C. A., Genest P. C., Setlow B., Setlow P. (1999). Formaldehyde kills spores of Bacillus subtilis by DNA damage and small, acid-soluble spore proteins of the alpha/beta-type protect spores against this DNA damage. J. Appl. Microbiol. 87 8–14. 10.1046/j.1365-2672.1999.00783.x [DOI] [PubMed] [Google Scholar]
- Lu K., Collins L. B., Ru H., Bermudez E., Swenberg J. A. (2010). Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol. Sci. 116 441–451. 10.1093/toxsci/kfq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke J. L., Shen J., Bruce K. E., Kehl-Fie T. E., Peng H., Skaar E. P., et al. (2014). The CsoR-like sulfurtransferase repressor (CstR) is a persulfide sensor in Staphylococcus aureus. Mol. Microbiol. 94 1343–1360. 10.1111/mmi.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maboudou P., Matheiu D., Bachelet H., Wiart J. F., Lhermitte M. (2002). Detection of oxidative stress. Interest of GC-MS for malondialdehyde and formaldehyde monitoring. Biomed. Chromatogr. 16 199–202. 10.1002/bmc.127 [DOI] [PubMed] [Google Scholar]
- Maden B. E. H. (2000). Tetrahydrofolate and tetrahydromethanopterin compared: functionally distinct carriers in C-1 metabolism. Biochem. J. 350 609–629. 10.1042/0264-6021:3500609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani J. C., Pietrusz R., Theorell H. (1970). Methanol activity of alcohol dehydrogenases from human liver, horse liver and yeast. Arch. Biochem. Biophys. 140 52–59. 10.1016/0003-9861(70)90009-3 [DOI] [PubMed] [Google Scholar]
- Marx C. J., Chistoserdova L., Lidstrom M. E. (2003). Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J. Bacteriol. 185 7160–7168. 10.1128/jb.185.24.7160-7168.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx C. J., Miller J. A., Chistoserdova L., Lidstrom M. E. (2004). Multiple formaldehyde oxidation/detoxification pathways in Burkholderia fungorum LB400. J. Bacteriol. 186 2173–2178. 10.1128/jb.186.7.2173-2178.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Sanders J. K. M., Crawford A., Hunter B. K. (1986). Formaldehyde metabolism by Escherichia coli – detection by invivo C-13 NMR-spectroscopy of S-(hydroxymethyl)glutathione as a transient intracellular intermediate. Biochemistry 25 4504–4507. 10.1021/bi00364a008 [DOI] [PubMed] [Google Scholar]
- Matsui T., Nambu S., Ono Y., Goulding C. W., Tsumoto K., Ikeda-Saito M. (2013). Heme degradation by Staphylococcus aureus IsdG and IsdI liberates formaldehyde rather than carbon monoxide. Biochemistry 52 3025–3027. 10.1021/bi400382p [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan A. G., Djoko K. Y., Chen N. H., Counago R. L. M., Kidd S. P., Potter A. J., et al. (2011). “Novel bacterial MerR-Like regulators: their role in the response to carbonyl and nitrosative stress,” in Advances in Microbial Physiology Vol. 58 ed. Poole R. K. (London: Academic Press Ltd-Elsevier Science Ltd; ), 1–22. [DOI] [PubMed] [Google Scholar]
- Meller E., Rosengar H., Friedhof A. J. (1974). Conversion of S-adenosylmethionine-C-14 to formaldehyde-C-14 and condensation with indoleamines – side reaction in N-methyltransferase assay in blood. Life Sci. 14 2167–2178. 10.1016/0024-3205(74)90099-x [DOI] [PubMed] [Google Scholar]
- Metz B., Kersten G. F. A., Hoogerhout P., Brugghe H. F., Timmermans H. A. M., de Jong A., et al. (2004). Identification of formaldehyde-induced modifications in proteins – reactions with model peptides. J. Biol. Chem. 279 6235–6243. 10.1074/jbc.M310752200 [DOI] [PubMed] [Google Scholar]
- Mitsui R., Kusano Y., Yurimoto H., Sakai Y., Kato N., Tanaka M. (2003). Formaldehyde fixation contributes to detoxification for growth of a nonmethylotroph, Burkholderia cepacia TM1, on vanillic acid. Appl. Environ. Microbiol. 69 6128–6132. 10.1128/aem.69.10.6128-6132.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L. K., Gutteridge J. M. C., Quinlan G. J. (2001). Thiols in cellular redox signalling and control. Curr. Med. Chem. 8 763–772. 10.2174/0929867013372904 [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Lesse A. J., Kirkham C., Zhong H., Sethi S., Munson R.S.Jr. (2011). A clonal group of nontypeable Haemophilus influenzae with two IgA proteases is adapted to infection in chronic obstructive pulmonary disease. PLoS ONE 6:e25923 10.1371/journal.pone.0025923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neculai A. M., Neculai D., Griesinger C., Vorholt J. A., Becker S. (2005). A dynamic zinc redox switch. J. Biol. Chem. 280 2826–2830. 10.1074/jbc.C400517200 [DOI] [PubMed] [Google Scholar]
- Newberry K. J., Fuangthong M., Panmanee W., Mongkolsuk S., Brennan R. G. (2007). Structural mechanism of organic hydroperoxide induction of the transcription regulator OhrR. Mol. Cell 28 652–664. 10.1016/j.molcel.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Newton G. L., Rawat M., La Clair J. J., Jothivasan V. K., Budiarto T., Hamilton C. J., et al. (2009). Bacillithiol is an antioxidant thiol produced in Bacilli. Nat. Chem. Biol. 5 625–627. 10.1038/nchembio.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norin A., VanOphem P. W., Piersma S. R., Persson B., Duine J. A., Jornvall H. (1997). Mycothiol-dependent formaldehyde dehydrogenase, a prokaryotic medium-chain dehydrogenase/reductase, phylogenetically links different eukaryotic alcohol dehydrogenases – Primary structure, conformational modelling and functional correlations. Eur. J. Biochem. 248 282–289. 10.1111/j.1432-1033.1997.00282.x [DOI] [PubMed] [Google Scholar]
- Nowak J. Z., Zelazowska E. (1987). Histamine levels and activity of histidine-decarboxylase (HD) and histamine-methyltransferase (HMT) in neonate and adult human-brain. Agents Actions 20 248–251. 10.1007/bf02074682 [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A., Fridovich I. (2000). The role of alpha,beta-dicarbonyl compounds in the toxicity of short chain sugars. J. Biol. Chem. 275 34853–34857. 10.1074/jbc.M005536200 [DOI] [PubMed] [Google Scholar]
- Osman D., Piergentili C., Chen J., Chakrabarti B., Foster A. W., Lurie-Luke E., et al. (2015). Generating a metal-responsive transcriptional regulator to test what confers metal-sensing in cells. J. Biol. Chem. 290 19806–19822. 10.1074/jbc.M115.663427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget M. S. B., Buttner M. J. (2003). Thiol-basedregulatory switches. Annu. Rev. Genet. 37 91–121. 10.1146/annurev.genet.37.110801.142538 [DOI] [PubMed] [Google Scholar]
- Panmanee W., Vattanaviboon P., Poole L. B., Mongkolsuk S. (2006). Novel organic hydroperoxide-sensing and responding mechanisms for OhrR, a major bacterial sensor and regulator of organic hydroperoxide stress. J. Bacteriol. 188 1389–1395. 10.1128/jb.188.4.1389-1395.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. J., Kidd S. P., Edwards J. L., Falsetta M. L., Apicella M. A., Jennings M. P., et al. (2009). Esterase D Is essential for protection of Neisseria gonorrhoeae against nitrosative stress and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 200 273–278. 10.1086/599987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. J., Kidd S. P., Jennings M. P., McEwan A. G. (2007). Evidence for distinctive mechanisms of S-nitrosoglutathione metabolism by AdhC in two closely related species, Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 75 1534–1536. 10.1128/IAI.01634-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras J., Vanophem P. W., Reijnders W. N. M., Vanspanning R. J. M., Duine J. A., Stouthamer A. H., et al. (1995). Isolation, sequencing, and mutagenesis of the gene encoding NAD-dependent and glutathione-dependent formaldehyde dehydrogenase (GD-FALDH) from Paracoccus denitrificans, in which GD-FALDH is essential for methylotrophic growth. J. Bacteriol. 177 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere M. L., Ukpabi G. N., Harry S. R., Stec D. F., Krull R., Wright D. W., et al. (2010). The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol. Microbiol. 75 1529–1538. 10.1111/j.1365-2958.2010.07076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuda S., Zhou Z. Y., Yamada Y. (1994). Structure of a novel disulfide of 2-(N-acetylcysteinyl)amido-2-deoxy-alpha-D-glucopyranosyl-myo-inositol produced by Streptomyces sp. Biosci. Biotechnol. Biochem. 58 1347–1348. 10.1271/bbb.58.1347 [DOI] [PubMed] [Google Scholar]
- Sanghani P. C., Davis W. I., Zhai L. M., Robinson H. (2006). Structure-function relationships in human glutathione-dependent formaldehyde dehydrogenase. role of Glu-67 and Arg-368 in the catalytic mechanism. Biochemistry 45 4819–4830. 10.1021/bi052554q [DOI] [PubMed] [Google Scholar]
- Schonberg A., Moubacher R. (1952). The strecker degredation of alpha-amino acids. Chem. Rev. 50 261–277. 10.1021/cr60156a002 [DOI] [Google Scholar]
- Shi Y. J., Lan F., Matson C., Mulligan P., Whetstine J. R., Cole P. A., et al. (2004). Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119 941–953. 10.1016/j.cell.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Siragusa R. J., Cerda J. J., Baig M. M., Burgin C. W., Robbins F. L. (1988). Methanol production from the degradation of pectin by human colonic bacteria. Am. J. Clin. Nutr. 47 848–851. [DOI] [PubMed] [Google Scholar]
- Skaar E. P., Gaspar A. H., Schneewind O. (2004). IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J. Biol. Chem. 279 436–443. 10.1074/jbc.M307952200 [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Varshavsky A. (1985). Formaldehyde-mediated DNA protein crosslinking – A probe for in vivo chromatin structures. Proc. Natl. Acad. Sci. U.S.A. 82 6470–6474. 10.1073/pnas.82.19.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteller G. (2008). “Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products,” in Maillard Reaction: Recent Advances in Food and Biomedical Sciences, eds Schleicher E., Somoza V., Shieberle P. (New York, NY: The New York Academy of Sciences; ), 128–133. [DOI] [PubMed] [Google Scholar]
- Staab C. A., Alander J., Brandt M., Lengqvist J., Morgenstern R., Grafstrom R. C., et al. (2008a). Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem. J. 413 493–504. 10.1042/bj20071666 [DOI] [PubMed] [Google Scholar]
- Staab C. A., Alander J., Morgenstern R., Grafstrom R. C., Hoog J.-O. (2009). The Janus face of alcohol dehydrogenase 3. Chem. Biol. Interact. 178 29–35. 10.1016/j.cbi.2008.10.050 [DOI] [PubMed] [Google Scholar]
- Staab C. A., Hellgren M., Hoog J. O. (2008b). Dual functions of alcohol dehydrogenase 3: implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell. Mol. Life Sci. 65 3950–3960. 10.1007/s00018-008-8592-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher U. H., Kidd S. P., Stafford S. L., Jennings M. P., Paton J. C., McEwan A. G. (2007). A pneumococcal MerR-like regulator and S-nitrosoglutathione reductase are required for systemic virulence. J. Infect. Dis. 196 1820–1826. 10.1086/523107 [DOI] [PubMed] [Google Scholar]
- Szende B., Tyihak E. (2010). Effect of formaldehyde on cell proliferation and death. Cell Biol. Int. 34 1273–1282. 10.1042/cbi20100532 [DOI] [PubMed] [Google Scholar]
- Tang X. J., Bai Y., Duong A., Smith M. T., Li L. Y., Zhang L. P. (2009). Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ. Int. 35 1210–1224. 10.1016/j.envint.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. (1969). Microsomal heme oxygenase – characterization of enzyme. J. Biol. Chem. 244 6388–6394. [PubMed] [Google Scholar]
- Thornalley P., Wolff S., Crabbe J., Stern A. (1984). The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalyzed by buffer ions. Biochim. Biophys. Acta 797 276–287. 10.1016/0304-4165(84)90131-4 [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. (1993). The glyoxalase system in health and disease. Mol. Aspects Med. 14 287–371. 10.1016/0098-2997(93)90002-U [DOI] [PubMed] [Google Scholar]
- Tulpule K., Dringen R. (2013). Formaldehyde in brain: an overlooked player in neurodegeneration? J. Neurochem. 127 7–21. 10.1111/jnc.12356 [DOI] [PubMed] [Google Scholar]
- Uotila L., Koivusal M. (1974a). Formaldehyde dehydrogenase from human liver – purification, properties, and evidence for formation of glutathione thiol esters by enzyme. J. Biol. Chem. 249 7653–7663. [PubMed] [Google Scholar]
- Uotila L., Koivusal M. (1974b). Purification and properties of S-formylglutathione hydrolase from human liver. J. Biol. Chem. 249 7664–7672. [PubMed] [Google Scholar]
- Veenhuis M., Van Dijken J., Harder W. (1983). The significance of peroxisomes in the metabolism of one-carbon compounds. Adv. Microbiol. Phys. 24 1–78. 10.1016/S0065-2911(08)60384-7 [DOI] [PubMed] [Google Scholar]
- Vogt R. N., Steenkamp D. J., Zheng R. J., Blanchard J. S. (2003). The metabolism of nitrosothiols in the mycobacteria: identification and characterization of S-nitrosomycothiol reductase. Biochem. J. 374 657–665. 10.1042/bj20030642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorholt J. A. (2002). Cofactor-dependent pathways of formaldehyde oxidation in methylotrophic bacteria. Arch. Microbiol. 178 239–249. 10.1007/s00203-002-0450-2 [DOI] [PubMed] [Google Scholar]
- Vorholt J. A., Marx C. J., Lidstrom M. E., Thauer R. K. (2000). Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182 6645–6650. 10.1128/jb.182.23.6645-6650.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., Gleisten M. P., Donohue T. J. (2008). Identification of proteins involved in formaldehyde metabolism by Rhodobacter sphaeroides. Microbiology 154 296–305. 10.1099/mic.0.2007/011346-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P. H., Wright S., Fan E. H., Lun Z. R., Gubisne-Harberle D. (2003). Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim. Biophys. Acta 1647 193–199. 10.1016/s1570-9636(03)00101-8 [DOI] [PubMed] [Google Scholar]
- Yu P. H., Zuo D. M. (1996). Formaldehyde produced endogenously via deamination of methylamine. A potential risk factor for initiation of endothelial injury. Atherosclerosis 120 189–197. [DOI] [PubMed] [Google Scholar]
- Yu R., Lai Y., Hartwell H. J., Moeller B. C., Doyle-Eisele M., Kracko D., et al. (2015). Formation, accumulation, and hydrolysis of endogenous and exogenous formaldehyde-induced DNA damage. Toxicol. Sci. 146 170–182. 10.1093/toxsci/kfv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurimoto H., Hirai R., Matsuno N., Yasueda H., Kato N., Sakai Y. (2005). HxlR, a member of the DUF24 protein family, is a DNA-binding protein that acts as a positive regulator of the formaldehyde-inducible hxlAB operon in Bacillus subtilis. Mol. Microbiol. 57 511–519. 10.1111/j.1365-2958.2005.04702.x [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Wishnok J. S., Blusztajn J. K. (1983). Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 225 320–324. [PubMed] [Google Scholar]
- Zhang L., Steinmaus C., Eastmond D. A., Xin X. K., Smith M. T. (2009). Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat. Res. 681 150–168. 10.1016/j.mrrev.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Zuo D. M., Yu P. H. (1994). Semicarbazide-sensitive amine oxidase and monoamine-oxidase in rat-brain microvessels, meninges, retina and eye sclera. Brain Res. Bull. 33 307–311. 10.1016/0361-9230(94)90198-8 [DOI] [PubMed] [Google Scholar]